Scoping paper on the potential risk(s) of Garcinia cambogia
Background and Introduction
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
Background
1. In March 2025, the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) published an opinion on their assessment of adverse reactions to the consumption of food supplements containing Garcinia cambogia (G. cambogia) (ANSES, (2025a); document in French). Their official webpage provided an overview and advised consumers to not consume food supplements containing G. cambogia (ANSES, 2025b).
2. Currently in the United Kingdom (UK), there are no safe levels or set limits established for the use of G. cambogia in food and drinks, including food supplements. In response to the ANSES review, the Food Standards Agency (FSA) and Food Standards Scotland (FSS) requests the Committee on Toxicity of Chemicals found in Food, Consumer Products and the Environment (COT) to perform a review of the opinion published by ANSES and assess the risk(s) associated with consumption of Garcinia cambogia in food supplements. In addition, the Committee is also requested to consider whether a safe level or maximum limit of G. cambogia for use in food and drink, including food supplements can be derived based on the current available data.
3. Points of consideration include:
i. What is the maximum dietary level of G. cambogia that can be added to/used in food and drink, including food supplements, to be consumed daily without appreciable health risk?
ii. Is there a link between consumption of food, drinks and food supplements, containing G. cambogia and adverse effects on health?
iii. What are possible subgroups of the general population that are more vulnerable or more sensitive to the adverse effects caused by G. cambogia?
4. The ANSES Opinion (including annexes) has been translated by the FSA via a third party for the sole purpose of supporting the COT in their assessment of G. cambogia in food supplements. It is not an official translation endorsed by the ANSES. Therefore, they are not reproduced here for copyright reasons. The original documents (in French) are available on the ANSES website: Avis relatif à l’évaluation des effets indésirables liés à la consommation de compléments alimentaires contenant du « Garcinia cambogia » The Secretariat has summarised the information presented in the ANSES Opinion from paragraph 17-91, as well as collated information from other regulatory authorities.
5. A literature search was carried out using the search string "Garcinia cambogia" AND "toxicity" in PubMed, Science Direct and Google Scholar. No filters or restrictions were used.
Introduction
6. The genus Garcinia, native to Asia and Africa, belongs to the Clusiaceae family and includes more than 300 species, such as G. cambogia. Various therapeutic effects have been attributed to this genus including anti-obesity, anti-ulcerogenic, antioxidant, anti-diabetes, anti-fungal, anti-inflammatory and anti-neoplastic (Chuah et al., 2013). The fruit has been consumed as a tea in Ayurvedic medicine for inflammation and stomach complaints, while the fruit rind has a history of traditional use as a food ingredient and preservative.
7. The European Novel Food List does not consider G. cambogia as a novel food; however, advises that other legislation may restrict the placing on the market as a food in the EU or in some Member States (EC, 2023).
8. The UK Medicines and Healthcare products Regulatory Agency (MHRA) deems that products containing G. cambogia are not classed as medicinal provided that they contain the whole herb and not the extract; hydroxycitric acid (HCA). While the whole herb is not regarded as medicinal and is known to have common food uses, when HCA is extracted from the herb its concentration will greatly exceed that found in the natural fruit and it is regarded as a medicinal substance with a known ability to modify physiological functions.
9. This only applies to ingested products. Neither whole G. cambogia nor its HCA extract is known to be capable of exerting a pharmacological effect when used topically, for example in cosmetics.
10. It is important to note that not all G. cambogia extracts are created using the same manufacturing process or have the same HCA inclusion rate in the final product. The anti-obesity properties of G. cambogia have been attributed to HCA, which is present in the rind or epicarp of the fruit at 10-30% by weight. Extracts can contain between 20–60% HCA (Semwal et al., 2015; Jakopin, 2019). HCA is susceptible to lactonization during manufacturing (evaporation and concentration), as such in commercially available samples of G. cambogia, HCA is present as its calcium salt for stability (Jena et al., 2002).
Chemical composition
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
11. The bioactive compounds which are extracted and isolated from G. cambogia are shown in Table 1 and Figure 1.
Table 1 - list of bioactive compounds from plant parts of G. cambogia and their related bioactivities (reproduced from Espirito Santo et al., 2020).
|
Xanthones |
Plant part |
Activity |
|
Garbogiol |
Roots |
Inhibition of α-glucosid |
|
Rheedia xanthone A |
Peel |
Not applicable* |
|
Oxy-guttiferone i |
Fruits |
Not applicable* |
|
Oxy-guttiferone k |
Fruits |
Not applicable* |
|
Oxy-guttiferone k2 |
Fruits |
Not applicable* |
|
Oxy-guttiferone m |
Fruits |
Not applicable* |
|
Benzophenones |
Plant part |
Activity |
|
Garcinol |
Peel |
Anticancer, anti-inflammatory, antiparasitic, action on nervous system |
|
Isogarcinol |
Peel |
Anticancer, anti-inflammatory, antiparasitic, action on nervous system |
|
Guttiferone i |
Fruits |
Not applicable* |
|
Guttiferone n |
Fruits |
Not applicable* |
|
Guttiferone j |
Fruits |
Not applicable* |
|
Guttiferone k |
Fruits |
Topoisomerase II inhibitor |
|
Guttiferone m |
Fruits |
Topoisomerase II inhibitor |
|
Organic acids |
Plant part |
Activity |
|
Heterocyclic amines |
Fruits |
Antiobesity |
|
Tartaric acid |
Fruits |
Not applicable* |
|
Citric acid |
Fruits |
Not applicable* |
|
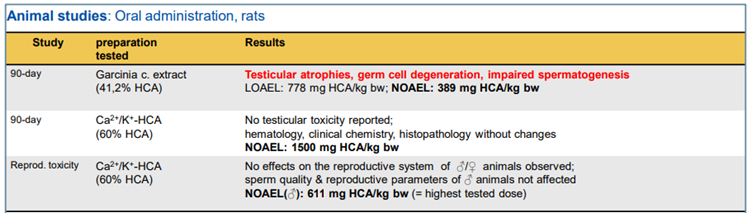
Malic acid |
Fruits |
Antimicrobial |
|
Garcinialactone |
Fruits |
Not applicable* |
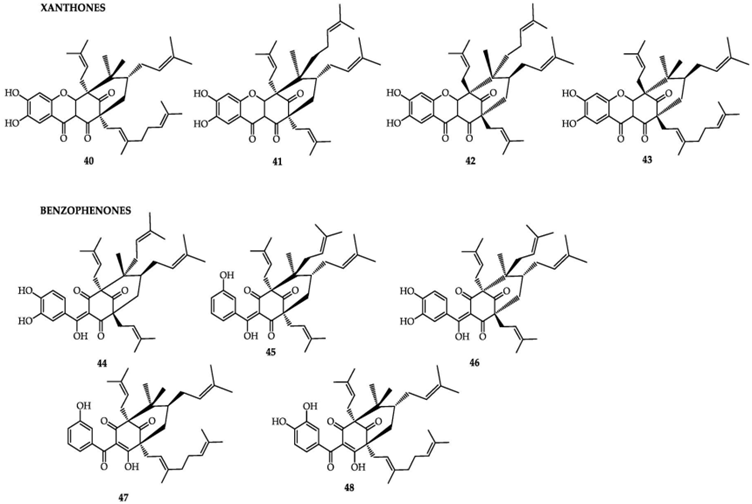
Figure 1 - chemical structures of the xanthone and benzophenone classes of bioactive compounds from G. cambogia. Xanthones: 40) oxy-guttiferone-i; 41) Oxy-guttiferone k; 42) Oxy-guttiferone k2 and 43) Oxy-guttiferone k. Benzophenones: 44) guttiferone-I; 45) guttiferone-j; 46) guttiferone-k; 47) guttiferone-n and 48) guttiferone-m (reproduced from Espirito Santo et al., 2020).
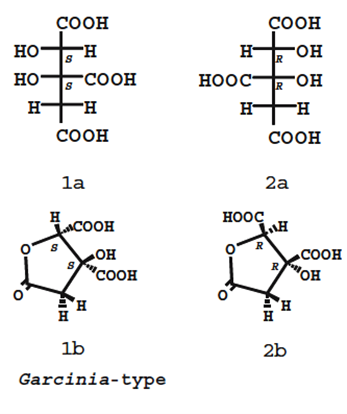
12. The HCA present in G. cambogia is a “potent” and “competent” inhibitor of adenosinetriphosphate (ATP) citrate lyase, which is a key enzyme in the synthesis of fatty acids, cholesterol, and triglycerides. It also regulates the level of serotonin, which has been associated with satiety, increased oxidation of fat, and decreased gluconeogenesis (Semwal et al., 2015; Preuss et al., 2004). HCA compromises a citric acid with a hydroxyl group at the second carbon. HCA has two diastereomers as there are two chiral centres, as such there are four stereoisomers of HCA, compromising two pairs of enantiomers (see Figure 2). Each of the stereoisomers can form a γ-lactone ring and in general solution, HCA is a mixture of non-lactone and lactone forms (Yamada et al., 2007).
Figure 2 - Structures of HCA derived from Garcinia ssp. Upper and bottom structures show the non-lactone and lactone forms, respectively. (2S,3S)-HCA (a mixture of 1a and 1b) is found in G. cambogia. The other isomers (2R,3R)-HCA (a mixture of 2a and 2b) and (2R,3S)-HCA have not been isolated from natural sources (reproduced from Yamada et al., 2007).
Data from authoritative bodies
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
13. Multiple authoritative bodies have reviewed the data on G. cambogia. For example, in European and international vigilance systems several reports of hepatic, digestive (pancreatitis), cardiac and muscular (rhabdomyolysis) damage as a result of consumption of supplements containing G. cambogia have been identified.
ANSES
14. It should be noted that paragraphs 15 and 16 were summarised from the relevant webpage of ANSES (ANSES, 2025b) rather than information from their opinion which is published in French.
15. The ANSES assessment highlighted 38 cases of adverse effects reported between 2009 and March 2024 (as identified in the French nutrivigilance scheme). They further “identified several drug interactions that can lead to an increase in adverse effects or cause medicines to lose their efficiency.”, from their analysis of other vigilance systems and literature review.
16. From their review of the literature, adverse effects were observed in people with various health issues like psychiatric disorders, pancreatitis or hepatitis, diabetes, obesity or hypertension. Those on certain medications that affect liver function, antiretroviral treatments or antidepressants were also seen to be negatively affected by consumption of G. cambogia. Those without any previous medical problems were also reported to have severe health effects following consumption of G. cambogia.
17. As mentioned in paragraph 4, the FSA commissioned a third party to translate the ANSES Opinion for the sole purpose of supporting the COT in their assessment of G. cambogia in food supplements. It is not an official translation endorsed by the ANSES. The following paragraphs provide a summary of the approach used by ANSES and their conclusions.
18. ANSES’ goal was to review the existing data on the physicochemical [and toxicological] properties of G. cambogia Desr., and analyse clinical cases from literature and vigilance reports to draw conclusions.
Classification and Taxonomy
19. G. gummi-gutta (L.) N. Robson is commonly known as the Malabar tamarind tree. In the scientific literature or on the labelling of marketed products it is referred to as G. cambogia Desr., It belongs to the Clusiaceae family and has 18 genera. The genus Garcinia was found to include nearly 404 species.
20. The French regulations allow the use of the fruit, fruit peel, and gum resin from G. gummi-gutta, as well as the fruit, fruit pulp, and pericarp (rind) of G. mangostana in food supplements.
21. ANSES observed that researchers and regulatory bodies utilise the name G. cambogia for G. gummi-gutta, which creates confusion. As such in their assessment, G. cambogia Desr., denotes G. gummi-gutta.
Botanical data and geographical distribution
22. ANSES described that the species of the genus Garcinia is widespread in tropical regions. G. cambogia Desr., is native to India, Sri Lanka and Nepal but has been introduced to other subtropical areas in Asia including China, Malaysia, Indonesia and the Philippines, as well as to West Africa and Polynesia.
Traditional uses of the plant and associated economic activities
23. ANSES found that the Indian populations had historic use of G. cambogia Desr., in several applications: medicinal, culinary, crafts and construction.
24. The fruits are edible; however, they are too acidic to eat raw and are processed into marmalade, vinegar or dried to use in condiments. The pericarp [rind] is sun-dried and are sold as is or ground into powder. It is traditionally used as a spice to act as a flavour enhancer and/or a preservative. Other food uses for the rind include non-alcoholic drinks and syrup. The bark has been used to produce fermented alcoholic drinks, whilst the seeds can be extracted for vegetable oil.
25. In Indian folk medicine, G. cambogia Desr., has been used to treat oedema, delayed menstruation, digestive disorders (in particular, chronic diarrhoea). The emetic properties of G. cambogia Desr., have been described as treatment for intestinal parasites. Various decoctions have been described to treat rheumatism and management of cardiovascular conditions.
26. It was found that the literature attributed these food and medicinal uses to other species of Garcinia, thus it is difficult to definitively associate them with G. cambogia Desr.
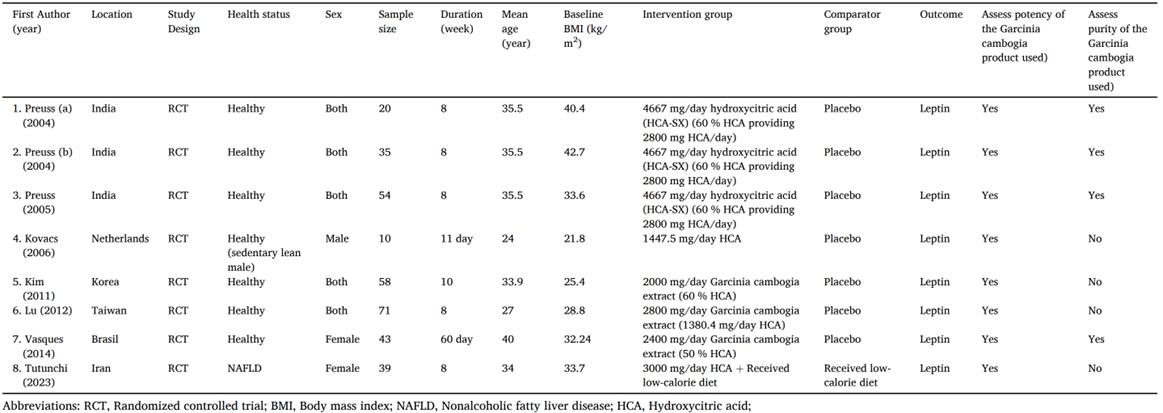
27. In 2014-2015, the Indian industry was reported to have an annual use of ~300 tonnes (dry weight) of G. cambogia Desr., fruit. Exported quantities ranged from 100 to 5,000 tonnes per year between 2005-2015, where more than half was destined for the United States of America, and the remainder exported to South Korea, Japan, Germany and Australia.
Chemical composition of the plant
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
28. The pericarp of G. cambogia Desr., contains 10 to 30% organic acids, calculated as citric acid equivalents, including less than 1% citric, oxalic, tartaric, and acetic acids; more than 4% malic acid; and up to over 15% HCA. It was identified that other Garcinia ssp., also contained HCA though typically at lower levels.
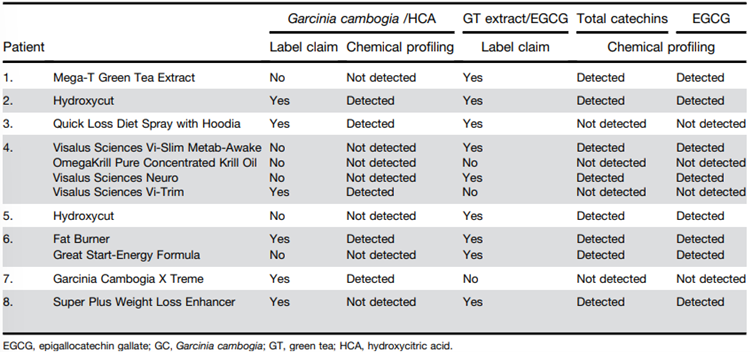
29. HCA (C6H8O8) is a derivative of citric acid that has an additional hydroxyl group on the second carbon, creating two chiral centres at positions C-2 and C-3. HCA exists as four stereoisomers: (-)-HCA (2S,3S), (+)-HCA (2R,3R), (+)-allo-HCA (2S,3R) et (-)-allo-HCA (2R,3S).
30. In G. cambogia Desr., this compound [HCA] appears in the (2S,3S) or (-)-HCA configuration and serves as a competitive inhibitor of ATP-citrate lyase, an enzyme that drives fatty acid synthesis. Each stereoisomer can cyclise to a γ-lactone. In G. cambogia Desr., HCA occurs in both its non-lactonic form and its lactonic form, known as garcinia lactone or (2S,3S)-3-hydroxy-5-oxo-2,3,4,5-tetrahydrofuran-2,3-dicarboxylic acid. From the literature, it was described that the lactonic form of (2S,3S)-HCA inhibits ATP citrate lyase less effectively than the non-lactonic form. It is also less bioavailable.
31. Industrial processes generally stabilise HCA as a single, double, or triple salt to prevent its cyclisation into the lactone form. The most common are calcium, magnesium, or potassium salts because they offer greater stability, higher solubility, and lower hygroscopicity compared to sodium salts.
32. The fruit of G. cambogia Desr., contains 6.25% carbohydrates, 3.25% protein, less than 0.0060% amino acids, and 0.34% lipids. The dried pericarp of G. cambogia Desr., contains 14.4 mg vitamin C. The bark has also been reported to contain certain B-group vitamins: 48 μg vitamin B1, 275 μg vitamin B2, 45 μg vitamin B3 and 8.8 μg vitamin B12 per 100 g. The dried fruit bark also contains minerals: 2.8 mg sodium, 26.6 mg potassium, 12.7 mg calcium, 14.4 mg magnesium, 9 mg iron and 5.3 mg phosphorus per 100g.
33. G. cambogia Desr., has been described to contain polyisoprenylated benzophenones (precursors of xanthones). Studies have isolated garcinol, isogarcinol from the plant’s bark and fruit. Both compounds have been shown to have antitumour, antimicrobial, antioxidant, anti-inflammatory, and central nervous system effects, especially in vitro and in animal models. Other isolated polyisoprenylated benzophenones are presented in the ANSES Opinion.
34. The xanthone content of the fruit was estimated to be 1.96%. The total phenol content is estimated to be 3.26%. Flavone heterosides (apigenin, luteolin) and flavanols (kaempferol, quercetin) have been detected in G. cambogia Desr., fruits.
35. Glycosylated derivatives of caffeic acid (caffeoyl glucose), esterified with quinic acid (dicaffeoyl-quinic acid) as well as p-coumaroyl-quinic acid have also been detected in fruits.
Regulatory status in different fields of use and geographic regions -
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
United States
36. Several clinical trials had not revealed any health risks, and G. cambogia Desr., was used as an ingredient in food supplements, most notably in the Hydroxycut® range sold in the U.S. in the early 2000s. However, shortly after they were marketed, reports of hepatic, muscular, cardiac and neurological damage, sometimes serious, were reported in the United States and Canada. In 2009, the United States Food and Drug Administration (US FDA) requested the withdrawal of G. cambogia Desr., from Hydroxycut® preparations. It should be noted that consumers continued to use these products for several years following withdrawal from the market, due to remaining stock or illegal sales.
Europe
37. Several European Union member states, including Belgium, Italy, and Hungary, have authorised the use of Garcinia cambogia Desr., in food supplements. Between 2008 and 2010, applicants submitted requests to the European Food Safety Authority (EFSA) for the evaluation of health claims related to the species G. cambogia Desr., including in extract form. These health claims relate to weight control, reducing fat storage and hunger, controlling blood sugar and cholesterol levels. These are said to still be awaiting assessments. In addition, HCA is also the subject of a health risk assessment by EFSA, which is yet to be published.
France
38. The French National Agency for the Safety of Medicines and Health Products (ANSM), classifies G.cambogia Desr., as a medical product due to its hypoglycaemic and lipid-lowering effects associated with HCA. However, given the lack of proven therapeutic benefit and an unfavourable benefit/risk ratio due to adverse effects reported in the U.S. and Canada, the ANSM Director General issued a ban on 12 April 2012. The decision prohibits the import, preparation, prescription, and dispensing of preparations containing G. cambogia, as well as its use in humans.
39. The French “plants” decree which establishes the list of plants authorised for use in food supplements and their conditions of use, does not include G. cambogia Desr.,; however, the General Directorate for Competition, Consumer Affairs and Fraud Control (DGCCRF) registered it by mutual recognition under the incorrect name G. gummi-gutta (L.) Roxb. The latter appears on the list of plants permitted in food supplements.
