Session IV Future Directions
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.Session IV Future Directions
Biotechnology and Biological Sciences Research Council (BBSRC) Integrative microbiome research and capability
156. Dr Louisa Jenkin (BBSRC) presented on BBSRC’s interests in microbiome research and what their current activities in this area are.
157. The UK Research and Innovation (UKRI) invest £8 billion per year in research and innovation partnering with academia and industry. The BBSRC support bioscience research through training and investments (£378m).
158. BBSRC interests in microbiome research are to understand the biological mechanisms of the role and function of the microbiome on health.
159. The microbiome fits under the remit of the BBSRC in their goal to improve food safety by better understanding the risks to human health from food at any stage of the food chain. This also involves understanding the unintended consequences of food innovation, processing and dietary change, which includes the microbiome.
160. The BBSRC does not conduct research focused on specific human diseases or abnormal conditions; animal models of human disease and human toxicology; or human-human transmission.
161. BBSRC held a workshop in 2020 on Microbiome Capability in which 45 participants attended from 38 research organisations, industry, networks and societies. The aim was to help the BBSRC to understand current and future microbiome community needs.
162. The speaker described the general reflections of the workshop, the biggest research challenges identified by the participants and what the UK’s capabilities in the area are. There is a need to build interdisciplinary approaches, and collaborations will be crucial, to understand the functions of microbiomes and the influence of key factors. It will be important to connect basic and applied research; look at all components of the microbiome; increase the variety of model organisms/ choose the right model system, including the use of synthetic communities to explore complex interactions; share data, software, tools and methodology within and between communities and fields as well as the integration of data, e.g. multi-omics; data standards, metadata, Findable, Accessible, Interoperable, and Reusable (FAIR).
163. Some of the biggest research challenges for the microbiome field include correlation and causation; sampling; scales, time series and longitudinal studies and functional annotation.
164. It was noted that the UK current technologies, resources and skills in the field include data and computing; infrastructure; multidisciplinary collaboration skills and expertise; as well as translation.
165. The workshop helped to inform new BBSRC activities: BBSRC-NSF/BIO lead agency 2024 and BBSRC-DFG Lead Agency Pilot 2023-2024. These aim to help understand host-microbe interactions, synthetic microbial communities and the microbiome as a whole. It also fed into other activities such as the KTN Microbiome Innovation Network road-mapping activity and strategy.
166. The speaker then stated some of BBSRC strategic investments and current activities including: the Quadram Institute, which is focusing on understanding how gut health, microbiology and food interact to promote physical and mental health across the life course and prevent disease including microbial safety in the food chain; and the Centre for Microbial Interactions to harness the huge breadth and capacity of our expertise within the extensive microbiology community at Norwich Research Park (NRP) to solve global challenges in food security, human health and climate change (which includes chemical interactions: AMR, natural products cell biology).
167. In Summer 2022, co-funded with the Food Standards Agency, a £2.2m Innovation Hub for Food Safety and UK Food Safety Research Network were launched to coordinate and fund cross-sectoral research and training activities that address current and emerging challenges in food safety. The aims include addressing microbial risk in the food chain, with the goal of introducing new capability, knowledge or skills to help reduce these risks.
168. BBSRC has recently announced £378m to support 16 new programmes, which cover a wide breadth of bioscience research. The programmes cover areas such as advanced genomics, crop resilience, animal health & welfare, food safety, food security, nutrition, lifelong health, preventing & controlling viral disease, and sustainable farming & agriculture.
169. The research themes include a variety of microbiome work: chemical interactions (antimicrobial resistance (AMR)), engineering microbial interactions (synthetic biology, biotechnology), microbial community interactions (microbiomes, infection, health), plant-microbe interactions (mutualism, symbiosis, pathogenesis), evolutionary interactions (biodiversity, genetics, genomics).
