Session III Possible ways to evaluate in the short to medium term and microbiome interventions for maintaining health and treating disease
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.Session III Possible ways to evaluate in the short to medium term and microbiome interventions for maintaining health and treating disease
A tiered approach to risk assess microbiome perturbations: illustrative case study on effects induced by application of beauty and personal care products
132. Dr Aline Métris (Unilever) introduced a tiered approach to risk assess the effects of beauty and personal care products on the skin and oral microbiome (Métris et al., 2022). The presentation was focussed on the skin microbiome and began by discussing the large biogeographical variability (i.e. elbows different from hands which are different from face) as well as significant inter-individual variation in the microbiome.
133. Although several dermatological conditions (including atopic dermatitis) are associated with dysbiosis of the skin microbiome, it is uncertain what precisely characterises a ‘healthy’ skin microbiome. A similar theme is reflected across many discussions on the microbiome and hence, at present, a relative, rather than an absolute, approach to risk assessment is more feasible. This is where an individual serves as their own control, whether in time or space.
134. With these considerations in mind, the speaker then discussed the Unilever Safety, Environmental and Regulatory Science (SERS, previously SEAC) Microbiology Team approach to tiered risk assessment (Figure 2) in the context of the skin microbiome.
135. A tiered approach is one in which evidence of risk at one level (or tier) prompts progression to the next level (or tier), as necessary, and in which each level is increasingly empirical and experimental in nature.
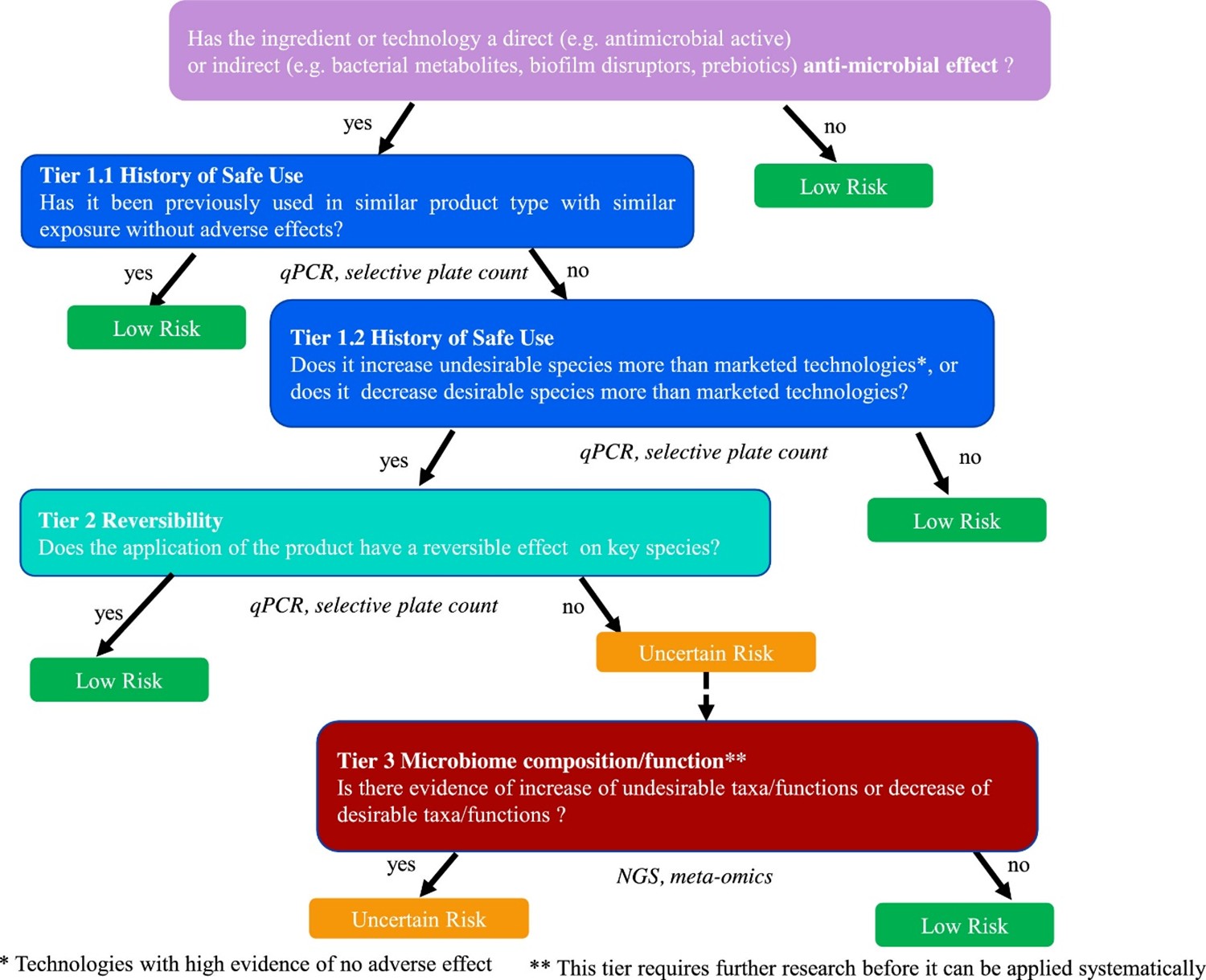
Figure 2. Tiered framework to assess the risk in response to the perturbation of the oral or skin microbiome by application of beauty and personal care products. The risk is classified into “low risk” (green boxes) and “uncertain risk” (orange box). The blue boxes refer to the first tier which is based on the notion of History of Safe Use, the second tier, depicted in turquoise, is based on the notion of reversibility of effect on key species and the third tier, in red, relies on Next Generation Sequencing (NGS) data describing taxa/functions. The data type used in each tier is shown under the corresponding box. Figure taken from Métris et al., 2022.
136. Questions such as ‘is the product a direct or indirect antimicrobial’ should guide whether an assessment is required at all. Tier 1 is based on evidence of history of safe use of a specific compound or other technologies that have the same or more pronounced effects on the microbiome than the compound under assessment. In the absence of history of safe use, one needs to progress to Tier 2.