40. Data from the Téléicare database show that the DGCCRF registered 340 food supplements containing G. cambogia Desr., between April 2016 and January 2023. Based on the information gathered from these products, the average HCA intake was 752 mg/day for products that contained only G. cambogia Desr. The range was 1.25 – 2,850 mg/day, with 747 mg/day as the median. For multi-ingredient dietary supplements (MIDS) containing HCA, the average HCA intake was 255 mg/day. The range was 0 – 2,070 mg/day, with 150 mg/day as the median.
41. HCA analyses were conducted separately by Service commun des laboratoires and shared between the DGCCRF and customs. The HCA intake was calculated to consider the manufacturer’s advice for use. The average daily intake was 412 mg/day. The range was 21 – 2,000 mg/day, with a median of 203 mg/day.
42. Seventy-four percent [of the registered 340 food supplement products] combined G. cambogia with other ingredients that are known to cause liver toxicity in experimental models and clinical studies. These included: green tea containing epigallocatechin gallate; curcuma containing curcumin; red yeast rice containing monacolin K and coumarin. The Plants Working Group (WG) identified other substances that were suspected to be hepatotoxic (as suggested by the literature). These included: conjugated linoleic acid (unspecified isomers), hydroxyanthracene derivatives, forskolin, salicin, methyl salicylate and salicylated derivatives, Equisetum arvense [horsetail], gingerol, ginsenosides, gymnemic acid, and parsley. The Plants WG and Human Nutrition Expert Committee further noted that 89% [of the registered 340 food supplement products] contained at least one other ingredient that is known to be hepatotoxic: chromium, caffeine or piperine.
Adverse effects linked to the consumption of G. cambogia Desr.
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
Data from French vigilance systems
43. Since launching in 2009 and up to March 2024, ANSES has received 38 reports of adverse reactions likely linked to food supplements containing G. cambogia (or G. gummi-gutta), the products labels did not display the full scientific names. All admissible cases (n=35/38) were hepatic, cardiovascular and digestive. Of the 35 reported cases, 18 had sufficient information to assess the product’s causal relationship with the observed adverse effects. The number of reports where the causal relationship of the product was very likely was (n = 1/18), likely (n = 7/18), possible (n = 8/18), doubtful (n = 1/18) or excluded (n = 1/18). It should be noted that the majority of the products are MIDS (n=16/18), with the remaining 2 stated to contain G. cambogia only.
44. The reported cases with a possible causal relationship associated with hepatic effects were further analysed by ANSES. Five of the six reported liver damage cases involved cytolytic hepatitis. In each case, the person either took G. cambogia alongside another potentially hepatotoxic ingredients in the supplement or used a hepatotoxic drug at the same time. In addition, all consumers have co-morbidities, or even risk factors for liver damage (obesity, significant and rapid weight loss).
45. Six additional case reports from the pharmacovigilance system were provided by the French ANSM. Three of which had enough information for the Nutrivigilance WG to assess the causal relationship of G. cambogia consumption with the observed adverse effects. The Nutrivigilance WG determined that 2 of the 6 cases had a possible or a very likely causal relationship.
46. Twenty out of 30 additional case reports from the toxicovigilance system were reviewed by the Nutrivigilance WG, they identified that the adverse effects were mostly cardiovascular (n=8/20; tachycardia) and digestive effects (n=6/20; abdominal pain, vomiting). Other effects included general symptoms (n=5/20; dizziness, malaise, fatigue, excessive sweating, dilated pupils) and skin reactions (itchy erythema).
Data from other vigilance system
Europe
47. In October 2020, ANSES contacted its European counterparts to gather more data on adverse effects potentially linked to the consumption of food supplements containing G. cambogia or G. gummi-gutta (under their truncated names). Of the 37 countries contacted, 21 responded. In brief, most of the 21 respondents (Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Estonia, Hungary, Iceland, Ireland, Latvia, Lithuania, Malta, Slovakia, Spain, Switzerland) were not aware of any cases of adverse reactions related to G. cambogia (truncated names).
48. In Belgium, the Federal Public Service for Public Health, Food Chain Safety and the Environment authorises the use of "gum-resin (from the whole fruit or the pericarp)" of Garcinia in food supplements. Companies have currently notified and obtained authorisation for over 300 such products on the Belgian market. In Hungary, manufacturers have used this plant in food supplements on the market for over 20 years, and in 2020, they notified 152 products containing it. Some of these contain HCA at a dose higher than the 1,000 mg considered acceptable in Hungary. In these two countries, despite the notification of several hundred dietary supplements containing G. cambogia, authorities have not received any reports of adverse reactions.
49. In contrast, Germany (which recorded at least 167 notified products containing G. cambogia in 2020), along with Italy, Norway, Slovenia, and the Netherlands, have reported cases. The effects were primarily hepatic (n=13), followed by cardiovascular (n=8), psychiatric (n=6), and neurological issues (n=6).
50. In 2018, the Slovenian National Institute of Public Health published information relating to dietary supplements for weight control, specifying the risks and benefits of the ingredients frequently used in their composition. Regarding G. cambogia, liver damage is mentioned.
51. In 2019, the Spanish Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) published a report on the risks linked to consuming dietary supplements containing G. gummi-gutta (AESAN, 2019). The report emphasised the existence of sufficient clinical evidence connecting the consumption of G. gummi-gutta to the occurrence of hepatic events. This agency also stressed the need to monitor psychiatric disorders (manic attacks, psychoses) reported in the literature in connection with the consumption of this plant.
Canada
52. From the 1st of January 1965 to 31st of August 2023, 93 reports involving at least one product containing one of the following ingredients: ‘garcinia', 'garcinia gummi-guta', 'garcinia gummigutta', 'garcinia gummi- guta extract', 'garcinia gummi-guta fruit', 'garcinia gummi-gutta gum resin' (names registered in the database), were recorded. The average age was 42 [note that 12% of the data did not have information on age], and over 80% were women. The mean BMI where available (62% missing data) was 29.9 (± 5.2) kg/m2. The commonly reported adverse reactions included general symptoms (headache, dizziness), digestive issues (abdominal pain, nausea, vomiting), cardiovascular problems (palpitations, high blood pressure), and psychiatric or neurological effects. Of these 93 reports, 4 were reports of liver damage.
United States
53. According to the US FDA-Medwatch database, authorities had received 40 adverse reaction reports up until the 14th of December 2023 [the start date was not provided]. Of these 40 adverse reaction reports, 35 involved women. The average age (with 17% of data missing) was 43 years (± 12.9). The adverse effects reported were mainly of a hepatic, digestive and cardiovascular nature. The Plants WG and the Human Nutrition Expert Committee reviewed the cases submitted by all foreign vigilance systems but could not establish a causal relationship due to a lack of information.
Data published in the literature
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
54. The clinical cases reported in the literature up to October 2024 were mainly hepatic effects (n = 35). Psychiatric (n = 8), cardiovascular (n = 3), digestive (n = 3), muscular (n = 2) and metabolic (n = 2) disorders were the subject of several publications. Authors of these publications utilised G. cambogia and did not further allow for accurate scientific identification.
Hepatotoxicity
55. Thirty-six cases of liver damage have been published, observations by ANSES were discussed in their Opinion.
56. In brief, hepatitis of the cytolytic type were observed (86%), followed by cholestatic hepatitis (8%) and mixed hepatitis (6%). Grade 2 hepatitis was observed with elevation of transaminases (greater than 3 times the normal range) in the majority of cases; however, some were life-threatening (n=6), or even fatal (n=1). 60% of the reported cases involved women aged 37 years on average (35 years as the median; 19 – 64 years age range).
57. In 20 of the 36 publications, co-morbidities were reported, including hypertension, heart or kidney failure, diabetes, haemochromatosis, allergy, hypothyroidism, adenoma, chronic pain, dyslipidaemia, or hepatic steatosis. Authors of the publications identified risk factors for certain pathologies, including high BMI, hypocaloric diets, intense physical activity, a history of hepatitis, rapid and significant weight loss, alcohol use, and smoking.
58. In 29 of the 36 publications, the composition of the food supplements was of sufficient detail to identify other ingredients. Within these 29, 24 involved dietary supplements containing G. cambogia in combination with other hepatotoxic ingredients: caffeine, piperine or chromium. Most cases involving multi-ingredient products have been reported in the United States in people who have consumed products from the Hydroxycut® range. The composition of Hydroxycut® products in reported cases is often vague or entirely unknown.
59. Besides these occasional published cases, research teams have also analysed database records of liver damage linked to toxic drugs or plant-based products.
60. As part of the US Drug Induced Liver Injury Network (DILIN), Vuppalanchi et al., (2022) published their analysis of the 22 cases related to G, cambogia hepatotoxicity. Five of the twenty-two cases could be attributed to G. cambogia alone, in combination with green tea (n=16/22) or Withania somnifera [ashwagandha] (n=1/22). Statistical tests comparing control groups—patients with liver injury linked to green tea supplements without G. cambogia (n=57) or to other herbal supplements (n=103) showed that liver damage caused by G. cambogia and green tea was clinically indistinguishable. Patients who developed liver damage from dietary supplements containing G. cambogia showed a significantly higher frequency of the HLA-B*35:01 allele compared to those whose liver injuries were linked to other herbal supplements or conventional medications. The authors concluded that this association suggested a mode of action that is mediated by the immune system.
61. Bessone et al., (2022) analysed the Latin America-Drug Induced Liver Injury (LATINDILI) database, they found that out of 367 cases, 8% were attributed to herbal food supplements. Camellia sinensis [green tea], Herbalife® products, and G. cambogia—mostly used for weight loss—were the most frequently identified as causes.
Psychiatric disorders
62. Four of the eight cases involved people with a history of psychiatric disorders. This was identified to be a potential risk factor, although adverse reactions have also occurred in individuals without any known psychiatric history.
Cardiovascular disorders
63. Two cases of cardiomyopathy and one case of giant cell myocarditis have been reported in the literature.
64. The Plants WG and the Human Nutrition Expert Committee noted that both cases of cardiomyopathy involved women with no prior medical history. The clinical observations were deemed to be severe; one led to cardiac arrest, and the other caused high blood pressure with cardiac dysfunction, requiring ongoing cardiology follow-up.
Digestive disorders
65. The Plants WG and Human Nutrition Expert Committee noted that acute lithiasis pancreatitis often occurs in cases of obesity and diabetes. These three cases of pancreatitis occurred in people with diabetes and hypertension. Two of the three cases involved a history of obesity and chronic hepatitis C.
Muscle disorders
66. Two cases of rhabdomyolysis following consumption of Hydroxycut® have been reported in the literature. During periods of consumption (prior to 2009 [when the US FDA requested the withdrawal of G. cambogia from the product – see paragraph 36]), ephedra, caffeine, chromium, G. cambogia and Gymnema sylvestre [gymnema] were part of the composition of Hydroxycut®. These cases involved women who were either overweight or had recently increased their physical activity.
Metabolic disorders
67. Roche et al., (2014) reported a case of hypoglycaemia (blood glucose at 33 mg/dl) leading to syncope in a 67-year-old woman. The woman had been taking the following medications: venlafaxine, lisinopril-hydrochlorothiazide and alprazolam. She had started taking a dietary supplement containing G. cambogia two days before the onset of hypoglycaemia. The symptoms disappeared after administration of glucose. The author suspected G. cambogia because of the temporality of the events and the nonrecurrence of similar events following discontinuation of the product.
68. Bystrak et al., (2017) reported a case involving digestive complications in a 56-year-old consumer with diabetes, the patient developed diabetic ketoacidosis. The authors suggested G. cambogia may have triggered ketosis by increasing ketone body production.
Other types of disorders
69. A 35-year-old woman with no known medical history or medication use reported an ocular disorder unilateral vision loss and eye pain after taking over 1,500 mg of HCA daily from G. cambogia extract for one week (Cho et al., 2019). These ocular complications resolved after the extract was discontinued and treatment with topical and oral steroids was initiated.
70. Other publications of adverse reactions suspected to be related to consumption of G. cambogia provided little descriptive information on the product label and in general e.g. dose, exposure duration, presence of other hepatotoxic ingredients. Two studies (Sikka et al., 2016; Graf et al., 2020) reported cases of thrombocytopenia in connection with taking dietary supplements containing G. cambogia. Li and Bordelon (2011) reported a case of nephropathy occurring in a 51-year-old woman with no previous medical history in association with one month's consumption of a dietary supplement containing G. cambogia.
Clinical trials
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
71. Several clinical trials have investigated the pharmacological effects of G. cambogia (Girola et al., 1996; Hayamizu et al., 2008; Heymsfield et al., 1998). Most patients either reported no adverse events or showed no significant difference from the control group.
72. Cheng et al., (2012) assessed the effects of a single oral HCA supplementation on postprandial glycogen synthesis in skeletal muscle in physically active men. Eight men aged on average 22 years (mean BMI 25.2 kg/m2) and in apparent good health were dosed with 500 mg of HCA, immediately after exercise. They noted an increase in fat oxidation after HCA supplementation and suggested this may present a risk of increased ketosis in patients with severe diabetes.
73. In another study (phase I observational study), 10 healthy men aged 26-56 years with no digestive disorders received 3,000 mg/day of HCA for 30 days. Although the measured parameters: anthropometric indices, clinical examinations, and serum testosterone levels remained unchanged, two subjects experienced anorexia and a third reported a headache (Hayamizu et al., 2003).
Drug interactions
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
74. Some reported cases in the literature and in vigilance systems raise suspicions of drug interactions involving G. cambogia and other medications.
Effects on P-glycoprotein (P-gp)
75. The Nutrivigilance WG suspects a drug interaction between antiretroviral treatments and the ingredients of the herbal tea blend Thé Catherine®, which includes G. cambogia, senna leaves and pods, and Chrysanthemum morifolium Ramat. A few days to weeks after starting the product, a 31-year-old woman with stage B3 HIV showed an increase in viral load. Ritonavir and darunavir (medication for HIV/AIDS) act as both substrates and inhibitors of P-glycoprotein (P-gp or MDR1; membrane efflux transporters). Bolla et al., (2021) investigated the effect of garcinol (a polyisoprenyl benzophenone present in G. cambogia Desr.,). on the P-gp transporter in rats. Concomitant administration of garcinol to digoxin, a P-gp substrate, decreased the area under the curve of digoxin measured in rat plasma. The authors suggested that this was due to increased expression of the gene encoding P-gp in the brain and digestive tract. Thus, co-administration of Thé Catherine® with ritonavir and darunavir may reduce exposure to these antiviral treatments and therefore contribute to an increase in viral load. An in vitro study on a 95% ethanolic extract of Garcinia cambogia in MDR1-MDCK epithelial cells showed an inhibitory effect at the highest dose tested (50 µg/mL) (Husain et al., 2023). Boonyong et al., (2024) investigated the effects of guttiferone K (a polyisoprenylated benzophenone found in G. cambogia Desr.) in Caco-2 cells and its P-gp function. Results showed that guttiferone K could inhibit P-gp.
Effects on cytochrome P450 monooxygenases (CYP)
76. A drug interaction was suspected in a case where a 38-year-old woman developed hypokalaemia and cardiorespiratory arrest with no relevant medical or family history. The cardiorespiratory arrest due to hypokalaemia occurred two to three days after taking the food supplement Eafit Ultra Slim Burner® (with G. cambogia), combined with Maté®, Spasfon®, and Primpéran®. The Nutrivigilance WG highlighted the large number of cofactors potentially responsible for adverse reactions, including Primpéran® (metoclopramide), known to lengthen the QT interval, and Maté®, which is rich in caffeine, known for its arrhythmogenic effects.
77. In the study by Bolla et al., (2021) garcinol exhibited strong inhibitory effects on CYP2D6 (IC50 = 9,5 µM) and CYP1A2 (IC50=7.6 µM). Significant inhibitions were also reported for CYP2C9 (IC50 = 8.0 µM), 2B6 (IC50 = 2.1 µM) and 3A4 (IC50=5.1 µM).
78. Yu et al., (2017) examined the inhibitory effects of a G. cambogia extract on CYP enzymes. The results showed significant dose-dependent inhibitory effects of G. cambogia extract on CYP2B6 activity, effects that did not appear with HCA, which was also tested.
79. An in vitro study using recombinant cytochromes found that a hydro-alcoholic extract (95% ethanol) of G. cambogia weakly inhibited CYP3A4, with an IC50 around 25 µg/mL (Husain et al., 2023). Another in vitro study on human hepatocytes showed that the same type of extract induced over 50% activity of CYP3A4 and CYP1A2 at concentrations below 10 µg/mL (Haron et al., 2023).
Effects on nuclear receptors PXR and AhR
80. In an in vitro study conducted by (Haron et al., 2023), an activating effect of the nuclear receptors PXR and AhR humans transfected into HepG2 cells (human hepatocytes) were also observed when exposed to a hydro-alcoholic extract (95% ethanol) of G. cambogia.
81. Another study describes the agonist effect of the same type of extract on PXR and AhR receptors in HepG2 liver cells and AhR-reporter cells respectively. The authors showed that G. cambogia was an activator of PXR and AhR (Husain et al., 2023).
Pharmacodynamic reactions
82. A 35-year-old woman developed serotonin syndrome marked by tremors, hot flushes, and diaphoresis after one month of taking a supplement containing G. cambogia, chromium, calcium, and potassium. She was also on escitalopram, baclofen, gabapentin, omeprazole, oxycodone, cannabinoids, silodosin, solifenacin, and diphenhydramine. Treatment was changed from escitalopram to sertraline, and the patient kept taking the dietary supplement. One week following change of medication, she was admitted to accident and emergency with a stammer and excessive sweating. On admission, she had tachycardia, hypertension, clonus, leucocytosis and hypokalaemia. The authors concluded that G. cambogia could be involved in a drug-drug interaction context without providing formal evidence (Lopez et al., 2014).
83. Roy et al., (2004) exposed Sprague-Dawley rats with a calcium-potassium salt of 60% HCA extract from G. cambogia (commercially known as Super Citrimax HCA-600-SXS) orally for 8 weeks (5 days a week) at a dose of 10 mg/kg and then performed transcriptomic analysis. A significant increase in the expression of genes encoding the serotonin receptors 5HT2A, 5HT3A, 5HT2B, 5HT4, and 5HT7, with expression levels in abdominal adipose tissue rising by a factor of 1.3.
84. Ohia et al., (2001) utilised the same extract (Super Citrimax HCA-600-SXS) in an ex vivo rat model (cortex sections) and it was shown to induce serotonin release at the highest concentration (300 µM) and an inhibition of the reuptake of this neurotransmitter (at 300 and 1 mM; following an 1 h exposure) (Ohia et al., 2002).
85. A review was carried out by Leite et al., (2021) on the concomitant use of plants with treatment with warfarin. Between 2016 and 2021, 114 medicinal plants were noted to interact with warfarin. G. cambogia (Gaertn.) Desr., (Incorrect designation) was identified as one of the plants that affected platelet activation by lowering adhesion, aggregation, and secretion, which raises the risk of bleeding.
86. Other authors have studied the toxicity of G. cambogia (Gaertn.) based products. Desr., (misnomer) and the mechanisms involved (Di Giacomo et al., 2023). Researchers examined several suspected hepatotoxic reactions linked to products containing G. cambogia (Gaertn.) Desr., based on reports collected through the Italian Phytovigilance System (IPS). Eight cases of hepatic adverse reactions associated with G. cambogia (Gaertn.) Desr., were reported to the IPS over a period of 20 years. One of these cases involves a fatal acute hepatitis in a 45-year-old woman who had taken a dietary supplement containing G. cambogia (Gaertn.) Desr., She was also taking montelukast to manage her asthma—a drug known to cause liver toxicity, frequently raising serum transaminase levels and, in rare cases, triggering hepatitis. An in vitro study was performed to assess the mechanisms possibly responsible for liver toxicity, focusing on modulation of oxidative stress and Nrf2 expression. Low cytotoxicity was observed. However, its combination with montelukast significantly reduced cell viability, increased intracellular levels of reactive oxygen species, and affected cytoplasmic expression of Nrf2, suggesting an impairment of antioxidant and cytoprotective defences.
Conclusions of the Plants WG and the Human Nutrition Expert Committee
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
87. The Plants WG and the Human Nutrition Expert Committee:
- Noted that the EU has not harmonised the lists of authorised plants and plant parts for G. cambogia Desr., nor their uses and doses in food supplements, or the related restrictions and warnings. Therefore, they recommended proper characterisation of the raw material, including measuring HCA and conducting a botanical identification utilising appropriate analytical techniques to discriminate between the different species.
- Highlighted that many of the reported studies in the literature and vigilance reports poorly characterise G. cambogia Desr., extracts and thus urge operators to clearly define their extracts by detailing the composition and specifying the exact production conditions, including the extraction and purification methods and the solvents used.
