Hepatotoxic case reports
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.This is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
DILIN network
135. The United States Drug-Induced Liver Injury Network (DILIN) have published several articles concerning herb related drug injury. Articles that referred to G. cambogia are summarised in chronological order.
136. In 2015, Navarro et al., noted that G. cambogia was implicated with herbal products in the DILIN database; however, the role of this herb in causing liver injury was difficult to assign since there was a lack of documentation of their chemical presence and purity, the possibility of contamination with other herbal products or mislabelling of the ingredients.
137. In 2022, Vuppalanchi et al., described the hepatotoxic effects of G. cambogia, either alone or in combination with green tea extract (catechins) consumption. Among the 1,418 patients enrolled in the DILIN from 2004-2018, it was identified that 22 cases of liver injury could be attributed to G. cambogia alone (n=5/22), in combination with green tea (n=16/22) or in combination with ashwagandha (n=1/22). Control groups consisted of 57 patients with liver injury from herbal and dietary supplements (HDS) containing green tea without G. cambogia and 103 patients from other HDS.
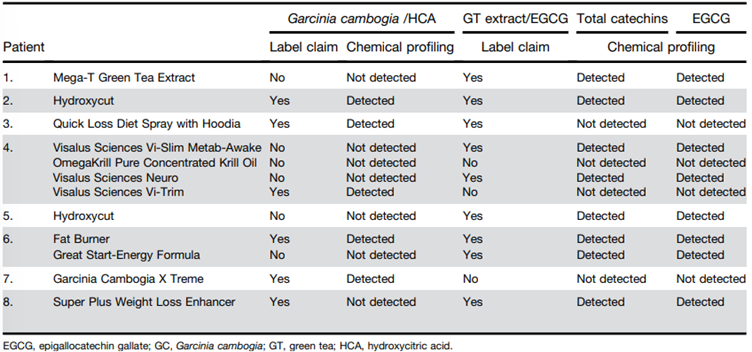
138. Patients who took G. cambogia (see Figure 6) were aged 17 and 54 years (n=22; 12 females and 10 males) with liver injury arising 13-223 days (median = 51 days) following the first dose. The doses for each product type were not made readily available, and it should be noted that although one of the products claimed the presence of Garcinia/HCA, it was not detected.
Figure 6 - Names and products linked to G. cambogia and green tea extract (catechins) induced liver injury (reproduced from Vuppalanchi et al., 2022).
139. Of these patients, one died, one required liver transplantation and 20 were hospitalised. The liver injury was hepatocellular with jaundice. Peak values of aminotransferases were significantly higher (2,001 ± 1,386 U/L) in the G. cambogia group (P < .018), the median time for improvement in total bilirubin (TB) was significantly lower compared with the control groups (10 vs 17 and 13 days; P = .03).
140. The presence of HLA-B∗35:01 allele was significantly higher in the G. cambogia containing HDS (55%) compared with patients because of other HDS (19%) (P = .002) and those with acute liver injury from conventional drugs (12%) (P = 2.55 × 10-6). The authors concluded that liver injury caused by G. cambogia and green tea was clinically indistinguishable and hypothesised that there is possible association immune-mediated mechanism of injury via HLA-B*35:01 allele.
LATINDILI
141. Bessone et al., (2025) published a “comprehensive analysis” of patients enrolled into the Latin American DILI Network over a decade. Chemicals suspected of causing DILI were classified according to the Anatomical Therapeutic Chemical classification. Causality was assessed using the Roussel Uclaf Assessment method. Overall, 468 idiosyncratic DILI cases were analysed, it was observed that 62% were women (mean age 49 years). Of the cases, 4.1% had a fatal outcome, and 24 patients (12%) developed chronic DILI. The most common drug classes were systemic anti-infectives (31%), musculoskeletal agents (12%), antineoplastic and immunomodulating agents (11%), and herbal and dietary supplements (9%). A total of 6 cases were attributed to G. cambogia.