137. Tier 2 is based on the reversibility of any change induced by the personal care product. In a longitudinal study, the microbiome is compared between baseline (i.e., before product application), after product application (intervention period) and after discontinuing product use (regression). After stopping using the product, do any observed changes during intervention return to baseline? Important considerations include the precise timing of the protocol (i.e., the length of intervention and regression time) and the methods used to profile the microbiome. Methods include 16S ribosomal RNA (rRNA) sequencing for microbiome profiling and Quantitative PCR (qPCR) for quantitative estimates of species of interest. However, functional changes and compositional changes may be related in relatively complex ways, meaning that this step requires careful analysis. If the observed change is not reversible then it is necessary to proceed to Tier 3.
138. Tier 3 involves an analysis of the microbiome functions implicated in the change and an understanding of which mechanisms are important for maintaining skin and oral health.
139. Finally, the speaker summarised the remaining scientific challenges by asking the questions:
- Which organisms/strains/species are biomarkers of health and disease?
- Which are the corresponding functions impacted and what might the effects be?
140. Known microbiome functions include resistance to colonisation of pathogens and interactions with host barrier functions and immunology. However, the specific modes of actions are not well understood. In silico and in vitro methods may be adopted to characterise microbiome functions (rather than taxa) and host-microbiome interactions.
Faecal Microbiota Transplant (FMT) Liver disease example
141. Dr Lindsey Ann Edwards (Kings College London) presented on how the microbiome can be modified using faecal microbiota transplantation in patients with end-stage cirrhosis.
142. The focus of the research is on modulating the microbiome to reduce intestinal barrier damage, inflammation and antimicrobial resistance. Simultaneously, reducing infections in chronic inflammatory diseases, including liver disease, lowers the requirement for the prescription for antibiotics.
143. Globally, 8 million people per year die due to infections; of those, over a million of these deaths are due to drug-resistant organisms, resistant to all known antibiotics. Antimicrobial resistance is escalating, and no new antibiotics have been discovered since the 1980’s. Therefore, the Lord O’Neil report predicts deaths due to antimicrobial resistance could reach over 50 million per year by 2050 if urgent action is not taken.
144. Liver cirrhosis patients (end-stage liver disease) have a microbiome dysbiosis leading to an outgrowth of pathogenic species that causes intestinal barrier damage and inflammation, and translocation of microbial species across the intestinal barrier, which can lead to systemic infections. This is combined with a disrupted immune system that is not able to fight those infections, which then leads to multiple organ failure. A dysbiotic microbiome causes intestinal inflammation, which can act as an environmental stressor to the microbes, which allows for the upregulation of virulence factors such as antimicrobial resistance genes, which progresses as the liver disease progresses. This increased risk of infections, particularly drug-resistant infections, is a major cause of deaths in patients with liver disease.