- Noted that only HCA has been investigated in clinical studies, other components may contribute to adverse reactions. They recommended conducting studies on constituents found in fruit extracts—particularly benzophenones and polyisoprenylated xanthones to investigate and clarify their potential role in adverse reactions.
- Noted that there is ambiguity in the plant parts present in G. cambogia Desr., food supplements.
- Advise individuals with psychiatric disorders, certain cardiometabolic diseases (diabetes, obesity, hypertension), and those with a history of pancreatitis or hepatitis to not consume food products (including supplements) containing G. cambogia Desr., based on the information from the literature and various vigilance systems.
- Further advise against the use of food supplements containing G. cambogia Desr., in children and pregnant or breastfeeding women [due to the lack of information].
- Recommend not combining the intake of G. cambogia Desr., with other hepatotoxic ingredients or foods (such as green tea extract, red yeast rice, turmeric or sources of coumarin).
- Recommended to avoid the use of this plant in combination with drugs known to affect liver function, anti-depressants, and antiretrovirals. More broadly, those taking drugs that are substrates of CYP3A4, CYP2B6, and Pg-P—to avoid consuming food supplements containing G. cambogia Desr. They also warn that G. cambogia may interact with substrates of CYP1A2, CYP2C9, and CYP2D6, and emphasise that these interactions remain a credible risk.
88. The Plants WG and the Human Nutrition Expert Committee reiterate the opinion of ANSES on slimming diets. Any weight-loss programme requires specialist medical support.
Conclusions of ANSES
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
89. In brief, ANSES highlighted the inconsistency of the regulatory status of the plant G. cambogia Desr., (also known as G. gummi-gutta (L.) N. Robson) in France. ANSES further noted that since 2012, the import, preparation, prescription and dispensing of medicines or preparations containing G. cambogia Desr., have been prohibited in France, as these preparations have not proven their efficacy and may expose the patient to health risks.
90. Based on the conclusions of the Human Nutrition Expert Committee and the Plants WG, ANSES advises against using G. cambogia Desr., products in individuals with psychiatric disorders, cardiometabolic conditions (diabetes, obesity, hypertension), or a history of pancreatitis or hepatitis. Use is also discouraged for those on liver-affecting drugs, antiretrovirals, or antidepressants. Given reports of severe adverse reactions in consumers without prior medical history. ANSES extends its recommendation to the entire population. It advises against consuming food supplements made from this plant or preparations containing it.
91. In general, ANSES highlights the need for European harmonisation regarding authorised plants, plant parts, uses and doses in food supplements, along with related restrictions and warnings.
AESAN
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
92. In 2019, the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) reviewed the risk associated with the consumption of food supplements that contain G. gummi-gutta.
93. They state that it is recommended to not exceed a daily dose of 3,000 mg of standardised extract at 50-60% HCA (dose equivalent to 1,500 – 1,800 mg of HCA), administered orally in three “shots”, 30-60 minutes before three main meals.
94. It was found that different supplements marked in Spain containing Garcinia and/or HCA were in the form of tablets, capsules, sachets or vials with differing compositions of G. cambogia and/or HCA content. The range of recommended daily allowances in these products was 30 – 2,070 mg of HCA.
95. AESAN noted that consumption of supplements containing Garcinia and/or HCA has been linked to toxic effects including hepatotoxicity, rhabdomyolysis, nephropathy, cardiovascular toxicity, hypomania and serotonin toxicity and psychosis; however, it has not been confirmed if Garcinia is the main toxicant, as it is often found in products with other ingredients.
96. AESAN concluded that there was “sufficient clinical evidence to establish a causal association between the consumption of garcinia and the duration of treatment, and the development of acute liver injury, with an obvious improvement in liver function after the withdrawal from the garcinia food supplement.” This conclusion was based on the literature reviewed by AESAN including Lunsford et al., (2016) (see paragraphs 152-154), Crescioli et al., (2018) (see paragraphs 142-148, 180), and Sharma et al., (2018) (see paragraphs 150-151), among others.
97. Further to this, AESAN highlighted the importance of regulatory authorities to develop systems for post-market surveillance and for healthcare professionals, researchers and citizens to report adverse effects.
Australian TGA
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
98. The Australian Department of Health, Disability and Ageing Therapeutic Goods Administration (TGA) have also been aware of an increasing number of cases in scientific literature, by consumers who had taken products containing G. gummi-gutta or HCA. One of the five cases which reported liver transplantation was an Australian case. In response, the TGA completed an investigation into the risk of liver injury for the ingredient G. gummi-gutta (G. cambogia) and its naturally occurring component HCA. They concluded that “available evidence shows that there may be a rare risk of liver injury from taking Garcinia gummi-gutta (Garcinia cambogia).” (TGA, 2024a).
99. The TGA states that they will continue to monitor the issue and considering further regulatory action following a consultation on proposed requirements for a label warning. The consultation has ended, and final changes commenced 1st of March 2025, and a 12-month transition period was given to ensure product compliance. These changes are: 1) replacing “rare” with “very rare”; 2) provisions relating to liver-related warnings, and 3) references to the plant part for G. gummi-gutta will be changed from “rind of the fruit” to “fruit peel” to align with the plant parts in the TGA Code Tables (TGA, 2024b).
BfR (German Federal Institute for Risk Assessment)
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
100. Although not an official published risk assessment, a slide deck on “Risk Assessment Approaches and Methodology – an Overview” presented HCA from G. cambogia extracts as a case study - is publicly available (Hirsch-Ernst, 2022). In this, it was noted that doses via supplements was up to 3,000 mg HCA/day.
101. Three animal studies (oral administration, rats) were summarised (see Figure 3). It was observed that at high doses male rats showed signs of testicular toxicity and/or impaired spermatogenesis (i.e. sperm quality and sperm count).
Figure 3 – Animal studies as summarised in Hirsch-Ernst, (2022) (reproduced from Hirsch-Ernst, 2022).
102. In human intervention studies that assessed the safety of HCA intake, the observed adverse effects were mostly non-specific and were in similar occurrence as in control groups. The parameters of potential effects on spermatogenesis were not investigated.
103. The risk assessment was as follows. It was unclear whether the reproductive toxicological effects observed in male rats were due to the possible impurities in the extracts or were compound-specific to high doses of HCA intake. The lowest no-observed adverse effect level (NOAEL) was determined to be 389 mg HCA/kg bw per day (G. cambogia extract, 41.2% HCA). The human data were found to be inadequate with regards to considerations for the male reproductive system. The lack of specifications for HCA preparations/G. cambogia extracts added to food supplements was noted, and as such “transferability” of the above NOAEL to other preparations of food supplements may not be appropriate. Lastly, uncertainties regarding the health assessment of prolonged HCA intakes, especially at high doses (3,000 mg HCA/day) were noted.
EFSA
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
104. The EFSA are currently in the process of writing a scientific opinion on the evaluation of the safety in use of hydroxycitric acid and plant preparations containing hydroxycitric acid (under EFSA query: EFSA-Q-2022-00805). The protocol of which has been published in 2023 (EFSA, 2023a). In brief, the protocol is based on a narrative review of the evidence and on expert knowledge. The overarching risk assessment questions are:
a) Is there a link between dietary exposure to HCA and adverse effects on health?
b) Is there a link between consumption of HCA in the plant preparations listed in Table 1 (of EFSA, 2023a, briefly covers G. gummigutta (L.) N.Robson, G. indica (Thouars) Choisy, G. mangostana L., Hibiscus sabdariffa L. with their respective plant parts e.g. fruit, resin, peel, flower, leaf, seed, bloom, twig) and adverse effects on health?
c) What is the maximum level of total chronic dietary exposure (i.e. over a substantial part of the lifespan) to HCA and HCA in plant preparations and foods containing HCA, which is unlikely to pose a risk of adverse effects to humans?
105. In 2023, EFSA issued a call for data specifically for: occurrence data (analytical data on the content of HCA in plant preparations and food, including food supplements); use of levels of supplements (use levels recommended by manufacturers for food supplements containing HCA) and biological and toxicological data (to support the assessment of a causal relationship between dietary exposure to HCA as single substance and/or in plant preparations and the a priori identified adverse effects, including data on absorption, digestion, absorption and metabolism for HCA and within the food matrix). The call for evidence ended on the 12th of January 2024 (EFSA, 2023b).
106. Publicly available minutes for the EFSA Scientific Panel on Nutrition, Novel Foods and Allergens show that the Working Group on substances other than vitamins and minerals last discussed the draft opinion on the 4th of June 2025 (EFSA, 2025).
Health Canada
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
107. An entry was found in Health Canada’s Licensed Natural Health Products Database for brand name G. Cambogia produced by New Roots Herbal Inc (Applicant) (Health Canada, 2024a). The accompanying monograph to which the Applicant attested to provides details on the dosage form and quantities. The route of administration is oral and can be taken by adults 18 years and older. The recommended dose is 2-3 grams of extract standardized to 50-60% hydroxycitric acid, per day; 1.5-2 grams of extract per single dose and is to be taken before meals.
108. These dose range were based on the studies by (Kim et al., 2011; Gatta et al., 2009; Ishii et al., 2003; Heymsfield et al., 1998).
109. Health Canada states that the monograph “is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorisation. It is not intended to be a comprehensive review of the medicinal ingredient.” (Health Canada, 2018, 2024b)
NCCIH
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
110. The United States National Centre for Complementary and Integrative Health (US NCCIH) states that “it may be unsafe to consume garcinia cambogia products, including multi-ingredient products containing garcinia cambogia extract. Several cases of liver damage have been reported. Some of these cases were severe, but this appears to be uncommon.”
111. Other reported side effects include headache, nausea, diarrhoea, and other gastrointestinal symptoms. Interactions between G. cambogia and some drugs affecting the liver and serotonin have been reported. Limited information is available on the safe use of G. cambogia during pregnancy or while breastfeeding (US NCCIH, 2025).
Data from literature search
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
112. As mentioned in paragraph 5, a literature search was carried out using the search string "Garcinia cambogia" AND "toxicity" in PubMed, Science Direct and Google Scholar. No filters or restrictions were used. These were in addition to the data reviewed by ANSES.
Toxicokinetics
113. van Loon et al., (2000) investigated the acute effects of ingestion of HCA (“6-30 times the reported dosage applied in human weight-loss studies) on plasma HCA availability. They further investigated whether systemic HCA availability altered fat oxidation rates and plasma metabolite concentrations at rest and during moderate-intensity exercise in “endurance-trained” humans by assessing total fat and oxidation rate calculations. Ten cyclists presumed to be male (sex based on information from a pilot study (n=3 males; these males were not included in the main study); aged 25± 2 years; BMI 22.1 ± 0.5 kg/m2) received a total of 0.5 g/kg bw of a liquid G. cambogia extract - Citrimax HCA-450-LS (48% HCA), which was divided over 4 boluses. This resulted in 18 ± 0.4 g HCA being ingested by every subject in the HCA trial. The placebo was water; the number of subjects in the control group were not detailed. It should be noted that beverages (HCA drink or water) were provided in a randomised order. A blood sample was taken at rest, and subjects were provided with either HCA or water to drink 45 mins before exercise. Blood samples were taken at 15 minutes intervals until t=0. Subjects received their second dose 15 minutes before exercise. Following a warm-up period (5 minutes), subjects started cycling at a moderate intensity of 50% Wmax for 2 h (t = 0–120). During exercise, subjects received another bolus of test drink at t = 30 and at t = 60. During exercise, blood samples were taken at 30-min intervals (t = 30 and 60).
114. In the pilot study, it was determined that plasma HCA concentrations increased over time after ingestion of a single dose of HCA solution 4.4g over a 3.5-hour period. Maximal values were attained after 60-90 minutes at 0.12 ± 0.03 mmol/L, after which the concentration decreased. No HCA was detected in the samples collected in the placebo trial.
115. In the main study, plasma HCA concentrations increased up to 0.08 ± 0.01 mmol/L (16.6 mg/L) after the ingestion of 4.4 ± 0.1 g HCA at t = -45 and t = -15 during resting conditions. Plasma HCA concentrations increased further up to 0.39 ± 0.02 mmol/L (82.0 ± 4.8 mg/L) after the ingestion of 4.4 ± 0.1 g HCA after 30 and 60 min of exercise. It was concluded by the authors that plasma HCA availability does not increase energy expenditure or stimulate skeletal fat muscle fat oxidation at rest or during exercise.
116. Loe et al., (2001) dosed fasting humans (n=4; 3 males and one female, subjects were anonymised; age range 21-42; weight range 52.3-86.2 kg) with 2 g of HCA (in the form of CitriMax HCA-600-SXP capsules, ~500 mg) to assess the [bio]availability in humans using a novel gas chromatography/mass spectrometry (GC/MS) method. The subjects were all “healthy, non-smokers that had no history of cardiovascular disease or diabetes”. Blood samples were collected 30 minutes after ingestion of the supplement and every 30 minutes thereafter over a period of 3.5 to 4 hours. A 50 mL blood sample from a control subject (who did not take the supplement) was also collected to construct a standard curve. The peak plasma HCA concentration was observed 2 hours after administration, measuring 8.4 µg/mL, which demonstrated that HCA absorption is relatively fast.
117. Cruz et al., (2021) aimed to determine the main pharmacokinetic parameters of G. cambogia extract/HCA in “healthy” women (n=16 fasted period and n=13/16 for fed period; ages 21-41 years with BMI 20.29–25.82 kg/m2), and to evaluate the food effects on HCA absorption. Subjects received 1,500 G. cambogia, of which 750 mg is HCA extract under 8 hours of fasting. In the fed period, a high-calorie breakfast (~600 calories) was given after dosing. Plasma HCA concentrations were significantly higher in the fasted state (1.21 µg/mL) compared to the fed state (0.40 µg/mL). Overall, plasma concentrations ranged from 0.05 – 2.74 µg/mL. Peak plasma concentration (Cmax) and area under the curve time concentration (AUC0-10hrs) were 3-fold and 2-fold lower (p<0.001, 0.01), in the fed-condition, respectively. The maximum concentration for both groups were similar with 2 hours as the median. In the presence of food, it was observed that, HCA elimination was reduced (5 hours vs 3 hours under fasted conditions). The authors further noted substantial inter-individual variation for the different pharmacokinetic parameters in both periods. The authors suggested that “HCA might suffer an active absorption uptake and intense adsorption on food.”
118. Heymsfield et al., (1998) evaluated the efficacy of G. cambogia for body weight and fat mass loss in “overweight but otherwise healthy adults” (aged 18-65 years; BMI range 27 - 38 kg/m2). The total daily dose of G. cambogia extract was 3,000 mg, of which 1,500 mg was HCA or a placebo was received. Both groups were prescribed a high-fibre, low energy diet. A total of 135 subjects were randomised to either active HCA (n=66) or placebo (n=69) groups; 42 (64%) in the active HCA group and 42 (61%) in the placebo group completed 12 weeks of treatment (P=.74). It was found that the co-administration of HCA with a high-fibre diet and low-energy may have inhibited the gastrointestinal absorption of HCA. There was no significant difference in group weight loss (mean [SD], 3.2 [3.3] kg vs 4.1 [3.9] kg; P=.14) between the exposed and placebo groups.
119. In the literature, (2S,3S)-HCA has been described to inhibit the ATP-citrate lyase enzyme (Jakopin, 2019). This is the primary enzyme responsible for the synthesis of cytosolic acetyl-CoA in many tissues. The product, acetyl-CoA, serves several important biosynthetic pathways, including lipogenesis and cholesterogenesis. In nervous tissue, ATP citrate-lyase may be involved in the biosynthesis of acetylcholine (NCBI, 2025). Thus, it is believed that HCA is widely distributed.
120. No other data were available for distribution, metabolism and excretion. It is also unclear if different Garcinia species influences the bioavailability of HCA.
Review articles
121. Andueza et al., (2021) performed a review to investigate the effectiveness and side-effects of nutritional supplements based on G. cambogia to promote weight loss. The efficacy of other Garcinia species was also provided. They utilised the Cochrane and PubMed databases; 13/51 and 29/53 articles were selected for detailed review, respectively. They concluded that toxicity cannot be reliably attributed to Garcinia, as it is typically present in MIDS. The authors were of the opinion that case reports describing adverse effects usually reflect the associations between the observed toxicity and the intake of the dietary supplement, rather than causality. Furthermore, the authors note that the use of these supplements should be discouraged for pregnant and lactating women, since HCA can affect the production of fatty acids and cholesterol, which can directly influence the production of sterols and steroid hormones.
122. Data from the LiverTox database noted that studies in rats and other animal models have suggested that G. cambogia and HCA do not have significant toxicities, although testicular toxicity was found with high doses (Saito et al., 2005). In humans, Garcinia has been linked to rare reports of serotonin syndrome, rhabdomyolysis and hepatic toxicity; however, the role of Garcinia as opposed to other components of MIDS typically used in humans is yet to be fully elucidated. The frequency of hepatic adverse reactions was estimated to be 1:10,000 and it was concluded that consumption of G. cambogia is likely a rare cause of clinically apparent liver injury (NCBI, 2019).
123. Márquez et al., (2012) noted that caution should be exercised when interpreting the results from human studies as other randomized, placebo-controlled clinical trials have not reported the same outcomes. Furthermore, most studies in humans have been conducted on small samples and mainly in the short term. None of them have shown whether these effects persist beyond 12 weeks of intervention. Therefore, there is still little evidence to support the potential effectiveness and long-term benefits of G. cambogia extracts. Regarding toxicity and safety, it is important to note that except in rare cases, studies conducted in experimental animals have not reported increased mortality or significant toxicity. Furthermore, at the doses usually administered, no differences have been reported in terms of side effects or adverse events (those studied) in humans between individuals exposed to G. cambogia and controls.
124. Chuah et al., (2013) “critically assessed” the evidence from the in vitro, in vivo, and clinical trials on the safety of Garcinia/HCA as a dietary supplement for treating obesity. The methodology in which the authors collected and reviewed studies in the literature was not described. The following endpoints were considered: cytotoxicity, genotoxicity, acute toxicity (oral, dermal, dermal irritation and eye irritation), sub-chronic (90 days) toxicity and reproductive and teratogenic toxicity. The authors summarised that G. cambogia/HCA is generally safe and a NOAEL up to 1, 240 mg/kg per day based on a developmental toxicity study in rats by Deshmukh et al., (2008a) (see Developmental toxicity section). In experimental animal studies at up to 233x the human equivalency dose of HCA (1, 500 mg/day of HCA), toxicological studies revealed no death, significant body weight changes, or gross necropsy findings in Albino rats (Ohia et al., 2001). The authors note that G. cambogia extract has been a widely used anti-obesity herbal supplement for decades, around the world without a birth defect or reproductive problem, as such, they are of the opinion that HCA is unlikely to cause reproductive or developmental toxicity. However, they acknowledged that most randomised clinical trials (RCTs) have been conducted on a small scale and with short exposure duration.
Genotoxicity
125. Lee & Lee (2007) evaluated the genotoxicity of HCA isolated from G. cambogia using three tests: the Ames test, the in vitro chromosomal aberration test, and an in vivo micronucleus test. The test item was described as “a natural, highly water-soluble, calcium-potassium salt of 60% HCA extract”; commercially known as Super CitriMax HCA-600-SXS (HCA-SX).
126. The Ames Salmonella mutation test was used according to the plate incorporation procedure described by Maron and Ames (1983). The five strains of Salmonella typhimurium (TA98, TA100, TA102, TA1535, and TA1537) were provided by Prof. B. N. Ames (University of California Berkeley). The assay was performed with and without metabolic activation using an S9 mixture. Negative and positive controls were used for each strain. The positive controls performed without metabolic activation were: 2-nitrofluorene (1 μg per plate) for TA98, sodium azide (1.5 μg per plate) for TA100 and TA1535, mitomycin (1 μg per plate) for TA102, and acridine mutagen (1 μg per plate) for TA1537. The positive controls performed with metabolic activation was 2-aminoanthracene (1 μg per plate) for all strains. Six concentrations of HCA-SX were examined with triplicate plates per dose: 0, 20, 200, 500, 2,500, and 12,500 μM/plate. HCA-SX did not induce mutagenic activity in any of the five bacterial strains tested, under any of the activation conditions examined.