170. Finally, the speaker presented relevant BBSRC investments into microbiome related work encompassing biofilms, AMR, infectious diseases, diet and health, gut immunology and the skin microbiome in healthy aging.
171. Moving forward, BBSRC is taking a one health approach to improve interdisciplinary working and the interconnectedness of soil, plant, animal and human health, considered alongside the complexities of wider health, social and environmental factors to avoid adverse effects, unintended consequences and promote nutrition security.
European Food Safety Authority (EFSA) Roadmap Microbiome
172. Dr Javier Moreno (Food science Research Institute (CIAL)/Spanish National Research Council (CSIC)) introduced the EFSA roadmap, which includes the impact of the microbiome on human, domestic animal, and plant health.
173. This project has performed a comprehensive and critical assessment of the evidence-based research about: i) the impact of dietary compounds on the human and some domestic animals (i.e., poultry, ruminants and pigs) gut microbiome; ii) the most representative in vitro and in vivo models of the human gut microbiota currently used in microbiome research studies; and iii) the methodology used to measure changes in the microbiota.
174. The approach used was to critically review the knowledge, data, and methods relevant for risk assessments. This, together with a number of experimental case studies, which looked at impacts on human and animal health, enabled identification of gaps in both the literature and research.
175. The draft proposed roadmap aims to advance research for addressing risk assessment needs, accounting for effects on/by the microbiome in humans, domestic animals, and the environment.
176. Drafting a roadmap for the gut microbiome is a real challenge. The gut microbiome plays an important role as a mediator and moderator of the effects of some dietary compounds on humans/animals. Incorporating these gut microbiome interactions in the risk assessment could help to fully understand potential hazards derived from their exposure.
177. The gut microbiome is a complex universe by itself, even more so when looking at its relationship with the host. It is a relatively novel research area that is evolving in parallel to technological developments and bioinformatics.
178. Knowledge gaps include: i) lack of data on the exposure of the gut microbiome to certain compounds and underlying mechanism(s); ii) lack of standardized models, iii) methodological limitations, iv) lack of guidance to evaluate microbiome-related data in safety evaluations.
179. The first part of the project was to establish how the gut microbiome acts as a moderator for dietary compounds in humans and animals. This needs to be inclusive of dietary compounds that are partially or not absorbed and incorporate the potential hazards of this into any further risk assessments.
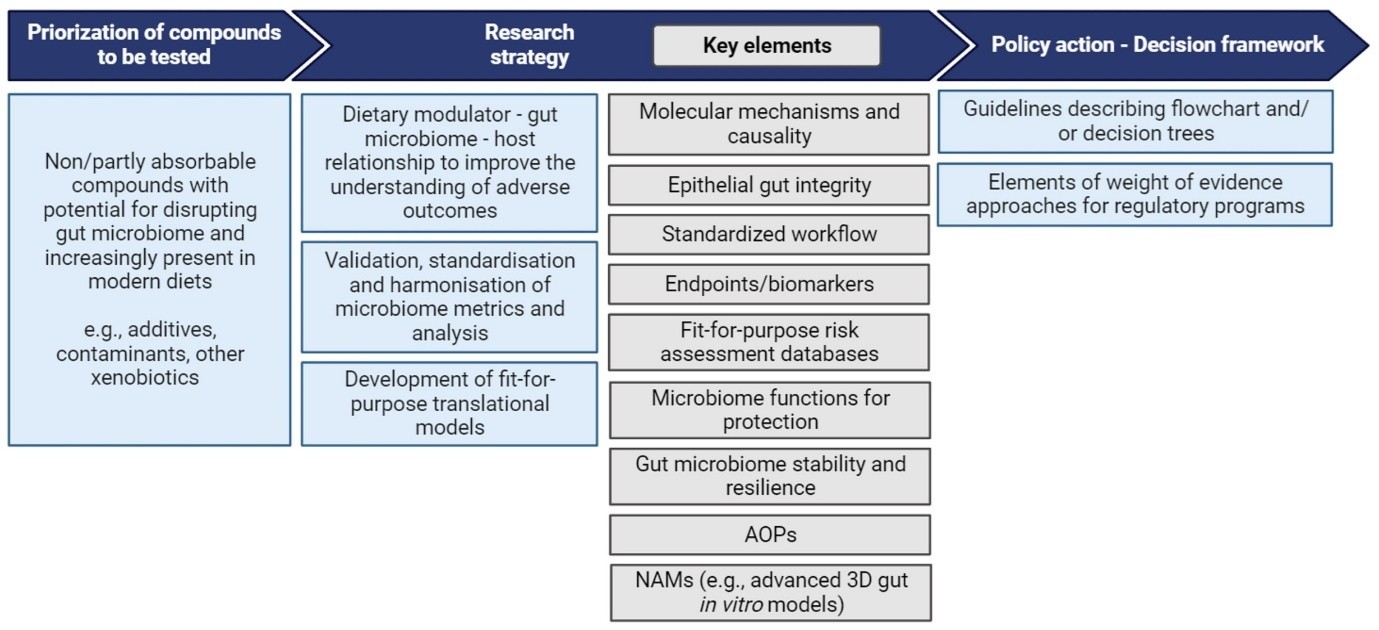
180. The stages of the roadmap (Figure 3) comprise the following steps:
1) Prioritisation of compounds to be assessed
2) Research strategy
3) Policy action- Decision framework.
