Overarching conclusions and recommendations - 2020 Workshop Report
In this guide
In this guide- Pragmatic guidelines / frameworks are needed for incorporating these models into risk assessment.
- We need to describe the uncertainty of these methods. There needs to be confidence in the prediction from these methods/models i.e., there is not necessarily a need for a full validation.
- Use case studies like the ones outlined in the workshop to move towards applicability and confidence in the models.
- Human biomonitoring data will be key to identifying a realistic snapshot of exposure scenarios. Incorporating this data in the in silico models could be provided to enhance accuracy in exposure scenarios.
- PBPK models could be used to provide relevant substances to benchmark against known human biomonitoring data.
- Big data need to be linked to human clinical data and biomonitoring data including the analysis of biofluids.
- Exposure data and exposure science will be key in developing in silico models in risk assessment.
- Explore the use of exposomics and the use of exposomics data alongside metabolomics.
- NAMs approaches used for the cosmetics could be applied in the same way for food ingredients/contaminants specifically for higher level exposures through Uncertainty Assessment.
- Transparency throughout the process i.e., Consumer facing engagement on new approach methods. There should be planning to take forward these new methods using social sciences research and technical research for integration.
- Finding a synergy to use / combine these new technologies and integrate these as part of our risk assessment methodologies with a validation process throughout.
Moving from research to risk assessment to regulatory setting and beyond
Food authorities should strive to incorporate the best scientific methods available (Kavlock et al., 2018).
In the recent EU Farm to Fork strategy and the EU Green Deal Food 2030 Pathways for Action (Food systems and data) it states that value should be placed on emerging technologies, tools, standards and infrastructure for use in food systems.
The Joint Research Centre (JRC) EU Reference Laboratory for alternatives to animal testing (EURL ECVAM) published its Status Report 2019 on the development, validation and regulatory acceptance of alternative methods used for scientific purposes stating: “Innovation, collaboration and education initiatives drive progress in alternatives to animal testing.”
NAMs and IATAs are rarely accepted by regulatory bodies and the key is how can these approaches be facilitated in a regulatory setting and supporting the technology available. However, the potential use through various case studies as a proof of principle concept is becoming apparent.
The future direction of safety assessment science will depend heavily on the evolution of the regulatory landscape. A key challenge, though, is whether the regulatory framework can keep pace with the increasing speed of scientific and technological developments (Worth et al., 2019).
This will need close collaboration between chemists, toxicologists, informaticians and risk assessors to develop, maintain and utilise appropriate models. Not only must the different disciplines come together, but also those scientists from industry, academia and regulatory agencies must recognise the commonalities (Cronin et al., 2018). The challenge is to respond to the growing need for adaptable, flexible and even bespoke computational workflows that meet the demands of industry and regulators, by exploiting the emerging methodologies of Tox21 and risk assessment.
The focus of the 7th annual Global Summit on Regulatory Science (GSRS17) was Emerging Technologies for Food and Drug Safety. In the GSRS17 meeting, it was said that “moving forward toward greater integration of emerging data and novel methodologies for chemicals risk assessment will need continuous efforts on capacity building. This will be accomplished through increased data accessibility and sharing, the maintenance and establishment of key partnerships, technical workshops and training sessions with international experts, and ongoing focus on data analysis tools development to address regulatory questions. It is also important to demonstrate proof of concept through various case studies and work collaboratively on the interpretation and application of new data for use in regulatory applications.” This is currently being done at an international level under the OECD and as the focus of the Accelerating the Pace for Chemical Risk Assessment initiative co-lead by the US EPA, the ECHA and Health Canada (Kavlock et al., 2018).
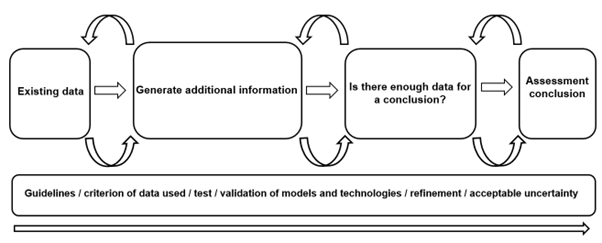
Figure 5. Diagram of concluding thoughts of workflow discussions around NAMs and IATA of the Exploring Dose Response workshop.
Ultimately, innovative technologies should be reviewed and evaluated once developed to be integrated as part of the risk assessment strategies for chemical testing for human health and the environment. Using a validation process via a science and evidence driven approach, to address the data gaps in the risk assessment process, will facilitate the acceptance and validity of these NAMs as well as pave the way for alternatives testing strategies with confidence (Figure 5). Furthermore, integration of these technologies as part of our risk assessment process to streamline our probabilistic rather than deterministic conclusions will be fundamental in the future of human and environmental safety.