Chemical composition
In this guide
In this guideThis is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
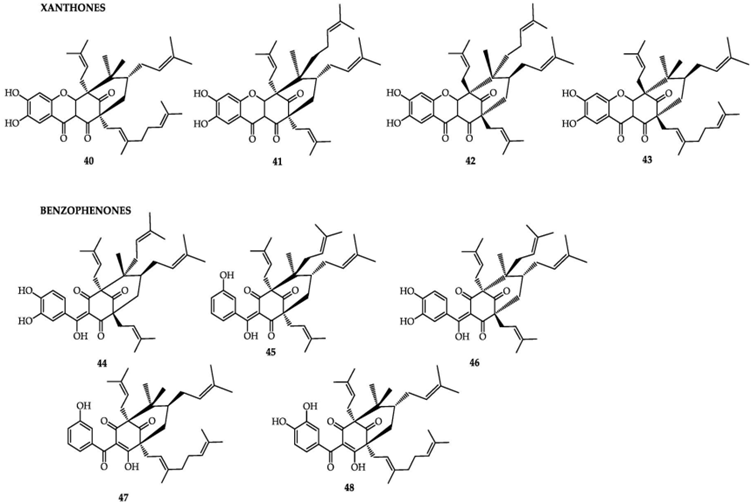
11. The bioactive compounds which are extracted and isolated from G. cambogia are shown in Table 1 and Figure 1.
Table 1 - list of bioactive compounds from plant parts of G. cambogia and their related bioactivities (reproduced from Espirito Santo et al., 2020).
|
Xanthones |
Plant part |
Activity |
|
Garbogiol |
Roots |
Inhibition of α-glucosid |
|
Rheedia xanthone A |
Peel |
Not applicable* |
|
Oxy-guttiferone i |
Fruits |
Not applicable* |
|
Oxy-guttiferone k |
Fruits |
Not applicable* |
|
Oxy-guttiferone k2 |
Fruits |
Not applicable* |
|
Oxy-guttiferone m |
Fruits |
Not applicable* |
|
Benzophenones |
Not applicable* |
Not applicable* |
|
Garcinol |
Peel |
Anticancer, anti-inflammatory, antiparasitic, action on nervous system |
|
Isogarcinol |
Peel |
Anticancer, anti-inflammatory, antiparasitic, action on nervous system |
|
Guttiferone i |
Fruits |
Not applicable* |
|
Guttiferone n |
Fruits |
Not applicable* |
|
Guttiferone j |
Fruits |
Not applicable* |
|
Guttiferone k |
Fruits |
Topoisomerase II inhibitor |
|
Guttiferone m |
Fruits |
Topoisomerase II inhibitor |
|
Organic acids |
Not applicable* |
Not applicable* |
|
Heterocyclic amines |
Fruits |
Antiobesity |
|
Tartaric acid |
Fruits |
Not applicable* |
|
Citric acid |
Fruits |
Not applicable* |
|
Malic acid |
Fruits |
Antimicrobial |
|
Garcinialactone |
Fruits |
Not applicable* |
Figure 1 - chemical structures of the xanthone and benzophenone classes of bioactive compounds from G. cambogia. Xanthones: 40) oxy-guttiferone-i; 41) Oxy-guttiferone k; 42) Oxy-guttiferone k2 and 43) Oxy-guttiferone k. Benzophenones: 44) guttiferone-I; 45) guttiferone-j; 46) guttiferone-k; 47) guttiferone-n and 48) guttiferone-m (reproduced from Espirito Santo et al., 2020).
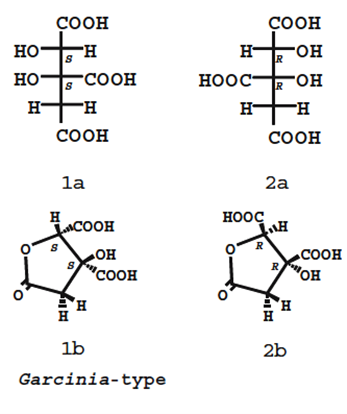
12. The HCA present in G. cambogia is a “potent” and “competent” inhibitor of adenosinetriphosphate (ATP) citrate lyase, which is a key enzyme in the synthesis of fatty acids, cholesterol, and triglycerides. It also regulates the level of serotonin, which has been associated with satiety, increased oxidation of fat, and decreased gluconeogenesis (Semwal et al., 2015; Preuss et al., 2004). HCA compromises a citric acid with a hydroxyl group at the second carbon. HCA has two diastereomers as there are two chiral centres, as such there are four stereoisomers of HCA, compromising two pairs of enantiomers (see Figure 2). Each of the stereoisomers can form a γ-lactone ring and in general solution, HCA is a mixture of non-lactone and lactone forms (Yamada et al., 2007).
Figure 2 - Structures of HCA derived from Garcinia ssp. Upper and bottom structures show the non-lactone and lactone forms, respectively. (2S,3S)-HCA (a mixture of 1a and 1b) is found in G. cambogia. The other isomers (2R,3R)-HCA (a mixture of 2a and 2b) and (2R,3S)-HCA have not been isolated from natural sources (reproduced from Yamada et al., 2007).