Other literature
142. Al-Khazraji et al., (2020) [abstract only – poster for symposium] claimed to report the first human case report of G. cambogia induced autoimmune hepatitis. A 39-year-old female with no past medical history presented with fatigue and dark coloured urine. Clinical exam demonstrated a palpable liver and scleral icterus. The patient reported using a “slimming herbal tea supplement” containing pure GC for weight loss 5 weeks prior to presentation [no further information on dose or HCA content]. Elevated transaminase levels were observed and were considered significant: alanine transaminase (ALT) 1803 IU/L; aspartate aminotransferase (AST) 1026 IU/L; alkaline phosphatase (ALP) 139 mg/dL and TB 5.2 mg/dL. Further liver work up revealed an elevated anti-nuclear antibody (ANA) 1:160 titer; anti-smooth muscle antibodies (ASMA) 1:320; and immunoglobulin G 1,814. The normal range for these parameters were not detailed in the paper. A liver biopsy was performed, which demonstrated moderate mixed inflammatory cells including lymphocytes, plasma cells, neutrophils and “rare” eosinophils in the portal tracts. Interface hepatitis and cholestasis was also noted. The authors stated that findings were consistent with DILI with a background of autoimmune hepatitis. The patient was treated with steroids (Prednisone 40 mg orally, daily), upon tapering of her steroids; her liver function began to rise, and she was started on immunosuppressive therapy for long term maintenance with “good response”. The authors further stated that G. cambogia liver injury can last for 2–3 months with normalizing of liver function tests by 5 months.
143. Crescioli et al., (2018) presented four cases of acute liver failure in women taking G. cambogia extract for weight loss, and a literature review of clinical evidence about hepatic toxicity in patients taking dietary supplements containing G. cambogia extract.
144. For Case 1, a 61-year-old woman presented to the emergency department with symptoms of abdominal pain, nausea, progressive weakness, jaundice, dark urine, and acholic stools. The patient’s anamnesis denoted cholecystectomy, mixed dyslipidemia, and hypothyroidism in treatment with levothyroxine. There was no history of alcoholism or exposure to hepatotoxins; she also denied paracetamol abuse. She reported taking one envelope/daily of SUPER ANANAS SLIM®, for a period of 2 months to lose weight. This contained extracts of G. cambogia (HCA 60%), Ananas comosus (bromelain 334 GDU; gelatine dissolving units), and Ilex paraguariensis [Yerba mate] (caffeine 2%). Laboratory tests revealed that ALT, AST, TB, ALP, gamma-glutamyl transferase (GGT), direct bilirubin and albumin were higher than the normal total range at 1,629 U/L, 1,121 U/L, 22.5 mg/dL, 16.7 mg/dL and 2.2 g/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL, 0-0.25 mg/dL and 3.5-5 g/dL, respectively. Serology was negative for hepatitis viruses or autoantibodies. A check-up 3-months prior had found these parameters to be normal. The abdominal computed tomography scan revealed a small peritoneal effusion, perihepatic lymphadenopathy, and a hepatic biopsy which was consistent with cholestatic hepatitis. Four weeks after the cessation of the supplement intake, patient symptoms and liver function tests gradually improved, and the patient was discharged with no need for liver transplant. Four months later, the levels of the previously tested markers reverted to normal values.
145. For Case 2, a 39-year-old woman presented to the emergency department with symptoms of jaundice, asthenia, loss of appetite, and right hypochondrial pain. Her anamnesis denoted arterial hypertension, obesity (BM 44.9 kg/m2), and hiatal hernia. Her medication at the time of admission were methyldopa 500 mg/day, domperidone 20 mg 3 times/day and omeprazole two 20 mg capsules/day. She also reported taking two dietary supplements for weight loss recommended by her dietician. The first, OBLESS®, contained in each capsule: C. aurantium [bitter orange] 140 mg of extract 10% (14 mg of synephrine), G. cambogia (72 mg of HCA), Orthosiphon stamineus [cat’s whiskers or Java tea] (0.2 mg of sinensetin), and Griffonia simplicifolia [African black bean] (75 mg of 5-hydroxy-l-tryptophan). The second dietary supplement was a magistral preparation containing in each capsule: C. aurantium [bitter orange] 350 mg of extract 6% (21 mg of synephrine), Rhodiola rosea [Roseroot or Goldenroot] 150 mg extract, and O. stamineus [cat’s whiskers or Java tea] 200 mg extract. The patient declared that she had been taking the first dietary supplement for the previous month (1 capsule/day) and the magistral preparation for 15 days (1 capsule/day), simultaneously. Laboratory tests performed revealed that ALT, AST and TB were higher than the normal ranges at 1,554 U/L, 1,071 U/L and 15.2 mg/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL, respectively. Serology was negative for hepatitis viruses, cytomegalovirus, and varicella-zoster virus. Non-specific antinuclear antibodies and biliary antibodies were positive. Abdominal ultrasound was normal and did not reveal steatosis. Liver biopsy was not performed. Supplement intake was ceased. After 12 days of hospitalisation, the patient was discharged with no need of supplementary therapies, and a diagnosis of acute cholestatic hepatitis related to consumption of OBLESS® was made.