Figure 3. Roadmap to advance research to address risk assessment needs to account for effects on/by gut microbiomes in humans. Figure taken from EFSA Roadmap for the integration of gastro-intestinal (GI) tract microbiomes (human and domestic animal) in risk assessments under EFSA's remit (2024).
181. The speaker then discussed the interactions between dietary modulators, the gut microbiome and the host (Garrido-Romero et al., 2024). There is evidence of some fatalities from chemical contaminants in food potentially due to detrimental effects on the human gut microbiome. These studies have been focused on the understanding of the mechanisms, which include:
- The gut microbiome can metabolise xenobiotics directly through microbial enzymatic biotransformation by specific bacterial enzymes. This could lead to the production of toxic metabolites that can then be absorbed in the small intestine and/or liver where they are detoxified and form conjugates. These are then secreted back into the intestinal lumen and into the microbiome community where the active toxic moiety is released, which can potentially cause carcinogenic or genotoxic effects.
- Another mechanism of xenobiotics is an indirect influence on host metabolic and transport pathways in the liver utilising microbial products.
- The inhibition or promotion of bacterial growth which can affect the composition and function of the gut microbiome and disrupt the homeostasis of the environment.
182. The speaker then emphasised that there is a need for internationally agreed minimal methodological requirements and reference materials for research and regulatory bodies to use when analysing the human gut microbiome. This needs to include information on sample collection, storage/processing of samples, handling and generating data, analysis workflows and fit-for-purpose data repositories for risk assessment.
183. There is a need to develop fit-for-purpose translational models to identify potential key events following exposure to harmful diet-derived compounds and predict the potential effects that can be had on the gut. Fit-for-purpose translational models need to be a considered as validated experimental models, because this is essential in the overall context of assessing effects on the gut microbiome. The gut microbiome can be a critical player in the metabolic and inflammation processes, immune reactions, and integrity of the gut barrier. Validated models can have short to medium exposure endpoints set as the markers for experiments. This can be using inflammatory markers, which assess the integrity of the epithelium, combined with markers produced by the bacteria that signal disruption.
184. Finally, the speaker discussed risk assessment and policy actions. Policy actions are included in the roadmap to help with regulator and innovator engagement to address these gaps in knowledge, and also to promote and prioritise funding areas for research and regulatory needs. The Roadmap encourages collaborative efforts to develop decision trees and end points that can be used by risk assessors and policy makers, enabling both safety and efficacy assessments related to microbiome-based products and tools. In particular, this will help close the gaps in early-microbiome regulatory guidance and ensure that it is fit for purpose in practice.
