Background and Objectives
In this guide
In this guide1. The future of food safety assessment of chemicals depends on our adaptability and flexibility whilst using the best scientific methodologies and strategies available in order to respond to the accelerating developments in science and technology. The vision is to be able to predict risk more rapidly and efficiently. In addition, it is about using the best science available and integrating innovative technologies into the chemical risk assessment process. This will be fundamental in the future for human and environmental safety.
2. Some of the current challenges faced by UK chemical risk assessors are: the large number of (groups of) chemicals that require assessment, lack of toxicological data on these chemicals and the speed, cost and ethical and moral considerations of traditional testing methods. One of the major recent scientific advancements is the development of New Approach Methodologies (NAMs) including but not limited to high throughput screening, omics and in silico computer modelling strategies (e.g., Artificial Intelligence (AI) and machine learning) for the evaluation of hazard and exposure. This also advocates the Replacement, Reduction and Refinement (3Rs) approach to animal testing.
3. NAMs are gaining traction as a systematic method to support the informed conclusions of chemical risk assessments.
4. For regulatory agencies to incorporate and implement these new predictive capabilities, brings both challenges and opportunities. Moving from research to risk assessment to the regulatory setting and beyond, there must be appropriate validation and acceptance of these new and emerging technologies.
5. In order to achieve this, the UK Food Standards Agency (FSA) and Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) are developing a UK roadmap towards acceptance and integration of these NAMs, including predictive toxicology methods using computer modelling, into safety and risk assessments for regulatory decision making.
6. A workshop was held online in October 2021 with the intention of gaining insights from a variety of perspectives to develop a UK Roadmap for NAMs in chemical risk assessment.
7. The background and objectives of the workshop were introduced to the participants.
8. Implementation of NAMs will not only require the historic 3Rs approach (i.e., replacement, reduction, and refinement of animal experiments) but also the expansion to the 6R principle: (to also include) reproducibility, relevance, and regulatory acceptance.
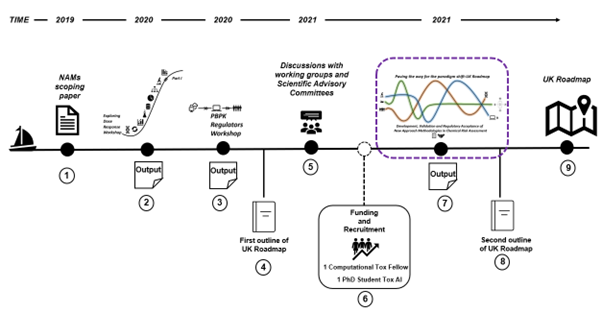
9. In support of this, the FSA and COT have produced a scoping paper on the available NAMs methodologies and strategies and organised three workshops (held in international, multidisciplinary settings and which included participation of attendees from regulatory agencies, government bodies, academia and industry). Furthermore, the FSA have funded a computational toxicology fellow at the University of Birmingham and a PhD Student (LIDo-TOX AI) in Kings College London (Figure 1).
10. The aim of the present workshop was to receive insights, comments and ideas from a wide variety of stakeholders and industry, academia and government, on the roadmap. This will allow it to be developed into a useful and engaging document that is beneficial to more than just the FSA and COT. This process would include a range of scientists, policy, and lawyers, will involve working in the international space and engaging with the public. Furthermore, the workshop should address issues such as: what is holding back the progress of NAMs being used in the regulatory space, including a range of areas such as socio-technical barriers and regulatory frameworks.
11. In order for us to create a roadmap that is inclusive and visionary we have taken into account a wide variety of opinions and ideas from different fields to ensure we capture all the views, opportunities and challenges that we may face in the integration of NAMs in chemical risk assessment.
12. Professor Alan Boobis (Imperial College London and Chair of the COT) further emphasised that this workshop is an opportunity to address what the Roadmap needs to include in order to help achieve the stated objectives and how do we get to where we want to be i.e., How do we gain acceptance of NAMs from risk assessors and regulators. Furthermore, how do we ensure that NAMs are fit for purpose.
Figure 1 The roadmap journey 1) Scoping paper: Environmental, health and safety alternative testing strategies: Development of methods for potency estimation” (TOX/2019/70) was reviewed by the COT in December 2019. 2) Output of the Exploring Dose Response Workshop (March 2020). 3) Output of the PBPK Regulators Workshop (December 2020). 4) First outline of UK Roadmap. 5) Output of the discussions with working groups and scientific advisory committees (regular reviews). 6) Funded a computational toxicology fellow (University of Birmingham) and a PhD student in artificial intelligence (Kings College London). 7) Paving the way for the paradigm shift - UK Roadmap Development, Validation and Regulatory Acceptance of New Approach Methodologies in Chemical Risk Assessment workshop (current workshop). 8) Second outline of the UK Roadmap. 9) Finalisation of the UK Roadmap.