146. For Case 3, a 47-year-old woman was admitted to the emergency department with symptoms of severe abdominal pain (right hypochondrial). Her anamnesis denoted hyperthyroidism (treated with levothyroxine 100 µg/day), arterial hypertension (treated with enalapril 20 mg/day), and mild obesity. She also reported taking of THERMO GIALLO®, as self-medication for weight control. Each capsule contains 50 µg chromium and 400 mg G. cambogia of which 50% was HCA (200 mg), the patient reported that she had been consuming 2 capsules/day for a month. Laboratory tests revealed elevation of ALT, AST and TB at 299 U/L, 67 U/L and 0.7 mg/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL, respectively. Serology was negative for hepatitis or autoantibodies. No clinical evidence of steatosis was observed, and a liver biopsy was not performed. During the patient’s hospital stay, after cessation of weight-loss supplement, the levels of TB spontaneously declined, and her symptoms and liver function tests “rapidly improved”, without the need of therapies. The patient was discharged with a diagnosis of acute hepatitis.
147. For Case 4, a 52-year-old woman was admitted to the emergency department and was diagnosed with acute hepatitis. No “significant” diseases and medication therapies were reported in anamnesis. However, she was taking two JILL COOPER BE SLIM® products (1 capsule/day for each product) for weight control, containing 400 mg G. cambogia of which 60% was HCA (240 mg), and 400 mg green coffee of which 50% was chlorogenic acid (200 mg), respectively. These products were purchased via T-commerce and were used for a month prior to the emergency department visit. Laboratory tests revealed elevation of ALT, AST, and TB at 1,819 U/L, 1,442 U/L and 14.7 mg/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL. Serologies for hepatitis viruses and autoantibodies were also negative. No clinical evidence of steatosis was observed, and a liver biopsy was not performed. During the following days [number of days not specified] and cessation of the supplement products, liver parameters “spontaneously declined” and acute hepatitis “completely resolved” with no need of supplementary therapies.
148. As previously mentioned, Crescioli et al., (2018) also performed a literature review related of four areas: i) general information for G. cambogia; ii) its use in humans for weight control; iii) the safety of G. cambogia, iv) G. cambogia dietary supplements. The criteria were filtered for human case reports and series only, published between January 2000 and October 2017. The final selection were 24 case reports and 8 case series reporting adverse effects in a total of 66 patients who consumed G. cambogia extract. Five studies reported single cases of myocarditis (Allen et al., 2014), cardiomyopathy (Joseph et al., 2014), serotonin toxicity (Lopez et al., 2014), hypoglycaemia (Roche et al., 2014), and thrombocytopenic purpura (Sikka et al., 2016). Two patients presented acute pancreatitis (Sidhu & Khehra, 2016) and diabetic ketoacidosis (Bystrak et al., 2017), three patients experienced rhabdomyolysis (Dehoney & Wellein, (2009); Hines et al., (2015); Mansi & Huang, (2004)), and in five studies the authors described adverse events of mania (Cotovio & Oliveira-Maia, (2017); Narasimha et al., (2013); Hendrickson et al., (2016)) and multiple psychotic symptoms (Nguyen et al., (2017); Wong et al., (2016)). The patients were mostly women (62%) with no relevant medical history. Seventeen out of the 32 studies described cases of acute liver injury, liver failure, and hepatotoxicity, observed in 50 patients who consumed G. cambogia dietary supplements or G. cambogia “pure extract” (Actis et al., (2007), Chen et al., (2010), Corey et al., (2016), Dara et al., (2008), Elinav et al., (2007), Fong et al., (2010), Jones & Andrew (2007), Kothadia & Olivera-Martinez, (2016), Lunsford et al., (2016), McDonnell et al., (2009), Melendez-Rosado et al., (2015), Schoepfer et al., (2007), Shim & Saab, (2008), Smith et al., (2016), Stevens et al., (2005), Stickel et al., (2009), Vitalone et al., (2011).
149. According to Crescioli et al., (2018), all patients with hepatic adverse effects consumed their food supplements according to the manufacturer’s recommendations and yet developed similar symptoms, including jaundice, weakness, abdominal pain, dark urine, nausea, and vomiting, which were the most reported. The duration of exposure varied with some patients taking supplements for a few days or weeks to more than one year. Of the 50 patients, the symptoms of 38 improved, while 8 required liver transplantation, 1 was diagnosed with liver cirrhosis and 2 died.
