BfR (German Federal Institute for Risk Assessment)
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
101. Although not an official published risk assessment, a slide deck on “Risk Assessment Approaches and Methodology – an Overview” presented HCA from G. cambogia extracts as a case study - is publicly available (Hirsch-Ernst, 2022). In this, it was noted that doses via supplements was up to 3,000 mg HCA/day.
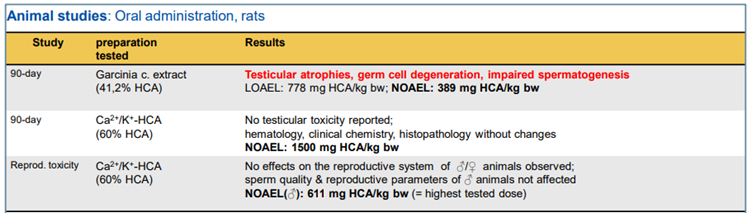
102. Three animal studies (oral administration, rats) were summarised (see Figure 3). It was observed that at high doses male rats showed signs of testicular toxicity and/or impaired spermatogenesis (i.e. sperm quality and sperm count).
Figure 3 – Animal studies as summarised in Hirsch-Ernst, (2022) (reproduced from Hirsch-Ernst, 2022).
103. In human intervention studies that assessed the safety of HCA intake, the observed adverse effects were mostly non-specific and were in similar occurrence as in control groups. The parameters of potential effects on spermatogenesis were not investigated.
104. The risk assessment was as follows. It was unclear whether the reproductive toxicological effects observed in male rats were due to the possible impurities in the extracts or were compound-specific to high doses of HCA intake. The lowest no-observed adverse effect level (NOAEL) was determined to be 389 mg HCA/kg bw per day (G. cambogia extract, 41.2% HCA). The human data were found to be inadequate with regards to considerations for the male reproductive system. The lack of specifications for HCA preparations/G. cambogia extracts added to food supplements was noted, and as such “transferability” of the above NOAEL to other preparations of food supplements may not be appropriate. Lastly, uncertainties regarding the health assessment of prolonged HCA intakes, especially at high doses (3,000 mg HCA/day) were noted.