Microbiome Strategic Roadmap (Regulatory and food)
185. Professor O’Brien (The Food Observatory and Ulster University) introduced the KTN Microbiome Special Interest Group (part of Innovate UK) to the audience. The objective is to bring together all stakeholders with an interest in the microbiome and to increase the translation of knowledge on the microbiome from science into action. Representatives of the food and pharmaceuticals industries, regulatory bodies, research funding councils and academia form this group, which has now been renamed as the Microbiome Innovation Advisory Board.
186. Two conferences organised by the Microbiome Innovation Advisory Board have already been held, one in Glasgow in 2023 and another one in Liverpool in 2024. The speaker explained that the future of this board is to hand the responsibility of delivering the recommendations for a microbiome strategy roadmap to a multi-university consortium group.
187. So far, three reports have been published by the Microbiome Innovation Advisory Board:
1) Microbiome Strategic roadmap
2) Human Intestinal Microbiome Therapies and Diagnostics – The Science, Opportunities and Challenges
3) Securing the future of microbiome research and innovation: the need for biobanking infrastructure in the UK
188. The presenter noted that the KTN Microbiome Strategic Roadmap is in line with the One Health approach to support unifying research activities in human, animal and plant health, and hopes to advance science translation and business application. The scope of this roadmap encompasses a wide range of applications, e.g. human intestinal and plant microbiome; animal, plant, crop and soil health; and live biotherapeutic products.
189. Within the nutrition and wellness areas, the speaker enumerated a few sub-areas such as immune modulation and foodborne diseases, and the most research driven areas i.e. metabolic health, women’s health and ageing.
190. The presenter went on to highlight that one of the goals of the KTN Microbiome Strategic Roadmap from a regulatory perspective is to anticipate and mitigate any unintended consequences of a product, to be ahead of any issues that might give raise to future human health concerns. The goal is “Ensuring regulations, rules and good regulatory practices encourage advances that target unmet needs, mitigate any unintended consequences of the developments and are based on good regulatory principles”. This goal is shared with the National Science and Technology Framework and National Vision for Engineering Biology.
191. The speaker then stated that the UK is well positioned to take advantage of opportunities represented by microbiome therapeutics and diagnostics. However, they also highlighted some of the challenges of this roadmap. For example, there is a gap between innovation and regulatory procedures, and also a lack of regulatory standards and definitions, which brings uncertainty. Another challenge is that assets resulting from microbiome research can impact multiple regulatory domains, e.g. food, agriculture, animal health, aquaculture, personal care. Additionally, the KTN Microbiome Strategic Roadmap could potentially disrupt traditional assumptions about definitions of health and disease.
192. The presenter reminded the audience of those methodological challenges and limitations when assessing the microbiome that had been highlighted throughout the session on numerous occasions. For example, a study that had used a bacterial fingerprint to predict vulnerability to cancer had misidentified bacterial sequences with human sequences. Another example was the reported major errors in a paper that identified cancers based on their microbiome, which led to the authors retracting their article. To address these challenges, improved biological standardisation in the omics area is needed.
193. The presenter went on to introduce the topic of Adverse Outcome Pathways (AOPs). He highlighted this with a proposed AOP that does not arise from a xenobiotic, but from a SARS viral protein that interacts with enterocyte ACE2 receptors, which leads to an alteration of the microbiota. In the areas of evidence used to evaluate this outcome, one is microbiome biodiversity. At least 17 different measures are cited by the paper, however there is no reference as to what constitutes an adverse effect.
194. As a concluding remark, the speaker acknowledged that there is a significantly high variability in the human microbiome and multiple modulating factors, not just the diet. In a way, every home has its own microbiome, and that is why it is difficult to define what is normal and abnormal, what is a result of adaptation, and whether this adaptation is reversible or adverse.