150. Sharma et al., (2018) presented a case report with “severe” liver toxicity following exposure to Hydroxycut®.
151. A 19-year-old man with no “significant” past medical history presented to a community medical centre with 2-day history of fever (103ºF/39.4ºC on presentation), severe fatigue, myalgia, arthralgias and an erythematous rash over his lower extremities. He started using Hydroxycut® [product formulation or HCA content not specified] approximately one week prior to presentation for fat burning and muscle building. He denied any smoking or alcohol use and was taking no other prescription or over the counter medication apart from Hydroxycut® and Myoflex® cream. His blood test revealed an AST level of 23 U/L, ALT of 81 U/L, alkaline phosphatase of 298 U/L white blood cell count of 31x109/L, haemoglobin level of 12.7 g/dL and a normal platelet count. TB was 7.3 mg/dL and he had a prothrombin time of 16.7 seconds. The paper did not provide details of the normal range for these parameters. Blood cultures, urine analysis, chest X-ray, abdominal ultrasound, computed tomography scan and magnetic resonance cholangiopancreatography results were normal. His liver appeared normal in size and texture and there was no evidence of mass, vascular compromise, stone disease, ascites or biliary ductal dilatation. The patient continued to have rising bilirubin levels over the next 3 days and despite antibiotics (Piperacillin and Tazobactum) had a persistent fever. On day 4, the patient was transferred to a hospital for possible urgent liver transplant evaluation due to rising TB of 12.7 mg/dL and a prothrombin time of 21.7 seconds. His hepatic profile showed AST at 27 U/L, ALT at 24 U/L, alkaline phosphatase at 152 U/L, bilirubin at 12.4 mg/dL, a prothrombin time of 15.4 seconds, ammonia at 38 µg/dL, a white blood cell count of 34.8 x 10(9)/L (71 neutrophils and 24 bands), haemoglobin level at 11.2 g/dL, and a platelet count of 237,000/µL. Repeat blood cultures and sputum cultures, as well as urine analysis showed no evidence of infection. Serologies for hepatitis A, B, and C, anti-mitochondrial antibody, anti-nuclear antibody, anti-smooth muscle antibody, F actin IgG, liver kidney Microsomal antibody-1, cytomegalovirus, Epstein Barr virus, Herpes Simplex virus, Group A streptococcal antigen, Coxsackie virus, Monospot virus and leptospirosis were all negative. Ceruloplasmin and Alpha-1 anti-trypsin levels were normal. Human immunodeficiency virus and Rapid plasma reagin tests were negative. Alpha-fetoprotein level was normal at 1 ng/mL. Iron studies were notable for mild iron deficiency and a ferritin level of 463 ng/mL. A comprehensive urine toxicity screen was negative for any drugs except for opiates which he was administered at the hospital for pain management. A liver biopsy done on the 7th day of hospitalization showed acute cholangitis with scant micro vascular fatty changes (<5%) and no evidence of lobulitis, hepatocytes necrosis, cholestasis, fibrosis, parasite, ova, vasculitis, thrombosis, viral inclusions or neoplasm. Infectious disease consultation was obtained, and Vancomycin was added to his antibiotic regimen. No infectious aetiology could be determined and the patient continued to have hyperbilirubinemia. He was started on Ursodiol 600 mg orally twice a day. His bilirubin level peaked at 18.6 milligram/dL, (direct at 10.2) and started to decrease after day 6 of hospitalization. Peak alkaline phosphatase was on day 3 of admission at 298 units/L and peak AST/ALT levels were 110/142 units/L respectively on days 11 and 13. All abnormal levels gradually started to decrease. Antibiotics were discontinued after a total of 14 days of therapy. The patient was discharged from hospital on day 17 with a bilirubin level of 6.8 mg/dL (direct at 3.2), AST level of 68 U/L, ALT level of 108 U/L, alkaline phosphatase level of 160 U/L. The patient had gradual recovery of liver functions and at 14 weeks after initial onset of symptoms his liver function tests had returned to normal.
152. The authors acknowledged that “whilst causation is difficult to prove in any drug induced injury, the temporal relationship after Hydroxycut® exposure and gradual improvement after withdrawing the involved medication plus the absence of any other aetiologies despite comprehensive testing would point to Hydroxycut® being the most likely possible cause for hepatotoxicity.”
153. Lunsford et al., (2016) reported “the first known case” of fulminant hepatic failure associated with dietary intake of a “pure” G. cambogia supplement.