127. For the chromosomal aberration test, the Chinese hamster ovary (CHO) cell line was provided by the Cancer Research Institute, Seoul National University, Korea. CHO cells were maintained under monolayer conditions in Eagle’s minimum essential medium supplemented with 10% foetal bovine serum, with L-glutamine and ampicillin at 37°C in a 5% CO2 atmosphere. For each treatment, 3 × 105 cells were cultured in duplicate in 5 mL of culture medium in a 2.5-L flask. After the cells were incubated for 24 h, they were treated with an HCA-SX 10 μL reaction volume. A 5-hr pulse treatment was then carried out with and without the S9 mixture (used at a final concentration of 10% in the treatment medium). Benzo[a]pyrene (BaP) was used as a positive control in the presence of S9. Mitomycin C (MMC) was used as a positive control in the absence of S9 mixture. Chromosomal aberration percentages included by HCA-SC in the treated groups were >3% and 4%, respectively. In the positive control groups, the percentage of structural chromosome aberrations in the BaP and MMC-treated groups were >21% with S9 and >25% without S9, respectively. HCA-SX was not observed to induce any cytotoxic effect.
128. For the micronucleus test, 7- to 8-week-old ICR male mice (n=5/group) were acclimatised for at least 7 days prior to the start of the test. HCA-SX were dissolved in dimethyl sulfoxide (DMSO). The groups were as follows: DMSO (negative control), 20, 100, 500, 2,500 or 12,500 HCA-SX μmol/kg dissolved/suspended in DMSO, mitomycin C at 2 mg/kg (positive control). Animals were administered the treatments by intraperitoneal injection and were sacrificed by cervical dislocation 24 hours after the test substance administration. Numbers of micronucleated cells were determined by counting the number of polychromatic erythrocytes (PCEs) from at least 1,000 PCEs per animal. The micronucleated polychromatic erythrocytes (MNPCEs) that contained micronuclei were counted from at least 1,000 PCEs. No mortality was observed. MNPCE/1,000 PCEs were induced at the highest HCA-SX dose (12,500 μmol/kg) and PCE/(PCE + NCE) ratios decreased with increasing dose.
129. The authors concluded that HCA-SX was not found to be genotoxic by bacterial or by chromosome aberration testing. It demonstrated “a weak mutagenic effect by micronucleus testing but did not induce structural chromosomal aberrations or significantly induce MNPCEs at the doses used.
130. The article by Lee & Lee (2007) (as summarised in paragraphs 125-129) was refuted by Lau et al., (2008), stating that for the in vivo micronucleus test, the authors: i) selected an inappropriate route of administration intraperitoneal rather than the oral route; ii) the use of vehicle for the test item (DMSO) was not justified and varied to that of the positive control (water), as DMSO may react with the test compound to induce an adverse effect; iii) a dose-range finding test was not performed and the range of selected doses (separated by a factor of 5) differed from conventional dose levels used in toxicological studies; iv) discrepancy with reporting of results; v) “very weak” statistical analysis, where a t-test was utilised without performing a one-way analysis of variance previously; a regression or correlation analyses was also not performed.
131. Ghosh & Mukherjee (2017) evaluated the in vitro genotoxicity of HCA (50.9% HCA in calcium salt of HCA) in human lymphocytes. The following methods were used: trypan blue dye exclusion test, MTT assay, Comet assay and a DNA diffusion assay. The trypan blue and MTT assay were performed according to the test methods of Tennant (1964) and Mosmann (1983), respectively, with modifications by Sinha et al., (2014). The Comet assay was performed following the method of Tice et al., (2000), with modifications based on Sinha et al., (2014). Cells were exposed to HCA (0, 10, 20, 40 or 100 µg/mL) for 3 hours or 24 hours and processed for cytotoxicity and genotoxicity analyses. The effects of HCA on erythrocytes were determined by a haemolysis test using the same doses and exposure duration. Results from the trypan blue and MTT assay in human lymphocytes demonstrated the absence of significant induction of cytotoxicity when compared to the positive control groups. However, as observed in the Comet assay, HCA induced DNA damage that was statistically significant at concentrations of 40 and 100 µg/mL. The authors noted that these concentrations were “almost identical to and approximately double the maximum permitted dose [author does not detail if this the human equivalent dose] (i.e. 900 – 2,800 mg/day or 15 – 47 mg/kg/day, respectively).” Oxidative stress, as a potential mechanism of DNA damage was evaluated using DCFH-DA dye. A significant increase in reactive oxygen species (ROS) production was found at concentrations of 40 and 100 µg/mL at both time points compared to the respective controls. As for the effects on the erythrocytes, no haemolytic potential was observed. The authors concluded that HCA-induced genotoxicity may not lead to apoptotic/necrotic cell death. The observed DNA damage can be attributed to oxidative stress, which was independent of mitochondrial ROS generation.
Human data
Clinical data
132. Onakpoya et al., (2010) performed a systematic review and meta-analysis of RCTs on the use of Garcinia extract (HCA) as a weight loss supplement. Twenty-three eligible trials were identified of which twelve were further analysed. Nine of the twelve trials provided data for statistical pooling (see Figure 4). The dosage of HCA and the duration of the study were varied, ranging from 1-2.8 grams daily and from 2 to 12 weeks, respectively. The adverse effects reported in the RCTs included headache, skin rash, common cold, and gastrointestinal symptoms. In most of the studies there were no “major” differences in adverse events between the HCA treatment and placebo groups. Except for one trial where gastrointestinal symptoms were twice as frequent in the HCA group compared to the placebo group (Heymsfield et al., 1998).
Figure 4 - Results table for studies with adequate data for meta-analysis (reproduced from Onakpoya et al., 2011).
133. Amini et al., (2024) performed a systematic review and meta-analysis of RCTs on the use of G. cambogia (HCA) on serum leptin concentrations. Eight studies were included in the meta-analysis (see Figure 5). Leptin is a peptide hormone that is produced and secreted by adipose tissue. It plays a role in appetite control, immune system modulation, insulin sensitivity, blood pressure regulation and energy homeostasis. The authors observed that several of the included studies found no adverse effects of supplementation. In one study, 38.4% of participants reported the following side effects during treatment: gastrointestinal symptoms, thirst, dizziness and diuresis with gastric discomfort being the commonly reported (Vasques et al., 2014). It was noted by the authors that the treatment period in the selected trials ranged from 11 days to 10 weeks and the long-term side effects of supplementation requires further evaluation as several case reports observed liver injury following G. cambogia supplementation.
Figure 5 - Demographic characteristics of the included studies (reproduced from Amini et al., 2024).
Hepatotoxic case reports
DILIN network
134. The United States Drug-Induced Liver Injury Network (DILIN) have published several articles concerning herb related drug injury. Articles that referred to G. cambogia are summarised in chronological order.
135. In 2015, Navarro et al., noted that G. cambogia was implicated with herbal products in the DILIN database; however, the role of this herb in causing liver injury was difficult to assign since there was a lack of documentation of their chemical presence and purity, the possibility of contamination with other herbal products or mislabelling of the ingredients.
136. In 2022, Vuppalanchi et al., described the hepatotoxic effects of G. cambogia, either alone or in combination with green tea extract (catechins) consumption. Among the 1,418 patients enrolled in the DILIN from 2004-2018, it was identified that 22 cases of liver injury could be attributed to G. cambogia alone (n=5/22), in combination with green tea (n=16/22) or in combination with ashwagandha (n=1/22). Control groups consisted of 57 patients with liver injury from herbal and dietary supplements (HDS) containing green tea without G. cambogia and 103 patients from other HDS.
137. Patients who took G. cambogia (see Figure 6) were aged 17 and 54 years (n=22; 12 females and 10 males) with liver injury arising 13-223 days (median = 51 days) following the first dose. The doses for each product type were not made readily available, and it should be noted that although one of the products claimed the presence of Garcinia/HCA, it was not detected.
Figure 6 - Names and products linked to G. cambogia and green tea extract (catechins) induced liver injury (reproduced from Vuppalanchi et al., 2022).
138. Of these patients, one died, one required liver transplantation and 20 were hospitalised. The liver injury was hepatocellular with jaundice. Peak values of aminotransferases were significantly higher (2,001 ± 1,386 U/L) in the G. cambogia group (P < .018), the median time for improvement in total bilirubin (TB) was significantly lower compared with the control groups (10 vs 17 and 13 days; P = .03).
139. The presence of HLA-B∗35:01 allele was significantly higher in the G. cambogia containing HDS (55%) compared with patients because of other HDS (19%) (P = .002) and those with acute liver injury from conventional drugs (12%) (P = 2.55 × 10-6). The authors concluded that liver injury caused by G. cambogia and green tea was clinically indistinguishable and hypothesised that there is possible association immune-mediated mechanism of injury via HLA-B*35:01 allele.
LATINDILI
140. Bessone et al., (2025) published a “comprehensive analysis” of patients enrolled into the Latin American DILI Network over a decade. Chemicals suspected of causing DILI were classified according to the Anatomical Therapeutic Chemical classification. Causality was assessed using the Roussel Uclaf Assessment method. Overall, 468 idiosyncratic DILI cases were analysed, it was observed that 62% were women (mean age 49 years). Of the cases, 4.1% had a fatal outcome, and 24 patients (12%) developed chronic DILI. The most common drug classes were systemic anti-infectives (31%), musculoskeletal agents (12%), antineoplastic and immunomodulating agents (11%), and herbal and dietary supplements (9%). A total of 6 cases were attributed to G. cambogia.
Other literature
141. Al-Khazraji et al., (2020) [abstract only – poster for symposium] claimed to report the first human case report of G. cambogia induced autoimmune hepatitis. A 39-year-old female with no past medical history presented with fatigue and dark coloured urine. Clinical exam demonstrated a palpable liver and scleral icterus. The patient reported using a “slimming herbal tea supplement” containing pure GC for weight loss 5 weeks prior to presentation [no further information on dose or HCA content]. Elevated transaminase levels were observed and were considered significant: alanine transaminase (ALT) 1803 IU/L; aspartate aminotransferase (AST) 1026 IU/L; alkaline phosphatase (ALP) 139 mg/dL and TB 5.2 mg/dL. Further liver work up revealed an elevated anti-nuclear antibody (ANA) 1:160 titer; anti-smooth muscle antibodies (ASMA) 1:320; and immunoglobulin G 1,814. The normal range for these parameters were not detailed in the paper. A liver biopsy was performed, which demonstrated moderate mixed inflammatory cells including lymphocytes, plasma cells, neutrophils and “rare” eosinophils in the portal tracts. Interface hepatitis and cholestasis was also noted. The authors stated that findings were consistent with DILI with a background of autoimmune hepatitis. The patient was treated with steroids (Prednisone 40 mg orally, daily), upon tapering of her steroids; her liver function began to rise, and she was started on immunosuppressive therapy for long term maintenance with “good response”. The authors further stated that G. cambogia liver injury can last for 2–3 months with normalizing of liver function tests by 5 months.
142. Crescioli et al., (2018) presented four cases of acute liver failure in women taking G. cambogia extract for weight loss, and a literature review of clinical evidence about hepatic toxicity in patients taking dietary supplements containing G. cambogia extract.
143. For Case 1, a 61-year-old woman presented to the emergency department with symptoms of abdominal pain, nausea, progressive weakness, jaundice, dark urine, and acholic stools. The patient’s anamnesis denoted cholecystectomy, mixed dyslipidemia, and hypothyroidism in treatment with levothyroxine. There was no history of alcoholism or exposure to hepatotoxins; she also denied paracetamol abuse. She reported taking one envelope/daily of SUPER ANANAS SLIM®, for a period of 2 months to lose weight. This contained extracts of G. cambogia (HCA 60%), Ananas comosus (bromelain 334 GDU; gelatine dissolving units), and Ilex paraguariensis [Yerba mate] (caffeine 2%). Laboratory tests revealed that ALT, AST, TB, ALP, gamma-glutamyl transferase (GGT), direct bilirubin and albumin were higher than the normal total range at 1,629 U/L, 1,121 U/L, 22.5 mg/dL, 16.7 mg/dL and 2.2 g/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL, 0-0.25 mg/dL and 3.5-5 g/dL, respectively. Serology was negative for hepatitis viruses or autoantibodies. A check-up 3-months prior had found these parameters to be normal. The abdominal computed tomography scan revealed a small peritoneal effusion, perihepatic lymphadenopathy, and a hepatic biopsy which was consistent with cholestatic hepatitis. Four weeks after the cessation of the supplement intake, patient symptoms and liver function tests gradually improved, and the patient was discharged with no need for liver transplant. Four months later, the levels of the previously tested markers reverted to normal values.
144. For Case 2, a 39-year-old woman presented to the emergency department with symptoms of jaundice, asthenia, loss of appetite, and right hypochondrial pain. Her anamnesis denoted arterial hypertension, obesity (BM 44.9 kg/m2), and hiatal hernia. Her medication at the time of admission were methyldopa 500 mg/day, domperidone 20 mg 3 times/day and omeprazole two 20 mg capsules/day. She also reported taking two dietary supplements for weight loss recommended by her dietician. The first, OBLESS®, contained in each capsule: C. aurantium [bitter orange] 140 mg of extract 10% (14 mg of synephrine), G. cambogia (72 mg of HCA), Orthosiphon stamineus [cat’s whiskers or Java tea] (0.2 mg of sinensetin), and Griffonia simplicifolia [African black bean] (75 mg of 5-hydroxy-l-tryptophan). The second dietary supplement was a magistral preparation containing in each capsule: C. aurantium [bitter orange] 350 mg of extract 6% (21 mg of synephrine), Rhodiola rosea [Roseroot or Goldenroot] 150 mg extract, and O. stamineus [cat’s whiskers or Java tea] 200 mg extract. The patient declared that she had been taking the first dietary supplement for the previous month (1 capsule/day) and the magistral preparation for 15 days (1 capsule/day), simultaneously. Laboratory tests performed revealed that ALT, AST and TB were higher than the normal ranges at 1,554 U/L, 1,071 U/L and 15.2 mg/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL, respectively. Serology was negative for hepatitis viruses, cytomegalovirus, and varicella-zoster virus. Non-specific antinuclear antibodies and biliary antibodies were positive. Abdominal ultrasound was normal and did not reveal steatosis. Liver biopsy was not performed. Supplement intake was ceased. After 12 days of hospitalisation, the patient was discharged with no need of supplementary therapies, and a diagnosis of acute cholestatic hepatitis related to consumption of OBLESS® was made.
145. For Case 3, a 47-year-old woman was admitted to the emergency department with symptoms of severe abdominal pain (right hypochondrial). Her anamnesis denoted hyperthyroidism (treated with levothyroxine 100 µg/day), arterial hypertension (treated with enalapril 20 mg/day), and mild obesity. She also reported taking of THERMO GIALLO®, as self-medication for weight control. Each capsule contains 50 µg chromium and 400 mg G. cambogia of which 50% was HCA (200 mg), the patient reported that she had been consuming 2 capsules/day for a month. Laboratory tests revealed elevation of ALT, AST and TB at 299 U/L, 67 U/L and 0.7 mg/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL, respectively. Serology was negative for hepatitis or autoantibodies. No clinical evidence of steatosis was observed, and a liver biopsy was not performed. During the patient’s hospital stay, after cessation of weight-loss supplement, the levels of TB spontaneously declined, and her symptoms and liver function tests “rapidly improved”, without the need of therapies. The patient was discharged with a diagnosis of acute hepatitis.
146. For Case 4, a 52-year-old woman was admitted to the emergency department and was diagnosed with acute hepatitis. No “significant” diseases and medication therapies were reported in anamnesis. However, she was taking two JILL COOPER BE SLIM® products (1 capsule/day for each product) for weight control, containing 400 mg G. cambogia of which 60% was HCA (240 mg), and 400 mg green coffee of which 50% was chlorogenic acid (200 mg), respectively. These products were purchased via T-commerce and were used for a month prior to the emergency department visit. Laboratory tests revealed elevation of ALT, AST, and TB at 1,819 U/L, 1,442 U/L and 14.7 mg/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL. Serologies for hepatitis viruses and autoantibodies were also negative. No clinical evidence of steatosis was observed, and a liver biopsy was not performed. During the following days [number of days not specified] and cessation of the supplement products, liver parameters “spontaneously declined” and acute hepatitis “completely resolved” with no need of supplementary therapies.
147. As previously mentioned, Crescioli et al., (2018) also performed a literature review related of four areas: i) general information for G. cambogia; ii) its use in humans for weight control; iii) the safety of G. cambogia, iv) G. cambogia dietary supplements. The criteria were filtered for human case reports and series only, published between January 2000 and October 2017. The final selection were 24 case reports and 8 case series reporting adverse effects in a total of 66 patients who consumed G. cambogia extract. Five studies reported single cases of myocarditis (Allen et al., 2014), cardiomyopathy (Joseph et al., 2014), serotonin toxicity (Lopez et al., 2014), hypoglycaemia (Roche et al., 2014), and thrombocytopenic purpura (Sikka et al., 2016). Two patients presented acute pancreatitis (Sidhu & Khehra, 2016) and diabetic ketoacidosis (Bystrak et al., 2017), three patients experienced rhabdomyolysis (Dehoney & Wellein, (2009); Hines et al., (2015); Mansi & Huang, (2004)), and in five studies the authors described adverse events of mania (Cotovio & Oliveira-Maia, (2017); Narasimha et al., (2013); Hendrickson et al., (2016)) and multiple psychotic symptoms (Nguyen et al., (2017); Wong et al., (2016)). The patients were mostly women (62%) with no relevant medical history. Seventeen out of the 32 studies described cases of acute liver injury, liver failure, and hepatotoxicity, observed in 50 patients who consumed G. cambogia dietary supplements or G. cambogia “pure extract” (Actis et al., (2007), Chen et al., (2010), Corey et al., (2016), Dara et al., (2008), Elinav et al., (2007), Fong et al., (2010), Jones & Andrew (2007), Kothadia & Olivera-Martinez, (2016), Lunsford et al., (2016), McDonnell et al., (2009), Melendez-Rosado et al., (2015), Schoepfer et al., (2007), Shim & Saab, (2008), Smith et al., (2016), Stevens et al., (2005), Stickel et al., (2009), Vitalone et al., (2011).
148. According to Crescioli et al., (2018), all patients with hepatic adverse effects consumed their food supplements according to the manufacturer’s recommendations and yet developed similar symptoms, including jaundice, weakness, abdominal pain, dark urine, nausea, and vomiting, which were the most reported. The duration of exposure varied with some patients taking supplements for a few days or weeks to more than one year. Of the 50 patients, the symptoms of 38 improved, while 8 required liver transplantation, 1 was diagnosed with liver cirrhosis and 2 died.
149.. Sharma et al., (2018) presented a case report with “severe” liver toxicity following exposure to Hydroxycut®.
150. A 19-year-old man with no “significant” past medical history presented to a community medical centre with 2-day history of fever (103ºF/39.4ºC on presentation), severe fatigue, myalgia, arthralgias and an erythematous rash over his lower extremities. He started using Hydroxycut® [product formulation or HCA content not specified] approximately one week prior to presentation for fat burning and muscle building. He denied any smoking or alcohol use and was taking no other prescription or over the counter medication apart from Hydroxycut® and Myoflex® cream. His blood test revealed an AST level of 23 U/L, ALT of 81 U/L, alkaline phosphatase of 298 U/L white blood cell count of 31x109/L, haemoglobin level of 12.7 g/dL and a normal platelet count. TB was 7.3 mg/dL and he had a prothrombin time of 16.7 seconds. The paper did not provide details of the normal range for these parameters. Blood cultures, urine analysis, chest X-ray, abdominal ultrasound, computed tomography scan and magnetic resonance cholangiopancreatography results were normal. His liver appeared normal in size and texture and there was no evidence of mass, vascular compromise, stone disease, ascites or biliary ductal dilatation. The patient continued to have rising bilirubin levels over the next 3 days and despite antibiotics (Piperacillin and Tazobactum) had a persistent fever. On day 4, the patient was transferred to a hospital for possible urgent liver transplant evaluation due to rising TB of 12.7 mg/dL and a prothrombin time of 21.7 seconds. His hepatic profile showed AST at 27 U/L, ALT at 24 U/L, alkaline phosphatase at 152 U/L, bilirubin at 12.4 mg/dL, a prothrombin time of 15.4 seconds, ammonia at 38 µg/dL, a white blood cell count of 34.8 x 10(9)/L (71 neutrophils and 24 bands), haemoglobin level at 11.2 g/dL, and a platelet count of 237,000/µL. Repeat blood cultures and sputum cultures, as well as urine analysis showed no evidence of infection. Serologies for hepatitis A, B, and C, anti-mitochondrial antibody, anti-nuclear antibody, anti-smooth muscle antibody, F actin IgG, liver kidney Microsomal antibody-1, cytomegalovirus, Epstein Barr virus, Herpes Simplex virus, Group A streptococcal antigen, Coxsackie virus, Monospot virus and leptospirosis were all negative. Ceruloplasmin and Alpha-1 anti-trypsin levels were normal. Human immunodeficiency virus and Rapid plasma reagin tests were negative. Alpha-fetoprotein level was normal at 1 ng/mL. Iron studies were notable for mild iron deficiency and a ferritin level of 463 ng/mL. A comprehensive urine toxicity screen was negative for any drugs except for opiates which he was administered at the hospital for pain management. A liver biopsy done on the 7th day of hospitalization showed acute cholangitis with scant micro vascular fatty changes (<5%) and no evidence of lobulitis, hepatocytes necrosis, cholestasis, fibrosis, parasite, ova, vasculitis, thrombosis, viral inclusions or neoplasm. Infectious disease consultation was obtained, and Vancomycin was added to his antibiotic regimen. No infectious aetiology could be determined and the patient continued to have hyperbilirubinemia. He was started on Ursodiol 600 mg orally twice a day. His bilirubin level peaked at 18.6 milligram/dL, (direct at 10.2) and started to decrease after day 6 of hospitalization. Peak alkaline phosphatase was on day 3 of admission at 298 units/L and peak AST/ALT levels were 110/142 units/L respectively on days 11 and 13. All abnormal levels gradually started to decrease. Antibiotics were discontinued after a total of 14 days of therapy. The patient was discharged from hospital on day 17 with a bilirubin level of 6.8 mg/dL (direct at 3.2), AST level of 68 U/L, ALT level of 108 U/L, alkaline phosphatase level of 160 U/L. The patient had gradual recovery of liver functions and at 14 weeks after initial onset of symptoms his liver function tests had returned to normal.