145. The microbiome within the intestine interacts with a single epithelial layer and a large mucosal immune system. In fact, 75% of the body's immune system is in the intestine. The epithelial layer and the mucosal immune system are responsible for preventing microbial translocation. Approximately 75% of the blood from the gut drains to the liver. If microbial translocation occurs, then the microbes can end up in the liver where there is also an immune system to try to tackle the infectious agents. This is the body’s final resort in stopping the systemic spread of infection and possible septicemia and death.
146. As liver disease patients are prone to risk of infection, they are prescribed prophylactic antibiotics even when no infection is detected. Many liver patients take them daily whether needed or not. This practice can exacerbate the situation as the antibiotic also acts as an environmental stressor and can lead to further antimicrobial resistance. The ATTIRE trial was published in the New England Journal of Medicine, finding that in the absence of an infection, there was ‘No evidence’ that prophylactic antibiotics prevent hospital-acquired infections in cirrhosis patients and recommended targeted antibiotic prescription on the development of an infection. However, as the risk of infection and death is high in end-stage liver disease, it is understandable that many liver clinicians are reluctant to cease the prophylactic prescription. Clear guidelines are required so this practice does not continue.
147. The NIHR-funded PROFIT study was a randomized placebo-controlled trial of Feacal microbiota transplant (FMT) in liver cirrhosis; the aim is to use a treatment that modifies the microbiome in order to reduce inflammation and the spread of antimicrobial resistance, as well as treat the cirrhosis. Donor stool was screened to determine no carriage of pathogens and was blended with glycerol, then administered into the duodenum via a naso-gastrointestinal tube inserted during endoscopy.
148. Exclusion criteria were that the recruited patients were not consuming alcohol and were not on any antibiotics. The recipients were mostly males in their 60s who had consumed diets high in animal protein and sugar, the donors were females in their 20-30s who followed either a vegan, vegetarian or omnivorous diet high in fruit and vegetables and complex carbohydrates.
149. Stool samples were taken at baseline before treatment and then 7-, 30- and 90-days post-treatment. Various techniques were employed to examine the effect of FMT on the microbiome itself and any changes in patient immunity and the intestinal barrier were studied, including plasma and faecal cytokines and intestinal barrier integrity markers of the gut. It was shown that FMT allowed for donor engraftment which is important to success.
150. It was shown that a mixture of a high-fibre, high-protein diet with FMT allowed for a more omnivorous microbiome. After one dose of the FMT, species richness increased from the baseline within the recipient; this did start to drop off at around 90 days, but did not return to the baseline level. The results suggested that multiple FMT doses would likely be needed. The new NIHR-funded PROMISE trial is dosing patients every 90 days.
151. Dr Edwards has discovered that FMT reduced pathogens, including those that cause epithelial barrier disruption, which are more prevalent in those that consume a western diet. This occurred in combination with the replacement by many anaerobic beneficial species of those consistent with a diet high in complex carbohydrate consumption, where microbial metabolic capacity was also replaced.
152. Dr Edwards has developed a method that allows for the quantification of mucosal cytokines by electrochemiluminescence. T helper 17 cell (TH17) cytokines and markers of barrier disruption were measured using this technique. It was found that the patients had levels of mucosal TH17 cytokines sufficiently high to damage the intestinal barrier, with low levels in the circulation, reducing the ability to fight infections. Treatment with FMT facilitates a reversal, which enables barrier repair and to fight against infections.
153. A mass-spectroscopy technique was developed by Dr Edwards to identify faecal proteins. Analysed pre- and post-FMT, in comparison to placebo controls, FMT resulted in changes in the levels of a number of proteins, of which 301 were quantified, 154 of human origin, and 147 bacterial. The human proteins were those involved in barrier response and repair. There was also evidence of a change in xenobiotic metabolism. However, this will require further research. Most of the bacterial-derived proteins were enzymes, including those involved in ammonia metabolism. In liver disease, the liver is unable to metabolise ammonia, therefore, the ammonia is retained in the blood and leads to hepatic encephalopathy, which can be fatal.
154. After FMT, a reduction in plasma ammonia is seen and there is an increase in faecal ammonia achieved by a change in metabolic pathways, which in turn leads to a better functioning of host immunity. In the future, boosting better immunity may reduce deadly infections and may limit the need for antibiotic prescriptions.
155. Dr Edwards found that faecal microbiota transplantation also reduced antimicrobial resistance gene carriage.