154. A 34-year-old Hispanic male presented with nausea, vomiting, abdominal pain, and dark urine. Testing revealed elevated transaminases and TB; however, imaging failed to demonstrate cirrhosis or anatomic abnormality. Hepatitis work-up, including testing for viral hepatitis, hemochromatosis, Wilson’s disease, and autoimmune hepatitis, was unremarkable with the exception of an elevated Ferritin level of 7,089 mg/dL. Genetic testing for hemochromatosis was negative. His medical history was only positive for occasional social alcohol use, and the drug toxicology testing was negative. He denied use of energy drinks, herbs, Chinese teas, or muscle milk. He was advised to discontinue alcohol use, which he did, and his symptoms initially seemed to abate. Six weeks later, the patient developed asterixis, jaundice, and confusion. Follow-up imaging was concerning for rapid onset of cirrhosis or infiltrative hepatocellular carcinoma. On admission, transaminases were elevated with AST 624 U/L, alanine ALT 520 U/L and TB of 34.7 mg/dL. Autoantibody titers demonstrated a positive antinuclear antibody, but no other positive autoantibodies. Evaluation of Wilson’s disease demonstrated normal ceruloplasmin and copper levels; however, 24-h urine copper was elevated. Serum ferritin but not transferrin was elevated. A liver biopsy was performed and demonstrated sub-massive necrosis with collapse of the hepatic architecture involving about 70% of the liver parenchyma. Mild lymphocytic inflammatory infiltration and minimal canalicular cholestasis were seen. No viral inclusions or other infectious agents were identified by histology or immunohistochemistry. No evidence of granuloma, tumour, or features of cirrhosis were demonstrated. Periodic acid-Schiff stain with diastase was negative for alpha-1 antitrypsin globules. Iron stain showed only mild iron deposition in Kupffer cells and hepatocytes. Quantitative tissue copper was within normal limits. Findings were suspected to be potentially related to drug-induced liver injury. After questioning, the patient confirmed intake of G. cambogia, purchased on the Internet. He was taking two 80 mg capsules of “Garcinia Cambogia 5:1 Extract” three times daily before meals for five months preceding initial presentation. Since not advised against intake, he continued the supplement after initial presentation. He denied any other medications or supplements and reported no alcohol intake for two months. An 80 mg tablet of a 5:1 concentrate of G. cambogia was determined to be equivalent to 400mg of standard preparation. Other listed ingredients include rice flour, gelatine, magnesium stearate, and silica. However, it was confirmed that the manufacturer does not perform assays to determine HCA concentration. The patient’s status declined and his mental status deteriorated; he was listed for liver transplantation and received an orthotopic liver transplant. Histopathologic examination of the explanted liver demonstrated near total hepatic necrosis with massive hepatocellular dropout and mixed inflammatory cell infiltrates, consistent with severe drug-induced liver injury.
155. The authors concluded that “while evidence from a case report rarely offers proof of causality, this case, in conjunction with known cases of hepatotoxicity and liver failure associated with other G. cambogia-containing supplements warrants a high index of suspicion.”
156. García-Cortés et al., (2016) reviewed the reported cases of hepatotoxicity by dietary supplements. Two case-studies were summarised relating to G. cambogia induced hepatotoxicity. Corey et al., (2015) reported a 52-year-old female requiring liver transplant after taking G. cambogia extract 936 mg (2 capsules per day) with 60% HCA (USA Nutra Labs) for 15 days. To note that the supplement also contained calcium (50 mg), chromium (200 µg), and potassium (50 mg) at the ingested amount.
157. Melendez-Rosado et al., (2015) reported a 42-year-old female with elevated transaminase levels (ALT at 1,277 U/L and AST at 2,792 U/L, which are 70 and 45 times the upper limit of normal, respectively) and coagulopathy. It should be noted that the woman had a medical history of hypertension, chronic kidney disease (stage V), diabetes mellitus type 2, chronic back pain, haemochromatosis, and obesity. Abdominal ultrasound showed mildly coarse hepatic echotexture with no intrahepatic or extrahepatic dilatation, and abdominal computed tomographic scan showed a surgically absent gallbladder and no hepatic parenchymal abnormalities. A week before presentation, the patient started taking “pure G. cambogia” for weight loss. The supplement was discontinued, and the patient was empirically treated with N-acetylcysteine due to concerns of acetaminophen toxicity. After several days of supportive care, the patient’s abdominal pain resolved, and liver enzymes recovered to baseline. The patient was discharged after 4 days. Four months later, the patient’s symptoms had not recurred, and normalisation of her liver function studies was noted. The authors were of the opinion that the development of acute abdominal pain with elevated liver enzymes in the setting of a baseline liver inflammation in combination with the use of acetaminophen and the introduction of a hepatotoxic herbal supplement makes the diagnosis of acute hepatitis secondary to G. cambogia “very likely”. No other information on the supplement, dose or exposure time could be obtained.