151. The authors acknowledged that “whilst causation is difficult to prove in any drug induced injury, the temporal relationship after Hydroxycut® exposure and gradual improvement after withdrawing the involved medication plus the absence of any other aetiologies despite comprehensive testing would point to Hydroxycut® being the most likely possible cause for hepatotoxicity.”
152. Lunsford et al., (2016) reported “the first known case” of fulminant hepatic failure associated with dietary intake of a “pure” G. cambogia supplement.
153. A 34-year-old Hispanic male presented with nausea, vomiting, abdominal pain, and dark urine. Testing revealed elevated transaminases and TB; however, imaging failed to demonstrate cirrhosis or anatomic abnormality. Hepatitis work-up, including testing for viral hepatitis, hemochromatosis, Wilson’s disease, and autoimmune hepatitis, was unremarkable with the exception of an elevated Ferritin level of 7,089 mg/dL. Genetic testing for hemochromatosis was negative. His medical history was only positive for occasional social alcohol use, and the drug toxicology testing was negative. He denied use of energy drinks, herbs, Chinese teas, or muscle milk. He was advised to discontinue alcohol use, which he did, and his symptoms initially seemed to abate. Six weeks later, the patient developed asterixis, jaundice, and confusion. Follow-up imaging was concerning for rapid onset of cirrhosis or infiltrative hepatocellular carcinoma. On admission, transaminases were elevated with AST 624 U/L, alanine ALT 520 U/L and TB of 34.7 mg/dL. Autoantibody titers demonstrated a positive antinuclear antibody, but no other positive autoantibodies. Evaluation of Wilson’s disease demonstrated normal ceruloplasmin and copper levels; however, 24-h urine copper was elevated. Serum ferritin but not transferrin was elevated. A liver biopsy was performed and demonstrated sub-massive necrosis with collapse of the hepatic architecture involving about 70% of the liver parenchyma. Mild lymphocytic inflammatory infiltration and minimal canalicular cholestasis were seen. No viral inclusions or other infectious agents were identified by histology or immunohistochemistry. No evidence of granuloma, tumour, or features of cirrhosis were demonstrated. Periodic acid-Schiff stain with diastase was negative for alpha-1 antitrypsin globules. Iron stain showed only mild iron deposition in Kupffer cells and hepatocytes. Quantitative tissue copper was within normal limits. Findings were suspected to be potentially related to drug-induced liver injury. After questioning, the patient confirmed intake of G. cambogia, purchased on the Internet. He was taking two 80 mg capsules of “Garcinia Cambogia 5:1 Extract” three times daily before meals for five months preceding initial presentation. Since not advised against intake, he continued the supplement after initial presentation. He denied any other medications or supplements and reported no alcohol intake for two months. An 80 mg tablet of a 5:1 concentrate of G. cambogia was determined to be equivalent to 400mg of standard preparation. Other listed ingredients include rice flour, gelatine, magnesium stearate, and silica. However, it was confirmed that the manufacturer does not perform assays to determine HCA concentration. The patient’s status declined and his mental status deteriorated; he was listed for liver transplantation and received an orthotopic liver transplant. Histopathologic examination of the explanted liver demonstrated near total hepatic necrosis with massive hepatocellular dropout and mixed inflammatory cell infiltrates, consistent with severe drug-induced liver injury.
154. The authors concluded that “while evidence from a case report rarely offers proof of causality, this case, in conjunction with known cases of hepatotoxicity and liver failure associated with other G. cambogia-containing supplements warrants a high index of suspicion.”
155. García-Cortés et al., (2016) reviewed the reported cases of hepatotoxicity by dietary supplements. Two case-studies were summarised relating to G. cambogia induced hepatotoxicity. Corey et al., (2015) reported a 52-year-old female requiring liver transplant after taking G. cambogia extract 936 mg (2 capsules per day) with 60% HCA (USA Nutra Labs) for 15 days. To note that the supplement also contained calcium (50 mg), chromium (200 µg), and potassium (50 mg) at the ingested amount.
156. Melendez-Rosado et al., (2015) reported a 42-year-old female with elevated transaminase levels (ALT at 1,277 U/L and AST at 2,792 U/L, which are 70 and 45 times the upper limit of normal, respectively) and coagulopathy. It should be noted that the woman had a medical history of hypertension, chronic kidney disease (stage V), diabetes mellitus type 2, chronic back pain, haemochromatosis, and obesity. Abdominal ultrasound showed mildly coarse hepatic echotexture with no intrahepatic or extrahepatic dilatation, and abdominal computed tomographic scan showed a surgically absent gallbladder and no hepatic parenchymal abnormalities. A week before presentation, the patient started taking “pure G. cambogia” for weight loss. The supplement was discontinued, and the patient was empirically treated with N-acetylcysteine due to concerns of acetaminophen toxicity. After several days of supportive care, the patient’s abdominal pain resolved, and liver enzymes recovered to baseline. The patient was discharged after 4 days. Four months later, the patient’s symptoms had not recurred, and normalisation of her liver function studies was noted. The authors were of the opinion that the development of acute abdominal pain with elevated liver enzymes in the setting of a baseline liver inflammation in combination with the use of acetaminophen and the introduction of a hepatotoxic herbal supplement makes the diagnosis of acute hepatitis secondary to G. cambogia “very likely”. No other information on the supplement, dose or exposure time could be obtained.
Animal studies
Subchronic effects
157. Shara et al., (2003) evaluated the dose- and time-dependent effects of HCA-SX in male and female Sprague-Dawley rats on body weight, hepatic and testicular lipid peroxidation, DNA fragmentation, liver and testis weight, expressed as such and as a percentage of body weight and brain weight, and histological changes over a period of 90 days. An animal research protocol (ARC# 0598) was obtained from Creighton University Medical Center. HCA-SX “a natural, highly water-soluble”, calcium-potassium salt of 60% HCA extract from G. cambogia – commercially known as SuperCitriMax HCA-600 SXS was dissolved in water and administered by gavage at 0, 0.2, 2.0 and 5% of feed intake. Control animals received the vehicle (water). Food and water consumption were measured 2-3 times weekly. Mortality/morbidity was assessed once daily throughout the study period. Clinical signs were evaluated once to twice daily. Body weights were recorded on day 1, twice weekly thereafter and before necropsy. Animals were sacrificed on days 30, 60 and 90 of treatment and the target organs were either processed immediately, preserved in 10% buffered formalin for histopathology, or stored at -80ºC. The number of animals per dose group was not explicitly stated by the authors; however, it was detailed that all result values were reported as a mean ± standard deviation from 5-7 samples.
158. By day 90, feed intake was reduced by 13.7, 26.7 and 25.6% in male rats following supplementation of HCA-SX at the aforementioned doses (0 (water), 0.2, 2.0 and 5% of feed intake), respectively, when compared to their corresponding controls. In females, the values were 16.3, 19.6 and 22.8%, respectively. Regarding changes in body weight, ~11.2, 12.4 and 15.8% reduction in body weight in male rats following supplementation at the tested dose levels, respectively. For females these values were ~11.1, 18.1 and 13.0%, respectively. These were considered as significant by the authors. Regarding changes in testicular weight (expressed as a percentage of body weight and brain weight), a small but not statistically significant increase in testis weight with increasing age was observed in control animals. The testis weights in the exposed groups were similar to the control animals. Regarding the effects of HCA-SX on hepatic and testicular lipid peroxidation, a time-dependent increase in hepatic lipid peroxidation was generally observed in all samples; however, these were not significant in the HCA-SX exposed groups. A small but non-significant increase in testicular lipid peroxidation was observed in the control group as well as the HCA-SX exposed group. Regarding the effects of HCA-SX on hepatic and testicular DNA fragmentation, the results showed that there were no significant HCA-SX treatment related effects on these parameters (in male rats for the latter) when compared with their respective controls. Histopathological analyses on the liver, brain and testis, revealed that HCA-SX exposure did not cause any morphological alterations in these tissues.
159. The authors were of the opinion that these results indicated that HCA-SX was “safe and efficacious in weight management” under the test conditions.
Reproductive toxicity
160. Deshmukh et al., (2008a) evaluated the effects of a novel calcium/potassium salt of HCA on the reproductive systems of male and female rats, the postnatal maturation and reproductive capacity of their offspring, and possible cumulative effects through multiple generations. The study was performed in compliance with a standard study protocol based on the US FDA Redbook Guidelines for Reproductive Studies IV.C.9.a, and Guidelines for Developmental Toxicity Studies IV.C.9.B., Feed Additive Safety (US FDA, 1993), and the OECD principles of Good Laboratory Practice.
161. The test article, HCA-SX, commercially known as Super CitriMax was mixed with powdered rodent diet to obtain three concentration levels. A small volume of diet premix was prepared, which was then mixed with the remaining portion of the diet in a mechanised ribbon blender for about 20 minutes to obtain the desired homogeneity of the test article concentration in diet. The experimental diets were prepared once a week based upon the results of the stability tests on HCA-SX. The diet preparation procedure was subsequently validated by conducting stability studies and homogeneity on exposed diets.
162. Sprague-Dawley rats (n=30/sex per dose group) were administered feed containing HCA at dose levels of 0, 1,000, 3,000, or 10,000 ppm for 10 weeks prior to mating, during mating, and, for females, through gestation and lactation, across two generations. The control group of animals were fed normal diet. The male and female rats of the F0 generation from each dose group were mated and allowed to deliver normally. At weaning, one male and one female pup from each litter from the control and treatment dose groups were selected for the F1 generation. The selected F1 animals were exposed to HCA-SX for 10 weeks before mating and then they were mated to produce a second generation F2a. During the period of study, animals were examined daily for signs of clinical toxicity and their body weight and feed consumption (g/rat/day) were recorded twice a week. N.B. A discrepancy was noted where the abstract states the latter was performed twice a week recording, whilst the methods section describes one weekly recording. For the parents (F0 and F1) and the offspring (F1 and F2a), reproductive parameters such as fertility and mating, gestation, parturition, litters, lactation, sexual maturity, and development of offspring were assessed. At termination, necropsy and histopathological examinations were performed on all animals.
163. Data on feed consumption by the parental male and female rats of both (F0 and F1) generations during the premating and mating periods, for both sexes, and during gestation and lactation in the case of female rats, did not reveal any “remarkable” treatment-related changes in the average daily feed intake by the male and female rats compared to the respective control groups, across the different dose levels for each of the F0 and F1 generations and also when compared across these two generations. Based on feed intake, the resulting dose of HCA-SX for the highest-dose groups of male and female was calculated as 813 and 1,205 mg/kg/day, respectively, for the F0 generation, while the same was respectively 1,018 and 1,524 mg/kg/day in the case of the F1 generation. The total daily dose of HCA-SX for all groups are provided in Table 2.
Table 2 – Daily dose of HCA-SX (mg/kg bw per day) during the premating period (reproduced from Deshmukh et al., (2008a).
|
HCA-SX level in diet (ppm) |
F0 Males (mg/kg bw per day) |
F0 Females (mg/kg bw per day) |
F1 Males (mg/kg bw per day) |
F1 Females (mg/kg bw per day) |
|
Control (0) |
0 |
0 |
0 |
0 |
|
Low (1,000) |
80 |
109 |
89 |
135 |
|
Mid (3,000) |
246 |
354 |
268 |
447 |
|
High (10,000) |
813 |
1,205 |
1,018 |
1,524 |
164. Dietary exposure to all animals and offspring at the F0 and F1 generations, did not reveal significant incidence of mortality or abnormal clinical signs. Compared to the respective controls, the HCA treatment groups at all dose levels, did not have different feed consumption or body weight. All deaths and abnormal clinical signs observed in the rats during F0 and F1 generations, such as transient/reversible spells of emaciation, abdominal breathing, respiratory rates, hypoactivity, circling disorder, and lacrimation, were considered to be incidental and not test substance related.
165. The average bodyweight and body weight gains on the parental F0 and F1 generations up to all stages including lactation of female rats of the exposed groups at all doses, did not reveal any significant differences when compared to the respective control groups. Although, a mild but significant lowering of body weight gain in F1 male offspring was noted in the highest dose level after they were weaned, between 3 to 7 weeks of their life. For the duration of the study, the difference in body weights persisted; however, it was not significant, and the percentage gain in body weights between control and exposed groups of male rats was found to be comparable on week 31 (it was slightly higher in the high dose group than the control group). The authors did not count this as an adverse effect while deciding the NOAEL, as it was considered to be a likely effect of the pharmacological activity of HCA-SX. There were other “occasional” instances of group mean values of exposed animals differing from those of the respective control; however, these were not considered of no toxicological significance due to the “small magnitude of variation”.
166. During the gestation period in all females exposed to HCA-SX, no treatment-related adverse effects on reproductive performance in terms of fertility and mating, gestation, parturition, and the litters born were observed. The values of male fertility indices for the exposed groups in the F0 and F1 generations did not differ significantly from those of the controls and also compared well with the historical control data at the test facility. The sperm motility of the F1 parents was lower than the F0 parents (in exposed groups). The authors did not consider this to be related to HCA-SX exposure, as the lowering was also observed in the concurrent control group of rats.
167. All the gross and microscopic findings of the parental organ weights, necropsy and histopathology were considered to be incidental as the incidence was found to be comparable among the control group and the treatment groups, without any dose-dependent trend.
168. Offspring observations are hereby summarised. The body weights of some of the pups selected as parents for the next generation were recorded at ~4 weeks after they were weaned at the end of their lactation; in male offsprings exposed at the highest dose (10,000 ppm), the group mean body weights were significantly lower than those of the control group; however, this effect was not considered as an adverse effect [was considered as a pharmacological effect]. When compared with their respective controls, data on survival and clinical observations recorded for the offspring of both generations F1 and F2a during the lactation period of 21 days did not reveal any remarkable differences. Nor were there any adverse effects on their litter sizes, sex ratio of litters, live birth indices and the viability of litters, which were calculated on days 4, 7, 14 and 21 of lactation from parental females exposed to HCA-SX at all dose levels. The sexual maturation [age at which there is balanopreputial separation in males and vaginal opening in females] was only measured for the F2a groups. It was observed that exposure to HCA-SX at any of the dose levels did not affect the age of sexual maturity of the offspring belonging to the F2a generation. Exposure of the parental animals of both the F0 and F1 generation to HCA-SX at the tested dose levels had no adverse effect on the physical development of their litters, compared to the respective control groups. The group mean values of absolute and relative weights of the brain, spleen, and thymus of the pups of the F1 and F2a generation did not significantly alter between the control and treatment groups. The study authors considered that all the gross and microscopic pathology findings in this study were accidental as the incidence was found to be comparable among the control group and the treatment groups, without any dose-dependent trend. HCA-SX treatment did not cause any significant histopathological changes in any organ.
169. The authors noted that exposure to HCA-SX did not affect reproductive performance as evaluated by sexual maturity fertility and mating, gestation, parturition, litter properties, lactation, and development of the offspring. Nor did it induce any systemic toxicity in the parental rats and their offspring at the tested concentration levels. Based on the results of this study, the authors determined a NOAEL to be greater than 10,000 HCA ppm in the diet or equivalent to 1,018 and 1,524 HCA mg/kg bw per day in male and female rats, respectively.
170. Saito et al., (2005) investigated the ability of G. cambogia extract to suppress body fat accumulation in developing male Zucker obese (fa/fa) rats. 6-week-old rats (n=6 per dose group) were fed diets containing HCA powder (41.2 wt%: ratio of free to lactone form was 36.6 to 63.4) at 0, 2, 10.1, 20.1 and 30.2 g HCA/kg diet for 92 or 93 days. All groups had free access to water. Each diet group was pair-fed to the 30.2 g HCA/kg diet group. On the last experimental day, the rats were allowed to consume three-quarters of the food intake of the previous day and were then killed by cardiac puncture. Liver, kidney, spleen, spleen, testis, and epididymal fat pads were excised, washed with isotonic saline and weighed. The liver, spleen and testis were fixed with 10% formalin neutral buffer solution, pH 7.4 and histopathological examinations were performed after haematoxylin-eosin staining. At the highest dose, there was significant suppression of epididymal fat accumulation in developing male Zucker obese rats, compared with the other groups. The higher dose groups (20.1 and 30.2 g HCA/kg diet) caused “potent” testicular atrophy and toxicity, whereas diets containing 10.1 g HCA/kg diet or less did not. The authors derived a NOAEL of 10.1 g HCA/kg diet equivalent to 389 mg HCA/kg BW per day.
171. Burdock et al., (2005) performed a “critical review” of the article by Saito et al., (2005) (as summarised in paragraph 170) and raised the following comments: (1) the form of HCA and toxicity; (2) experimental study design and results; (3) Zucker rat model and testicular toxicity; and (4) dietary ingredients and testicular toxicity. The form of HCA can vary its toxic profile, dietary supplements containing HCA consist of various salts, including HCA–sodium, –calcium, –potassium, –magnesium, or combinations thereof. Depending on the salt(s) used and the extraction process, the solubility, bioavailability, bioefficacy and lactone content can vary considerably.
172. Limitations on the experimental design were noted: the amount of food received by pair-fed animals was approximately 10% less (compared to ad libitum fed control, which was not included). Burdock et al., further state that HCA is known to affect satiety, effectively preventing consumption of food at high levels. Secondly, the investigators reported severe diarrhoea at the highest use levels (1,244 mg HCA/kg/day), which may have further affected the feed intake in this group and other groups (as a result of pair feeding). Thirdly, Saito et al., (2005) stated that Zucker obese rats (or models with higher lipogenic properties) may be insensitive to HCA at usual dietary levels; however, a biphasic effect of HCA on fat accumulation in the liver was noted. The selection of Zucker rat model was thought to be inappropriate since serum concentrations of testosterone in obese male Zucker rat at the age of 2-, 3- and 4-months were lower when compared to lean male Zucker rats. Testes morphology of Zucker rats was also found to be different from lean rats.
173. It was not made clear by Saito et al., (2005), whether dietary imbalance or nutritional imbalance (use of high levels of the extract, pair feeding) may have contributed to the observed testicular toxicity at two highest doses of G. cambogia extract.
Developmental toxicity
174. Deshmukh et al., (2008b) conducted a follow-up study (summarised in paragraphs 160-169) to evaluate maternal toxicity and effects on the developing embryo in Sprague-Dawley rats (effects included death, structural abnormalities, and altered or retarded growth) when exposed to HCA-SX. The study was performed as per the previous protocol in Deshmukh et al., (2008a). A total of 30 males and 30 female pups per dose group (except the 3,000 ppm (mid) dose group where 25 of each sex were available), including the concurrent control group. The animals in this study were selected randomly after weaning from each F2b litter of the F1 generation from the two-generation reproductive toxicity study (as summarised in paragraphs 160-169). Therefore, the rats in the treatment group were exposed directly to HCA-SX via feed, prior to which they had indirect exposure to HCA during lactation. The dietary exposure levels were the same as those employed for the two-generation reproductive toxicity study: 0, 1,000, 3000, or 10,000 ppm. Following mating at maturity, the pregnant rats were observed twice daily for clinical signs of adverse effects, and body weight and feed consumption were recorded. On day 20 of gestation, animals were subjected to a necropsy and caesarean section to examine the uterus, ovaries, and foetuses for assessment of different parameters of pregnancy and embryo-foetal defects.
175. The daily amount of HCA-SX consumed at dietary feed levels of 1,000, 3,000 or 10,000 (ppm) (equivalent to 0.1%, 0.3% and 1.1%) were calculated as 103, 352, or 1,240 mg/kg bw per day, respectively.
176. Comparison indices of sperm-positive females (mating behaviour), maternal deaths during pregnancy, number of pregnant/non-pregnant females, pregnancy rate (%), and females with resorptions (%) were evaluated between the control group and the HCA-SX exposed groups. At the dose levels administered, no adverse effects were observed. Maternal body weight changes during gestation were recorded for the following periods: days 0, 7, 14 and 20. The group mean values of body weight gain for each period did not show any significant differences between the exposed and control groups. On the 20th day of gestation, a significant decrease (by 13%) in mean body weight of the rats maintained on the highest dose (10,000 ppm) was noted. During the study, no treatment-related clinical effects were observed in any of the groups. However, the following incidences were reported: i) wryneck and a transient period of emaciation were observed in females during the course of the study [was deemed to be not dose related by the authors]; ii) on day 17 of gestation, one pregnant female from the highest dose group died – profuse haemorrhage [by vaginal bleeding] was identified as the probable cause of death. No other mortalities were noted in any of the groups during the course of the study. Observations made on the gravid uteri of females euthanised on day 20 of gestation did not reveal any “remarkable” alterations indicative of adverse effects of HCA-SX on absolute uterus weight, number of: corpora lutea, implantations, live and dead implants, early and late resorptions, and post-implantation losses (%). Observations made on the litters of females euthanised on day 20 of gestation did not reveal any remarkable treatment-related alterations in litter size, number of foetuses, sex ratios and foetal weights. The group mean litter size from the highest-dose group of HCA-SX was significantly lower than the control group (p <0.05). However, these observations deemed to be not of biological significance by the authors as the changes were smaller in magnitude compared to the variation observed in the historical control data.
177. Observations in the foetal groups are summarised. The number of foetuses examined in the control, 1,000, 3,000 and 10,000 ppm dose groups were 226, 227, 158 and 160, respectively. The only “major malformation” was an omphalocele [birth defect, where the intestine or other abdominal organs stick out of the belly button], which was found in two foetuses: one in the control and high-dose groups. This was considered incidental by the authors. Two other “minor malformations” were observed. An incidence of a small haematoma at the tip of the tail in three foetuses: one in the control, low-dose and mid-dose groups. One foetus in the high-dose group was small (runt). No treatment-related significant incidence of soft tissue alterations in foetuses of dams exposed to HCA-SX at the tested doses were noted (n=108, 106, 74, 76 for the control and each HCA-SX dose groups, respectively). Small incidences of “minor anomalies” were observed including, globular heart and unilateral enlargement of the ventricle of the heart, mottled lungs, unilateral displacement of adrenal, and unilateral hypoplasia of kidney in foetal soft tissues. These were considered as incidental and of no toxicological significance by the authors. The only abnormal finding classified under “major anomalies” was unilateral cerebral hypoplasia observed in one of the foetuses in the low-dose group; this was considered to be incidental by the authors, in light of its isolated incidence (0.94%). There was no incidence of any significant and treatment related skeletal abnormalities in foetuses of dams exposed to HCA-SX. Any abnormalities were considered incidental or of no toxicological significance due to either being a normal variant, minor in nature or the incidence was in isolation and/or comparable to the control dose group.
178. The authors concluded that HCA-SX was not found to be teratogenic in Sprague-Dawley rats at dietary exposure levels of 1,000, 3000, and 10,000 ppm, equivalent to the dose levels of 103, 352, or 1,240 mg/kg/day, respectively. Based on the results of the study, the NOAEL of HCA-SX was determined by the authors at 1,240 mg/kg per day.
Mode of action
179. The cause of hepatotoxicity from G. cambogia is unclear. In vitro studies suggest that HCA may be toxic to the liver in high doses, but the rare instances of acute liver injury that occur with G. cambogia suggest an idiosyncratic form of injury. The possibility of mislabelling or adulteration with hepatotoxic herbal products has been identified as an issue in herbal related injury.
180. In the literature review by Crescioli et al., (2018), it was noted that HCA mechanism of toxicity is not clearly defined; however, HCA increases hepatic collagen accumulation, lipid peroxidation, mRNA levels of genes related to oxidative stress (superoxide dismutase and glutathione peroxidase), and inflammatory responses (tumour necrosis factor- α and monocyte chemoattractant protein-1). It was suggested that certain patients could have genetic predisposition leading to hepatotoxicity, such as cytochrome P450 polymorphisms promoting toxic accumulation of metabolites. The information suggested that there is a potential causal relationship between G. cambogia product exposure and development of herb induced liver injury (HILI); however, these were limited by the lack of data on factors influencing the severity of HILI, especially for cases derived from the literature.
Exposure
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
Brief market analysis
181. A brief market analysis was carried out using the search term on Google: “Garcinia cambogia buy UK”. This information was collated into Tables 3 and 4. The FSA’s Exposure Assessment Team (EAT) have quality assured this data by reviewing the transcription of the information in the table is correct according to the weblinks.
182. The Holland & Barret website states that “Most studies have been based on 900 mg to 3,000mg of HCA of garcinia cambogia daily. Dosage recommendations on commercially available pills are generally lower, around 300mg – 1,600mg daily.” (Holland & Barrett, 2019). At the time of writing, two products (each available in different pack sizes) were available that contained G. cambogia in MIDS: G. cambogia and green coffee bean capsules (600 mg G. Cambogia (fruit) per day (2 capsules)) in 60 or 100 capsule bottles; protein shakes (150 mg G. cambogia extract/55g per scoop; instructions state to add 2 scoops per serving and recommends 1-3 servings daily – total of up to 900 mg G. cambogia extract/day) in 1 or 2 kg tubs (Holland & Barrett, 2025).
183. Other online retailers such as Amazon, Superdrug, Grape Tree, Natur House and various body building supplement & nutrition websites offer G. cambogia capsule products with daily doses of HCA ranging from 80 – 10,000 mg. However, the product description and labelling are inconsistent with some listings simply stating G. cambogia extract without describing whether it is HCA nor the standardised HCA content. A powdered product of G. Cambogia extract was also available, where half a teaspoon serving gave a dose of 500 mg HCA (60% standardised (Oils and Herbs, 2025). It may be possible that other (non-)UK retailers offer products with higher concentrations of HCA than identified in this discussion paper.
Estimated exposure
184. A comprehensive exposure assessment using consumption data from years 1-11 of the National Diet and Nutrition Survey (NDNS) (Bates et al., 2014; Bates et al., 2016; Roberts et al., 2018; Bates et al., 2020) was not possible, for the following reasons:
- Specific food supplements containing G. cambogia are not in the NDNS.
- Specific food/drink products containing G. cambogia are not in the NDNS. Whilst consumption estimates for food/drink products containing G. cambogia could be based on consumption of “normal” versions of the food in NDNS, it was noted these products are uncommon; this approach may have resulted in a significant overestimation of exposure.
- The use of the rind for culinary purposes was not in scope for the exposure assessment. In this instance, using the identified dose for use as a supplement was considered the most appropriate approach.
185. Therefore, the exposure estimates provided for the identified products of the market analysis are based on the recommended dose/serving size and the average body weights of adults (aged 19-64 years) and toddlers (aged 1.5-3 years) from the NDNS (78.6 and 14.6 kg, respectively).
186. Most products in the market analysis included warning information that recommended the product was suitable for adults only. However, consumption by other age groups could not be ruled out completely. Therefore, toddlers have been chosen as the age group for worst case exposure estimates.
187. Table 3 provides dosage information for G. cambogia only supplements, whilst Table 4 provides dosage information for multi-ingredient G. cambogia dietary supplements, as sold in UK online retailers at the time of the assessment.
188. Table 5 and Table 6 provide the exposure estimates for adults (G. cambogia only and MIDS products respectively), while Table 7 and Table 8 provide the exposure estimates for toddlers (G. cambogia only and MIDS products respectively). There are exposure estimates for both G. cambogia and HCA, where possible. In some cases, insufficient information was provided to perform an exposure assessment for one or the other.
Table 3 - Dosage information for G. cambogia only supplements, as sold in UK online retailers at the time of the assessment.
|
Supplement name |
Supplement type |
Recommended daily serving |
G. cambogia concentration per unit (capsule/ tablet/ scoop etc) (mg) |
G. cambogia concentration in a daily serving (maximum serving size*) (mg) |
HCA content % (mg in maximum daily serving*) |
|
BodyBuilding Warehouse Pure Garcinia cambogia |
Capsules |
1-2 capsules |
500 |
1000 |
60% (600) |
|
UK Health House Garcinia Cambogia - High Strength - 1000mg Tablets 60% HCA Max |
Tablets |
1-2 tablets |
1000 |
2000 |
60% (1200) |
|
HERBASENSE GARCINIA CAMBOGIA EXTRACT – 60% HCA by HPLC |
Powder |
3 spoons (1.5g) |
1500 |
4500 |
60% (2700) |
|
NOW Foods, Garcinia, 120 Tablets |
Tablets |
1-3 tablets |
1000 |
3000 |
50% (1500) |
|
Fitimins Garcinia Cambogia Extract 1000mg Capsule |
Capsules |
2 capsules |
1000 |
2000 |
60% (1200) |
|
BioTechUSA HCA - 100 caps |
Capsules |
3 capsules |
1000 |
3000 |
60% (1800) |
|
Woods Supplements Garcinia Cambogia 1000mg |
Capsules |
1 capsule |
1000 |
1000 |
Not provided |
|
Paradise Herbs, Garcinia Extract, 500 mg, 60 Vegetarian Capsules |
Capsules |
1-3 capsules |
500 |
1500 |
50% (750) |
|
Inlife Garcinia 1600 MG Capsules - 120 Capsules |
Capsules |
2-3 capsules |
1600 |
4800 |
>60% (>2880) |
|
Himalaya, Organic Garcinia, 60 Caplets |
Caplets |
2 caplets |
600 |
1200 |
66% (792) |
|
Swanson Garcinia Cambogia 5:1 Extract, 80mg - 60 caps |
Capsules |
1 capsule |
80 |
80 |
Not provided |
|
Sotya Garcinia |
Capsules |
3 capsules |
250 |
750 |
60% (450) |
|
DR WAKDE'S Garcinia Fruit Powder - 100g (3.5oz) | Pure, Raw & Dried Powder | Ayurvedic Herb | Vegan | Nothing Added, Nothing Removed | Same Day Dispatch |
Powder |
1-2 teaspoons (5-10g) |
5,000 (5g teaspoon)** |
10,000** |
Not provided |
|
Garcinia Cambogia 1500mg | 120 Vegan Capsules | High Strength 20:1 Whole Fruit Powder | Premium Quality Supplement | by Horbaach |
Capsules |
1 capsule |
1500 |
1500 |
Not provided |
|
Black Swan Hydroxycitric Acid Capsules - 500mg HCA Garcinia Cambogia Enhanced Mood Weight Management Metabolic Support Natural Food Supplement - 30 Capsules 1 Month Supply |
Capsules |
1-2 capsules |
Not provided** |
Not provided** |
Percentage content not provided** (1000) |
* Maximum serving assumes the maximum amount recommended on the packaging or website of the supplement. E.g. if recommended to take 1-2 capsules per day, this table assumes 2 capsules would be taken. Another example is if the website suggests a serving of 2 capsules a day, but the packaging suggests 3, then the highest suggestion has been used.
**An assumption has been made regarding the value, see Table 1 (Annex A of TOX/2025/41) for more details.
Table 4 Dosage information for multi-ingredient G. cambogia dietary supplements, as sold in UK online retailers at the time of the assessment.
|
Supplement name |
Supplement type |
Recommended daily serving |
G. cambogia concentration per unit (capsule/ tablet/ scoop etc) (mg) |
G. cambogia concentration in a daily serving (maximum serving size*) (mg) |
HCA content % (mg in maximum daily serving*) |
|
Swiss Bioenergetics Garcinia cambogia |
Capsules |
3-6 capsules |
500 |
3000 |
Not provided |
|
Nutralie Garcinia cambogia complex fat burner |
Capsules |
3 capsules |
667 |
2000 |
60% (1200) |
|
Holland & Barrett Garcinia Cambogia & Green Coffee Bean 100 Capsules |
Capsules |
2 capsules |
300 |
600 |
Not provided |
|
HERBASENSE Garcinia Cambogia Plus Jar – 60% HCA by HPLC + Green Coffee Bean + Green Tea Extracts |
Powder |
3 spoons (1.5g) |
1500 |
4500 |
60% (2700) |
|
Sensilab Essentials Garcinia Cambogia 1,800mg High Dose - Vegan, 90 Capsules |
Capsules |
3 capsules |
600 |
1800 |
60% (1080) |
|
Sensilab Garcinia Slim
|
Capsules |
2 capsules |
209 |
417 |
60% (250) |
|
GARCINIA ULTRA BLEND (with Acai & Green Tea) 60 Vegetarian Capsules |
Capsules |
1-2 capsules |
100 |
200 |
55% (110) |
|
Garcinia Cambogia - 90 Capsules - 1500mg Daily Dosage - Premium Quality Supplement - UK formulated - Vegetarian & Vegan Suitable - Optimum Strength For Maximum Results - Garcinia Clean For Men & Women |
Capsules |
3 capsules |
500 |
1500 |
Not provided |
|
Apple Cider Vinegar with Cayenne Pepper, Turmeric & Ginger Root + Garcinia Cambogia with Calcium, Potassium, Chromium, etc. - UK Formulated Food Supplements Capsules – Both Vegan & Vegetarian Suitable |
Capsules |
3 capsules |
1500** |
4500** |
15% (675)** |
|
Prowise Healthcare Essentials Garcinia Cambogia High Dose - Vegan, 180 Capsules |
Capsules |
2 capsules |
1000 |
2000 |
Not provided |
|
Troo Health Care Garcinia Cambogia Complex Supplement - 90 Capsules | UK Manufactured |
Capsules |
3 capsules |
500 |
1500 |
Not provided |
|
Garcinia Cambogia 1500mg Daily Dosage –with Added Chromium Picolinate for Rapid Absorption – 30 Day Supply of The Super Strength Whole Fruit – Manufactured in The UK |
Capsules |
3 capsules |
500 |
1500 |
Not provided |
*Maximum serving assumes the maximum amount recommended on the packaging or website of the supplement. E.g. if recommended to take 1-2 capsules per day, this table assumes 2 capsules would be taken. Another example is if the website suggests a serving of 2 capsules a day, but the packaging suggests 3, then the highest suggestion has been used.
**An assumption has been made regarding the value, see Table 1 (Annex A of TOX/2025/41) for more details.
Table 5 - Exposure estimates for adults from G. cambogia only supplements, as sold in UK online retailers at the time of the assessment.
|
Supplement name |
G. cambogia concentration in a daily serving (maximum serving size*) (mg) |
HCA content % (mg in maximum daily serving*) |
Exposure to G. cambogia (maximum daily serving*) (mg/ kg bw/day) |
Exposure to HCA (maximum daily serving*) (mg/ kg bw/day) |
|
BodyBuilding Warehouse Pure Garcinia cambogia |
1000 |
60% (600) |
13 |
7.6 |
|
UK Health House Garcinia Cambogia - High Strength - 1000mg Tablets 60% HCA Max |
2000 |
60% (1200) |
25 |
15 |
|
HERBASENSE GARCINIA CAMBOGIA EXTRACT – 60% HCA by HPLC |
4500 |
60% (2700) |
57 |
34 |
|
NOW Foods, Garcinia, 120 Tablets |
3000 |
50% (1500) |
38 |
19 |
|
Fitimins Garcinia Cambogia Extract 1000mg Capsule |
2000 |
60% (1200) |
25 |
15 |
|
BioTechUSA HCA - 100 caps |
3000 |
60% (1800) |
38 |
23 |
|
Woods Supplements Garcinia Cambogia 1000mg |
1000 |
Not provided |
13 |
Not provided |
|
Paradise Herbs, Garcinia Extract, 500 mg, 60 Vegetarian Capsules |
1500 |
50% (750) |
19 |
9.5 |
|
Inlife Garcinia 1600 MG Capsules - 120 Capsules |
4800 |
>60% (>2880) |
61 |
37 |
|
Himalaya, Organic Garcinia, 60 Caplets |
1200 |
66% (326) |
15 |
10 |
|
Swanson Garcinia Cambogia 5:1 Extract, 80mg - 60 caps |
80 |
Not provided |
1.0 |
Not provided |
|
Sotya Garcinia |
750 |
60% (450) |
9.5 |
5.7 |
|
DR WAKDE'S Garcinia Fruit Powder - 100g (3.5oz) | Pure, Raw & Dried Powder | Ayurvedic Herb | Vegan | Nothing Added, Nothing Removed | Same Day Dispatch |
10,000** |
Not provided |
130 |
Not provided |
|
Garcinia Cambogia 1500mg | 120 Vegan Capsules | High Strength 20:1 Whole Fruit Powder | Premium Quality Supplement | by Horbaach |
1500 |
Not provided |
19 |
Not provided |
|
Black Swan Hydroxycitric Acid Capsules - 500mg HCA Garcinia Cambogia Enhanced Mood Weight Management Metabolic Support Natural Food Supplement - 30 Capsules 1 Month Supply |
Not provided** |
Percentage content not provided** (1000) |
Not provided |
13 |
* Maximum serving assumes the maximum amount recommended on the packaging or website of the supplement. E.g. if recommended to take 1-2 capsules per day, this table assumes 2 capsules would be taken. Another example is if the website suggests a serving of 2 capsules a day, but the packaging suggests 3, then the highest suggestion has been used.
**An assumption has been made regarding the value, see Table 1 (Annex A of TOX/2025/41) for more details.
Table 6 - Exposure estimates for adults from multi-ingredient G. cambogia dietary supplements, as sold in UK online retailers at the time of the assessment.
|
Supplement name |
G. cambogia concentration in a daily serving (maximum serving size*) (mg) |
HCA content % (mg in maximum daily serving*) |
Exposure to G. cambogia (maximum daily serving*) (mg/ kg bw/day) |
Exposure to HCA (maximum daily serving*) (mg/ kg bw/day) |
|
Swiss Bioenergetics Garcinia cambogia |
3000 |
Not provided |
38 |
Not provided |
|
Nutralie Garcinia cambogia complex fat burner |
2000 |
60% (1200) |
25 |
15 |
|
Holland & Barrett Garcinia Cambogia & Green Coffee Bean 100 Capsules |
600 |
Not provided |
7.6 |
NA |
|
HERBASENSE Garcinia Cambogia Plus Jar – 60% HCA by HPLC + Green Coffee Bean + Green Tea Extracts |
4500 |
60% (2700) |
57 |
34 |
|
Sensilab Essentials Garcinia Cambogia 1,800mg High Dose - Vegan, 90 Capsules |
1800 |
60% (1080) |
23 |
14 |
|
Sensilab Garcinia Slim
|
417 |
60% (250) |
5.3 |
3.2 |
|
GARCINIA ULTRA BLEND (with Acai & Green Tea) 60 Vegetarian Capsules |
200 |
55% (110) |
2.5 |
1.4 |
|
Garcinia Cambogia - 90 Capsules - 1500mg Daily Dosage - Premium Quality Supplement - UK formulated - Vegetarian & Vegan Suitable - Optimum Strength For Maximum Results - Garcinia Clean For Men & Women |
1500 |
No provided |
19 |
Not provided |
|
Apple Cider Vinegar with Cayenne Pepper, Turmeric & Ginger Root + Garcinia Cambogia with Calcium, Potassium, Chromium, etc. - UK Formulated Food Supplements Capsules – Both Vegan & Vegetarian Suitable |
4500** |
15% (675)** |
25 |
8.6 |
|
Prowise Healthcare Essentials Garcinia Cambogia High Dose - Vegan, 180 Capsules |
2000 |
Not provided |
25 |
Not provided |
|
Troo Health Care Garcinia Cambogia Complex Supplement - 90 Capsules | UK Manufactured |
1500 |
Not provided |
19 |
Not provided |
|
Garcinia Cambogia 1500mg Daily Dosage –with Added Chromium Picolinate for Rapid Absorption – 30 Day Supply of The Super Strength Whole Fruit – Manufactured in The UK |
1500 |
Not provided |
19 |
Not provided |
* Maximum serving assumes the maximum amount recommended on the packaging or website of the supplement. E.g. if recommended to take 1-2 capsules per day, this table assumes 2 capsules would be taken. Another example is if the website suggests a serving of 2 capsules a day, but the packaging suggests 3, then the highest suggestion has been used.
**An assumption has been made regarding the value, see Table 1 (Annex A of TOX/2025/41) for more details.
Table 7 - Exposure estimates for toddlers from G. cambogia only supplements, as sold in UK online retailers at the time of the assessment.
|
Supplement name |
G. cambogia concentration in a daily serving (maximum serving size*) (mg) |
HCA content % (mg in maximum daily serving*) |
Exposure to G. cambogia (maximum daily serving*) (mg/ kg bw/day) |
Exposure to HCA (maximum daily serving*) (mg/ kg bw/day) |
|
BodyBuilding Warehouse Pure Garcinia cambogia |
1000 |
60% (600) |
68 |
41 |
|
UK Health House Garcinia Cambogia - High Strength - 1000mg Tablets 60% HCA Max |
2000 |
60% (1200) |
140 |
82 |
|
HERBASENSE GARCINIA CAMBOGIA EXTRACT – 60% HCA by HPLC |
4500 |
60% (2700) |
310 |
180 |
|
NOW Foods, Garcinia, 120 Tablets |
3000 |
50% (1500) |
210 |
100 |
|
Fitimins Garcinia Cambogia Extract 1000mg Capsule |
2000 |
60% (1200) |
140 |
82 |
|
BioTechUSA HCA - 100 caps |
3000 |
60% (1800) |
210 |
120 |
|
Woods Supplements Garcinia Cambogia 1000mg |
1000 |
Not provided |
68 |
Not provided |
|
Paradise Herbs, Garcinia Extract, 500 mg, 60 Vegetarian Capsules |
1500 |
50% (750) |
100 |
51 |
|
Inlife Garcinia 1600 MG Capsules - 120 Capsules |
4800 |
>60% (>2880) |
330 |
200 |
|
Himalaya, Organic Garcinia, 60 Caplets |
1200 |
66% (326) |
82 |
54 |
|
Swanson Garcinia Cambogia 5:1 Extract, 80mg - 60 caps |
80 |
Not provided |
5.5 |
Not provided |
|
Sotya Garcinia |
750 |
60% (450) |
51 |
31 |
|
DR WAKDE'S Garcinia Fruit Powder - 100g (3.5oz) | Pure, Raw & Dried Powder | Ayurvedic Herb | Vegan | Nothing Added, Nothing Removed | Same Day Dispatch |
10,000** |
Not provided |
680 |
Not provided |
|
Garcinia Cambogia 1500mg | 120 Vegan Capsules | High Strength 20:1 Whole Fruit Powder | Premium Quality Supplement | by Horbaach |
1500 |
Not provided |
100 |
Not provided |
|
Black Swan Hydroxycitric Acid Capsules - 500mg HCA Garcinia Cambogia Enhanced Mood Weight Management Metabolic Support Natural Food Supplement - 30 Capsules 1 Month Supply |
Not provided** |
Percentage content not provided** (1000) |
Not provided |
68 |
* Maximum serving assumes the maximum amount recommended on the packaging or website of the supplement. E.g. if recommended to take 1-2 capsules per day, this table assumes 2 capsules would be taken. Another example is if the website suggests a serving of 2 capsules a day, but the packaging suggests 3, then the highest suggestion has been used.
**An assumption has been made regarding the value, see Table 1 (Annex A of TOX/2025/41) for more details.
Table 8 - Exposure estimates for toddlers from multi-ingredient G. cambogia dietary supplements, as sold in UK online retailers at the time of the assessment.
|
Supplement name |
G. cambogia concentration in a daily serving (maximum serving size*) (mg) |
HCA content % (mg in maximum daily serving*) |
Exposure to G. cambogia (maximum daily serving*) (mg/ kg bw/day) |
Exposure to HCA (maximum daily serving*) (mg/ kg bw/day) |
|
Swiss Bioenergetics Garcinia cambogia |
3000 |
Not provided |
210 |
Not provided |
|
Nutralie Garcinia cambogia complex fat burner |
2000 |
60% (1200) |
140 |
82 |
|
Holland & Barrett Garcinia Cambogia & Green Coffee Bean 100 Capsules |
600 |
Not provided |
41 |
Not provided
|
|
HERBASENSE Garcinia Cambogia Plus Jar – 60% HCA by HPLC + Green Coffee Bean + Green Tea Extracts |
4500 |
60% (2700) |
310 |
180 |
|
Sensilab Essentials Garcinia Cambogia 1,800mg High Dose - Vegan, 90 Capsules |
1800 |
60% (1080) |
120 |
74 |
|
Sensilab Garcinia Slim
|
417 |
60% (250) |
29 |
17 |
|
GARCINIA ULTRA BLEND (with Acai & Green Tea) 60 Vegetarian Capsules |
200 |
55% (110) |
14 |
7.5 |
|
Garcinia Cambogia - 90 Capsules - 1500mg Daily Dosage - Premium Quality Supplement - UK formulated - Vegetarian & Vegan Suitable - Optimum Strength For Maximum Results - Garcinia Clean For Men & Women |
1500 |
No provided |
100 |
Not provided
|
|
Apple Cider Vinegar with Cayenne Pepper, Turmeric & Ginger Root + Garcinia Cambogia with Calcium, Potassium, Chromium, etc. - UK Formulated Food Supplements Capsules – Both Vegan & Vegetarian Suitable |
4500** |
15% (675)** |
310 |
46 |
|
Prowise Healthcare Essentials Garcinia Cambogia High Dose - Vegan, 180 Capsules |
2000 |
Not provided |
140 |
Not provided
|
|
Troo Health Care Garcinia Cambogia Complex Supplement - 90 Capsules | UK Manufactured |
1500 |
Not provided |
100 |
Not provided
|
|
Garcinia Cambogia 1500mg Daily Dosage –with Added Chromium Picolinate for Rapid Absorption – 30 Day Supply of The Super Strength Whole Fruit – Manufactured in The UK |
1500 |
Not provided |
100 |
Not provided
|
* Maximum serving assumes the maximum amount recommended on the packaging or website of the supplement. E.g. if recommended to take 1-2 capsules per day, this table assumes 2 capsules would be taken. Another example is if the website suggests a serving of 2 capsules a day, but the packaging suggests 3, then the highest suggestion has been used.
**An assumption has been made regarding the value, see Table 1 (Annex A of TOX/2025/41) for more details.
Uncertainties in the exposure assessment
189. It should be highlighted that the UK market analysis of G. cambogia dietary supplements was performed in brief and as such, may not include all products that are available online. Thus, products with higher dosage recommendations and resulting in higher estimated exposures may exist on the UK market.
190. Some assumptions have been made where dosage recommendations are varied. In cases where the dosage is given as a range (e.g. 1-3 capsules per day), the highest possible amount has been assumed to be consumed (e.g. 3 capsules per day). Also, where there is a discrepancy between the packaging image and website instructions, the highest dosage has been assumed e.g. if the website suggests a serving of 2 capsules a day, but the packaging suggests 3, then the value of 3 has been used.
191. Some assumptions have been made where G. cambogia or HCA content has not been explicitly stated. E.g. for pure G. cambogia it is assumed that 5g-10g of the powder is equal to 5000-10,000 mg of G. cambogia (1g = 1000mg). I.e. it is assumed that no other ingredient(s) are present.
192. As stated in paragraph 186, most supplements in the market analysis, provided warnings on the website/ packaging recommending that that the product is only suitable for adults. However, exposures for other age groups could not be completely excluded. Toddlers have been selected as the age group for worst case exposure estimates, which are expressed on a per kg bodyweight basis. This is expected to be a conservative assessment and likely to be protective of other age groups under the age of 18 due to the average bodyweight of toddlers being lower than older children.
193. Infants have not been included in the assessment as it is assumed that supplements intended for weight loss and provided as small capsules or powders that could cause a choking hazard would not be used for infants.
Uncertainties
194. The following uncertainties have been noted:
- Dietary supplement labels often do not list the full scientific name/specie of G. cambogia nor the part of the plant that the extract(s) is/are derived from. This represents an uncertainty as it does not allow accurate determination of the components of the food product (including food supplements).
- G. cambogia products have inconsistent labelling. Some are simply marketed as G. cambogia or G. cambogia extract without describing whether it is HCA nor the standardised HCA content. This poses an issue for both exposure and risk assessment as the HCA content is unknown.
- The percentage of standardised HCA in G. cambogia extract or the salt form of HCA in food supplement products is not always disclosed and there is inconsistency in labelling of said products. Depending on the amount of concentrate and the form of HCA salt, the toxicological profile could potentially be different.
- G. cambogia (and/or its extract; HCA) is more commonly present in MIDS that have other active compounds that may or may not be fully disclosed, which does not allow for a comprehensive risk assessment.
- If G. cambogia (and/or its extract) is present in MIDS there is a potential synergistic effect of the mixture, the potential risk(s) of which is unknown.
- Consumers may use more than one HIDS and/or in combination with prescription drugs. Incorrect dosing is also possible should the consumer not follow the manufacturers’ instructions.
- Considerations for the presence of contaminants such as heavy metals, extraction solvents (from the manufacturing process), pesticides or any other chemicals that could pose a risk to health have not been considered in this assessment.
- Case reports in the literature are limited to certain brands and formulations of G. cambogia and may not always provide the holistic state of the consumer: granularity on duration of exposure and dose, health status of the consumer (e.g., do they have suspected liver injury or immunocompromised or any other, fasting or non-fasting, physically active or not active), concomitant with other drugs or MIDS. Thus, identification of sensitive population and establishment of a mode of action for the toxicity observed in these cases is always not possible.
- Possible drug-drug interactions have been reported in some case-studies (e.g., anti-depression (serotonergic drugs), drugs affecting the liver). However, there is little scientific information to be able to fully assess this potential risk. Furthermore, it is unknown if other types of medication can cause adverse drug interaction.
- The long-term safety of G. cambogia (and/or its extract; HCA) has not been established.
Risk Characterisation
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
195. The presence of HCA in food supplements is considered medicinal according to the UK MHRA (see paragraph 8). From the Brief market analysis described in the Exposure section, it is clear that some products are medicinal.
196. Data from nutrivigilance programs/schemes of authoritative bodies and literature have reported the following adverse effects following consumption of supplements containing G. cambogia: hepatic, digestive (pancreatitis), cardiac and muscular (rhabdomyolysis) damage, psychiatric, metabolic disorders, as well as drug-drug interactions. However, the mode of action of G. cambogia and its extracts have yet to be fully elucidated.
Questions to the Committee
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
197. The Committee are asked to consider the following questions:
a) Does the Committee agree with the conclusions reached by ANSES?
b) Based on the current data, is the Committee able to identify a no-observed effect level or any other options, to derive a health-based guidance value?
i) If no, what additional information would the Committee require to provide a risk assessment?
c) Does the Committee have any other comments?
October 2025
Secretariat
Abbreviations
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
|
AESAN |
Spanish Scientific Committee of the Spanish Agency for Food Safety and Nutrition |
|
AhR |
Aryl hydrocarbon receptor |
|
AIDS |
Acquired immune deficiency syndrome |
|
ALP |
alkaline phosphatase |
|
ALT |
Alanine transaminase |
|
ANA |
Anti-nuclear antibody |
|
ANSES |
French Agency for Food, Environmental and Occupational Health and Safety |
|
ANSM |
French National Agency for the Safety of Medicines and Health Products |
|
ASMA |
Anti-smooth muscle antibodies |
|
ASP |
Aspartate aminotransferase |
|
ATP |
Adenosinetriphosphate |
|
BaP |
Benzo[a]pyrene |
|
BMI |
Body mass index |
|
CHO |
Chinese hamster ovary cell line |
|
COT |
Committee on the Toxicity of Chemicals in Food, Consumer Products and the Environment |
|
CYP |
Cytochrome P450 |
|
DCFH-DA |
2′− 7′- dichlorodihydrofluorescein diacetate |
|
DGCCRF |
General Directorate for Competition, Consumer Affairs and Fraud Control |
|
DILIN |
US Drug Induced Liver Injury Network |
|
DMSO |
Dimethyl sulfoxide |
|
EAT |
Exposure assessment team |
|
FSA |
Food Standards Agency |
|
FSS |
Food Standards Scotland |
|
GGT |
Gamma-glutamyl transferase |
|
HCA |
Hydroxycitric acid |
|
HDS |
Herbal and dietary supplements |
|
HepG2 |
Hepatocellular carcinoma cell line |
|
HIV |
Human immunodeficiency virus |
|
IC50 |
Half maximal inhibitory concentration |
|
IPS |
Italian Phytovigilance System |
|
LATINDILI |
Latin America-Drug Induced Liver Injury database |
|
MDCK-MDR1 |
Madin-Darby Canine Kidney-Human Multidrug Resistance gene-1 cell line |
|
MHRA |
Medicines and Healthcare products Regulatory Agency |
|
MIDS |
Multi-ingredient dietary supplements |
|
MMC |
Mitomycin C |
|
MNPCE |
Micronucleated polychromatic erythrocytes |
|
MTT |
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
|
NCE |
Normochromatic erythrocytes |
|
NDNS |
National Diet and Nutrition Survey |
|
NOAEL |
No observed adverse effect level |
|
PLA |
Product licence application |
|
PXR |
Pregnane X receptor |
|
RCT |
Randomised clinical trial |
|
ROS |
Reactive oxygen species |
|
TB |
Total bilirubin |
|
TGA |
Australian Department of Health, Disability and Ageing Therapeutic Goods Administration |
|
UK |
United Kingdom |
|
US FDA |
United States Food and Drug Administration |
|
US NCCIH |
United States National Centre for Complementary and Integrative Health |
|
WG |
Working Group |
|
WHO |
World Health Organisation |
References
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
AESAN. (2019) Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) on the risk associated with the consumption of food supplements that contain Garcinia gummi-gutta as an ingredient. Reference number: AESAN-2019-005. Revista del Comité Científico de la AESAN, 30, pp: 11-28. 01_Informe_garcinia_Ing_CCAESAN_Nº_30.indd Accessed: 11/06/2025.
Al-Khazraji, A., Novikov, A., Ahmed, M., Singh, B., Syed, U., Sharma, R., Bansal, R., Baum, J., Aron, J, Gurram, K. and Walfish, A. (2020) S2605 First Case of Garcinia Cambogia (GC)-Induced Autoimmune Hepatitis (AIH). The American Journal of Gastroenterology. 115:S1368-S1369, October 2020. October 2020 - Volume 115 - Issue : Official journal of the American College of Gastroenterology | ACG
Amini, M. R., Salavatizadeh, M., Kazeminejad, S., Javadi, F., Hajiaqaei, M., Askari, G. and Jekmatdoost, A. (2024) The effects of Garcinia cambogia (hydroxycitric acid) on serum leptin concentrations: A systematic review and meta-analysis of randomized controlled trials. Complementary Therapies in Medicine 84; 103060. https://doi.org/10.1016/j.ctim.2024.103060.
Andueza, N., Giner, R. M. and Portillo, M. P. (2021) Risks Associated with the Use of Garcinia as a Nutritional Complement to Lose Weight. Nutrients 2021, 13, 450. https://doi.org/10.3390/nu13020450.
ANSES. (2025a) Opinion on the assessment of adverse reactions to the consumption of food supplements containing Garcinia cambogia (PDF in French). Avis relatif à l’évaluation des effets indésirables liés à la consommation de compléments alimentaires contenant du « Garcinia cambogia »
ANSES. (2025b) Do not consume food supplements containing Garcinia cambogia. Do not consume food supplements containing Garcinia cambogia | Anses - Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail Accessed: 09/06/2025.
Bates, B., Lennox, A., Prentice, A., Bates, C., Page, P., Nicholson, S. and Swan, G. (2014) National Diet and Nutrition Survey (NDNS): results from Years 1 to 4 (combined) of the rolling programme for 2008 and 2009 to 2011 and 2012. NDNS: results from Years 1 to 4 (combined) - GOV.UK Accessed: 14/10/2024.
Bates, B., Cox, L., Nicholson, S., Page, P., Prentice, A., Steer, T. and Swan, G. (2016) National Diet and Nutrition Survey (NDNS): results from Years 5 and 6 (combined) of the Rolling Programme (2012/2013 – 2013/2014). NDNS: results from Years 5 and 6 (combined) - GOV.UK Accessed: 14/10/2024.
Bates, B., Collins, D., Jones, K., Page, P., Roberts, C., Steer, T. and Swan, G. (2020) National Diet and Nutrition Survey Results from years 9, 10 and 11 (combined) of the Rolling Programme (2016/2017 to 2018/2019). National Diet and Nutrition Survey Accessed: 14/10/2024.
Beecheno, M., Silver, B. and Titus, M. (2016). Natural weight loss supplements – Are they psychoactive? Australian & New Zealand Journal of Psychiatry 50 (7): 700- 701. https://doi.org/10.1177/0004867416634869.
Bessone, F., García-Cortes, M., Medina-Caliz, I., Hernandez, N., Parana, R., Mendizabal, M., Schinoni, M. I., Ridruejo, E., Nunes, V., Peralta, M., Santos, G., Andres, M., Chiodi, D., Tagle, M., Montes, P., Carrera, E., Arrese, M., Lizarzabal, M. I., Alvarez-Alvarez, I., Caballano-Infantes, E., Niu, H., Pinazo, J., Cabello, M. R., Lucena, M. I. Andrade, R. J. (2022) Herbal and Dietary Supplements-Induced Liver Injury in Latin America: Experience From the LATINDILI Network. Clin Gastroenterol Hepatol 20(3):e548-e563. https://doi.org/10.1016/j.cgh.2021.01.011.
Bessone, F., Hernandez, N., Medina-Caliz, I., García-Cortes, M., Schinoni, M. I., Mendizabal, M., Chiodi, D., Nunes, V., Ridruejo, E., Pazos, X., Santos, G., Fassio, E., Parana, R., Reggiardo, V., Tanno, H., Sanchez, A., Tanno, F., Montes, P., Tagle, M., Arrese, N., Brahm, J., Girala, M., Lizarzabal, M. I., Carrera, E., Zerega, A., Bianchi, C., Reyes, L., Arnedillo, D., Cordone, A., Gualano, G., Jaurequizahar, F., Rifrani, G., Robles-Díaz, M., Ortega-Alonso, A., Pinazo-Bandera, J. M., Stephens, C., Sanabria-Cabrera, J., Bonilla-Toyos, E., Niu, H., Alvarez-Alvarez, I., Lucena, M. I. and Andrade, R. J. (2025) Drug-induced Liver Injury in Latin America: 10-year Experience of the Latin American DILI (LATINDILI) Network. Clinical Gastroenterology and Hepatology, Volume 23, Issue 1, 89 – 102. https://doi.org/10.1016/j.cgh.2024.06.030.
Bolla, L., Srivastava, P., Ravichandiran, V. and Nanjappan, S. K. (2021) Cytochrome P450 and P-gp mediated herb-drug interactions and molecular docking studies of garcinol. Membranes 11 (12). https://doi.org/10.3390/membranes11120992.
Burdock, G., Bachi, M. and Bagchi. D. (2005) Garcinia cambogia toxicity is misleading. Food and Chemical Toxicology Volume 43, Issue 11, November 2005, Pages 1683-1684. https://doi.org/10.1016/j.fct.2005.05.011.
Boonyong, C., Lertnitikul, N., Pattamadilok, C., Sitthigool, S., Suttisri, R., Jianmongkol, S. and Wongsakul, A. (2024) In vitro and in silico effects of the polyisoprenylated benzophenones guttiferone K and oblongifolin C on P-glycoprotein function. Journal of Applied Pharmaceutical Science 14 (2): 102-108. https://doi.org/10.7324/JAPS.2024.158877.
Bystrak, T., Cervera-Hernandez, M., Reddy, N., King, Z. and Bratberg. J. (2017) Garcinia Cambogia, Diabetic Ketoacidosis, and Pancreatitis. Rhode Island Medical Journal 100: 48-50.
Cheng, I. S., Huang, S. W., Lu, H. C., Wu, C. L., Chu, Y. C., Lee, S. D., Huang, C. Y. and Kuo, C. H. (2012) Oral hydroxycitrate supplementation enhances glycogen synthesis in exercised human skeletal muscle. Br J Nutr 107 (7): 1048-55. https://doi.org/10.1017/s0007114511003862.
Cho, H-K., Han, Y. S. and Park, J. M. (2019) Ocular complications of Garcinia cambogia extract diet pills: Case report. European Journal of Ophthalmology 30 (6): NP21-NP26. https://doi.org/10.1177/1120672119872364.
Corey, R., Werner, K. T., Singer, A., Moss, A., Smith, M., Noelting, J. and Rakela, J. (2016) Acute liver failure associated with Garcinia cambogia use. Ann Hepatol 15 (1): 123-6. https://doi.org/10.5604/16652681.1184287.
Cotovio, G. and Oliveira-Maia, A. J. (2016) Hypomania induced by a Garcinia cambogia supplement. Australian & New Zealand Journal of Psychiatry 51 (6): 641- 642. https://doi.org/10.1177/0004867416667827.
Chuah, L. O., Ho, W. Y., Beh, B. K. and Yeap, S. W. (2013) Updates on Antiobesity Effect of Garcinia Origin (−)-HCA. Evidence-Based Complementary and Alternative Medicine Volume 2013, Article ID 751658. https://doi.org/10.1155/2013/751658.
Crescioli, G., Lombardi, N., Bettiol, A., Marconi, E., Risaliti, F., Bertoni, M., Ippolito, F. M., Maggini, V., Gallo, E., Firenzuoli, F. and Vannaci, A. (2018) Acute liver injury following Garcinia cambogia weight-loss supplementation: case series and literature review. Intern Emerg Med 13, 857–872 (2018). https://doi.org/10.1007/s11739-018-1880-4.
Cruz, A. C., Pinto, A. H. S., Costa, C. D. D., Oliveira, L. P., Oliveira-Neto, J. R. and Cunha, L. C. (2021) Food-Effect on (−) – Hydroxycitric Acid Absorption After Oral Administration of Garcinia cambogia Extract Formulation: a Phase I, Randomized, Cross-Over Study. J Pharm Sci, 110:693- 697. https://doi.org/10.1016/j.xphs.2020.10.035.
Deshmukh, N. S., Bagchi, M., Yasmin, T., and Bagchi, D. (2008a). [abstract only] Safety of a Novel Calcium/Potassium Salt of Hydroxycitric Acid (HCA-SX): I. Two-Generation Reproduction Toxicity Study. Toxicology Mechanisms and Methods, 18(5), 433–442. https://doi.org/10.1080/15376510802084030.
Deshmukh, N. S., Bagchi, M., Yasmin, T., & Bagchi, D. (2008b) [abstract only]. Safety of a Novel Calcium/Potassium Saltof (-)-Hydroxycitric Acid (HCA-SX): II. Developmental Toxicity Study in Rats. Toxicology Mechanisms and Methods, 18(5), 443–451. https://doi.org/10.1080/15376510802055022.
Di Giacomo, S., Di Sotto, A., Percaccio, E., Scuotto, E., Battistelli, C., Mazzanti, G., Menniti-Ippolito, F. and Ippoliti, I. (2023) Interaction of Garcinia cambogia (Gaertn.) Desr. and Drugs as a Possible Mechanism of Liver Injury: The Case of Montelukast. Antioxidants 12 (9): 1771.
EC. (2023) Food and Feed Information Panel Database Version 5.3.1. EU Novel Food status Catalogue. Garcinia cambogia Desr. Search Novel Food status Catalogue | Food and Feed Information Portal Database | FIP Accessed: 17/06/2025.
EFSA. (2023a) Protocol for the Scientific Opinion on the evaluation of the safety in use of hydroxycitric acid and plant preparations containing hydroxycitric acid. EFSA Supporting Publications Volume 20, Issue 9 8244E. https://doi.org/10.2903/sp.efsa.2023.EN-8244.
EFSA. (2023b) Call for data for the Scientific Opinion on the evaluation of the safety in use of plant preparations containing hydroxycitric acid. Call for data for the Scientific Opinion on the evaluation of the safety in use of plant preparations containing hydroxycitric acid | EFSA Accessed: 04/07/2025.
EFSA. (2025) Scientific Panel on Nutrition, Novel Foods and Allergens 23rd Working Group meeting on substances other than vitamins and minerals (Art.8(2) of Regulation (EC) No 1925/2006). Minutes - Substances other than vitamins and minerals Accessed: 04/07/2025.
Espirito Santo, B. L. S. D., Santana, L. F., Kato Junior, W. H., de Araújo, F. O., Bogo, D., Freitas, K.C., Guimarães, R. C. A., Hiane, P. A., Pott, A., Filiú, W. F. O., Arakaki Asato, M., Figueiredo, P. O. and Bastos, P. R. H. O. (2020) Medicinal Potential of Garcinia Species and Their Compounds. Molecules. 2020 Oct 1;25(19):4513. https://doi.org/10.3390/molecules25194513.
García-Cortés, M., Robles-Díaz, R., Ortega-Alonso, A., Medina-Caliz, I. and Andrade, R. J. (2016) Hepatotoxicity by Dietary Supplements: A Tabular Listing and Clinical Characteistics. Int. J. Mol. Sci. 2016, 17(4), 537. https://doi.org/10.3390/ijms17040537.
Ghosh, I. and Mukherjee, A. (2017) In vitro cyto-genotoxicity of hydroxycitric acid: A weight-loss dietary supplement. Journal of Exploratory Research in Pharmacology 2017;2(2):41-48. http://dx.doi.org/10.14218/JERP.2017.00003.
Girola, M., Bernardi, M. and Contos, S. (1996) Dose effect in lipid lowering activity of a new dietary integrator (Chitosan, Garcinia cambogia extract, and Chrome). Acta Toxicol Ther 17: 25-40.
Graf, C., Elmassry, M., Chu, V. M., Pawar, D., Tijani, L. (2020) Plexus Slim®-Induced Immune Thrombocytopenic Purpura. Cureus 12 (11): e11413. https://doi.org/10.7759/cureus.11413.
Guu, T. W., Tsai, S. T. and Su, K. P. (2020) Autoimmune psychosis in Taiwan: A case report and review of literature. Brain Behav Immun Health 3: 100055. https://doi.org/10.1016/j.bbih.2020.100055.
Haron, M. H., Dale, O., Martin, K., Avula, B., Chittiboyina, A. G., Khan, I. A., Gurley, B. J. and Khan, S. I. (2023) Evaluation of the Herb-Drug Interaction Potential of Commonly Used Botanicals on the US Market with Regard to PXR- and AhR-Mediated Influences on CYP3A4 and CYP1A2. Journal of Dietary Supplements 20 (5): 763-776. https://doi.org/10.1080/19390211.2022.2110351.
Hayamizu, K., Ishii, Y., Kaneko, I., Shen, M., Okuhara, Y., Shigematsu, N., Tomi, H., Furuse, M., Yoshino, G. and Shimasaki, H. (2003) Effects of garcinia cambogia (Hydroxycitric Acid) on visceral fat accumulation: a double-blind, randomized, placebo-controlled trial. Curr Ther Res Clin Exp 64 (8): 551-67. https://doi.org/10.1016/j.curtheres.2003.08.006.
Hayamizu, K., Tomi, H., Kaneko, I., Shen, M., Soni, M. G. and Yoshino, G. (2008) Effects of Garcinia cambogia extract on serum sex hormones in overweight subjects. Fitoterapia 79 (4): 255-61. https://doi.org/10.1016/j.fitote.2007.12.003.
Health Canada. (2018) Natural Health Product Malabar tamarind – Garcinia gummi-gutta. Product information Accessed: 09/06/2025.
Health Canada. (2024a) Product information Natural Product Number (NPN): 80054640. Product information Accessed: 09/06/2025.
Health Canada. (2024b) Malabar tamarind - Garcinia gummi-gutta. Malabar tamarind - Garcinia gummi-gutta Accessed: 09/06/2025.
Hendrickson, B. P., Shaikh, N., Occhiogrosso, M. and Penzner, J. B. (2016) Mania Induced by Garcinia cambogia: A Case Series. Prim Care Companion CNS Disord 18 (2). https://doi.org/10.4088/PCC.15l01890.
Heymsfield, S. B., Allison, D. B., Vasselli, J. R., Pietrobelli, A., Greenfield, D. and Nunez, C. (1998) Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. JAMA 280(18):1596-600. https://doi.org/10.1001/jama.280.18.1596.
Hirsch-Ernst, K. (2022) BfR-BVL Joint Meeting: “Super(?)foods and Supplements – Risky or Healthy?" 1st July 2022. Risk Assessment Approaches and Methodology – an Overview. Risk assessment approaches and methodology – an overview; Presentation from 01.07.2022 Accessed: 13/08/2025.
Holland & Barrett. (2021) Garcinia cambogia: Benefits, uses & side effects. Garcinia Cambogia: Benefits, Uses & Side Effects | Holland & Barrett Accessed: 18/06/2025.
Holland & Barrett. (2025) Searching for "garcinia". garcinia | Holland & Barrett Accessed: 18/06/2025.
Husain, I., Dale, O. R., Martin, K., Gurley, B. J., Adams, S. J., Avula, B., Chittiboyina, A. G., Khan, I. A. and Khan, S. I. (2023) Screening of medicinal plants for possible herb-drug interactions through modulating nuclear receptors, drug-metabolizing enzymes and transporters. Journal of Ethnopharmacology 301. https://doi.org/10.1016/j.jep.2022.115822.
Ishii, Y., Kaneko, I., Shen, M., Hayamizu, K., Shigematsu, N., Tomi, H., Yoshino, G. and Shimaski, H. (2003) Safety of Garcinia cambogia extract in healthy volunteers: High-dose administration study II. Journal of Oleo Science 2003; 52(12): 663-671. Safety of Garcinia cambogia extract in healthy volunteers: High-dose administration study II pdf
Jakopin, Z. (2019) Risks associated with fat burners: A toxicological perspective. Food and Chemical Toxicology 123, pp. 205-224. https://doi.org/10.1016/j.fct.2018.10.051.
Jena, B. S., Jayaprakasha, G. K., Singh, R. P. And Sakariah, K. K. (2002) Chemistry and Biochemistry of (-)-Hydroxycitric Acid from Garcinia. J. Agric. Food Chem. 2002, 50, 10-22. https://doi.org/10.1021/jf010753k.
Lau, F. C., Bagchi, M. and Bagshi, D. (2008) Letter to the Editor. Journal of Toxicology and Environmental Health, Part A: Current Issues, 71:5, 348-349. https://doi.org/10.1080/15287390701738525.
Lee, K. H. and Lee, B. Y. (2007) [abstract only] Evaluation of the Genotoxicity of (−)-Hydroxycitric Acid (HCA-SX) Isolated from Garcinia cambogia. Journal of Toxicology and Environmental Health, Part A, 70(5), 388–392. https://doi.org/10.1080/15287390600882192.
Leite, P. M., Martins, M. A. P., Carvalho, M. D. G. and Castilho, R. O. (2021) Mechanisms and interactions in concomitant use of herbs and warfarin therapy: An updated review. Biomed Pharmacother 143: 112103. https://doi.org/10.1016/j.biopha.2021.112103.
Li, J. W. and Bordelon. P. (2011) Hydroxycitric acid dietary supplement-related herbal nephropathy. Am J Med 124 (11): e5-6. https://doi.org/10.1016/j.amjmed.2011.03.015.
Loe, Y. C., Bergeron, N., Rodriguez, N. And Schwarz, J. M. (2001) Gas chromatography/mass spectrometry method to quantify blood hydroxycitrate concentration. Anal Biochem. 2001 May 1;292(1):148-54. https://doi.org/10.1006/abio.2001.5046.
Lopez, A. M., Kornegay, J. and Hendrickson. R. G. (2014) Serotonin toxicity associated with Garcinia cambogia over-the-counter supplement. J Med Toxicol 10 (4): 399-401. https://doi.org/10.1007/s13181-014-0390-7.
Lunsford, K. E., Bodzin, A. S., Reino, D. C., Wang, H. L. And Busuttil, R. W. (2016) Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J Gastroenterol. 2016 Dec 7;22(45):10071–10076. https://doi.org/10.3748/wjg.v22.i45.10071.
Márquez, F., Babio, N., Bulló, M. and Salas-Salvadó, J. (2012) [abstract only] Evaluation of the Safety and Efficacy of Hydroxycitric Acid or Garcinia cambogia Extracts in Humans. Critical Reviews in Food Science and Nutrition, 52(7), 585–594. https://doi.org/10.1080/10408398.2010.500551.
Melendez-Rosado, J., Snipelisky, D., Matcha, G. and Stancampiano, F. (2015). Acute hepatitis induced by pure Garcinia cambogia. J Clin Gastroenterol. 2015 May-Jun;49(5):449-50. https://doi.org/10.1097/MCG.0000000000000303.
Mosmann, T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods.1983 Dec 16;65(1-2):55-63. https://doi.org/10.1016/0022-1759(83)90303-4.
Narasimha, A., Shetty, P. H., Nanjundaswamy, M. H., Viswanath, B. and Bada Math, S. (2013). Hydroxycut - Dietary supplements for weight loss: Can they induce mania? Aust N Z J Psychiatry 47 (12): 1205-6. https://doi.org/10.1177/0004867413493522.
Navarro, V. J., Khan, I., Björnsson, E., Seef, L. B., Serrano, J. and Hoofnagle, J. H. (2017) Liver injury from herbal dietary supplements. Hepatology 65(1):p 363-373. https://doi.org/10.1002/hep.28813.
NCBI. (2019) LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Garcinia Cambogia. Last Update: February 13, 2019. Garcinia Cambogia - LiverTox - NCBI Bookshelf Accessed: 18/06/2025.
NCBI. (2025) National Library of Medicine National Center for Biotechnology Information. ACLY ATP citrate lyase [Homo sapiens (human)]. ACLY ATP citrate lyase [Homo sapiens (human)] - Gene - NCBI Accessed: 12/06/2025.
Nguyen, D. C., Timmer, T. K., Davison, B. C. and McGrane. I. R. (2019) Possible Garcinia cambogia-Induced Mania With Psychosis: A Case Report. J Pharm Pract 32 (1): 99- 102. https://doi.org/10.1177/0897190017734728.
Ohia, S. E., Awe, S. O., LeDay, A. M., Opere, C. A. and Bagchi. D. (2001) Effect of hydroxycitric acid on serotonin release from isolated rat brain cortex. Res Commun Mol Pathol Pharmacol 109 (3-4): 210-6.
Ohia, S. E., Opere, C. A., LeDay, A. M., Bagchi, M., Bagchi, D. and Stohs, S. J. (2002) Safety and mechanism of appetite suppression by a novel hydroxycitric acid extract (HCASX). Mol Cell Biochem 238 (1-2): 89-103. https://doi.org/10.1023/a:1019911205672.
Oils and Herbs. (2025) Organic Garcinia Cambogia Extract Powder. https://oilsandherbs.co.uk/product/garcinia-cambogia-extract-powder/. Accessed: 19/06/2025.
Onakpoya, I., Hung, S, K., Perry, R., Wider, B. and Ernst, E. (2011) The Use of Garcinia Extract (Hydroxycitric Acid) as a Weight loss Supplement: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. J Obes.14;2011:509038. https://doi.org/10.1155/2011/509038.
Preuss, H.G., Rao, C.V., Garis, R., Bramble, J.D., Ohia, S.E., Bagchi, M. and Bagchi, D., 2004. An overview of the safety and efficacy of a novel, natural (-)-hydroxycitric acid extract (HCA-SX) for weight management. Journal of medicine, 35(1-6), pp.33-48.
Roberts, C., Steer, T., Maplethorpe, N., Cox, L., Meadows, S., Nicholson, S., Page, P. and Swan, G. (2018) National Diet and Nutrition Survey (NDNS): results from years 7 and 8 (combined) for 2014 to 2015 and 2015 to 2016. NDNS: results from years 7 and 8 (combined) - GOV.UK Accessed: 14/10/2024.
Roche, B., Chenoweth, J. A., Radke, J. B., Poppenga, R. H., Sutter, M. E. (2014) Hypoglycemia associated with Garcinia Cambogia ingestion. Clin Toxicol 52:736.
Roy, S., Rink, C., Khanna, S., Phillips, C., Bagchi, D., Bagchi, M. and Sen, C. K. (2004) Body weight and abdominal fat gene expression profile in response to a novel hydroxycitric acid-based dietary supplement. Gene Expr 11 (5-6): 251-62. https://doi.org/10.3727/000000003783992289.
Saito, M., Ueno, M., Ogino, S., Kubo, K., Nagata, J. and Takeuchi, M. (2005) High dose of Garcinia cambogia is effective in suppressing fat accumulation in developing male Zucker obese rats, but highly toxic to the testis. Food and Chemical Toxicology 43, pp.411-419. https://doi.org/10.1016/j.fct.2004.11.008.
Semwal, R. B., Semwal, D. K., Vermaak, I. and Viljoen, A. (2015) A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 102: 134-48. https://doi.org/10.1016/j.fitote.2015.02.012.
Shara, M., Ohia, S. E., Yasmin, T., Zardetto-Smith, A., Kincaid, A., Bagchi, M., Chatterjee, A., Bagchi, D. and Stohs, S. J. (2003) Dose- and time-dependent effects of a novel (-)-hydroxycitric acid extract on body weight, hepatic and testicular lipid peroxidation, DNA fragmentation and histopathological data over a period of 90 days. Molecular and Cellular Biochemistry 254: 339–346, 2003. https://doi.org/10.1023/a:1027358106407.
Sharma, T., Wong, L., Tsai, N. and Wong, R. D. (2018) Hydroxycut® (herbal weight loss supplement) Induced Hepatotoxicity: A Case Report and Review of Literature. Hawaii Med J. 2010 Aug;69(8):188–190.
Sikka, G., Gupta, A., Bachan, M. and Khan, Z. (2016). “Uber-Trim” Causes Immune Thrombocytopenic Purpura: A Life-Threatening Case Report. Chest 150 (4, Supplement): 392A. https://doi.org/https://doi.org/10.1016/j.chest.2016.08.405.
Sinha, S., Jothiramajayam, M., Ghosh, M. and Mukherjee, A. (2014) Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem Toxicol 2014; 68:71-77. https://doi.org/10.1016/j.fct.2014.02.036
Tice, R. R., Argurell, E., Anderson, D., Burlinson, B., Hartmann, A. and Kobayashi, H. (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 2000; 35:206-221. https://doi.org/10.1002/(SICI)1098-2280(2000)35:3%3C206::AID-EM8%3E3.0.CO;2-J
Tennant, J. R. (1964) Evaluation of the trypan blue technique for determination of cell viability. Transplantation 1964; 2:685-694. https://doi.org/10.1097/00007890-196411000-00001.
TGA. (2024a) Medicines containing Garcinia gummi-gutta (Garcinia cambogia) or hydroxycitric acid (HCA). Medicines containing Garcinia gummi-gutta (Garcinia cambogia) or hydroxycitric acid (HCA) | Therapeutic Goods Administration (TGA)
https://www.tga.gov.au/news/safety-alerts/medicines-containing-garcinia-gummi-gutta-garcinia-cambogia-or-hydroxycitric-acid-hca. Accessed: 09/06/2025.
TGA. (2024b) Proposed changes to the Permissible Ingredients Determination: Low-negligible risk changes 2024-25. Feedback updated 2 Dec 2024. Proposed changes to the Permissible Ingredients Determination: Low-negligible risk changes 2024-25 - Therapeutic Goods Administration - Citizen Space Accessed: 09/06/2025.
US NCCIH. (2025) Garcinia Cambogia. Garcinia Cambogia: Usefulness and Safety | NCCIH Accessed: 09/06/2025.
van Loon, L. J. C., van Rooijen, J. J. M., Niesen, B., Verhagen, H., Saris, W. H. M. and Wagenmakers, A. J. M. (2000) Effects of acute (−)-hydroxycitrate supplementation on substrate metabolism at rest and during exercise in humans. Am J Clin Nutr, 72:1445-1450. https://doi.org/10.1093/ajcn/72.6.1445.
Vasques, C. A. R., Schneider, R., Klein-Júnior, L. C., Falavigna, A., Piazza, I. and Rossetto, S. (2014) Hypolipemic Effect of Garcinia cambogia in Obese Women. Phytother. Res. 2014, 28, 887–891. https://doi.org/10.1002/ptr.5076.
Vuppalanchi, R., Bonkovsky, H. L., Ahmad, J., Barnhart, H., Durazo, F., Fontana, R. J., Gu, J., Khan, I., Kleiner, D. E., Koh, C., Rockey, D. C., Philipps, E. J., Li, Y-J., Serrano, J., Stolz, A., Tillman, H. L., Seeff, L. B., Hoofnagle, J. H. and Navarro, V. J. (2022) Garcinia cambogia, Either Alone or in Combination With Green Tea, Causes Moderate to Severe Liver Injury. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1416-e1425. https://doi.org/10.1016/j.cgh.2021.08.015.
Yamada, T., Hida, H. and Yamada, Y. (2007) Chemistry, physiological properties, and microbial production of hydroxycitric acid. Appl Microbiol Biotechnol. 2007 Jul;75(5):977-82. https://doi.org/10.1007/s00253-007-0962-4.
Yu, J. S., Choi, M. S., Park, J. S., Rehman, S. U., Nakamura, K. and Yoo, H. H. (2017) Inhibitory Effects of Garcinia cambogia Extract on CYP2B6 Enzyme Activity. Planta Med 83 (11): 895-900. https://doi.org/10.1055/s-0043-104934.