Mission and Vision: Objectives and outline of workshop - 2020
In this guide
In this guideObjectives of the workshop
The application of these alternative strategies to human health risk assessment requires effective collaboration between scientists including chemists, toxicologists, informaticians and risk assessors. As such, this multi-disciplinary workshop drew upon delegates and speakers from industry, academia, and regulatory agencies with a diverse range of experience.
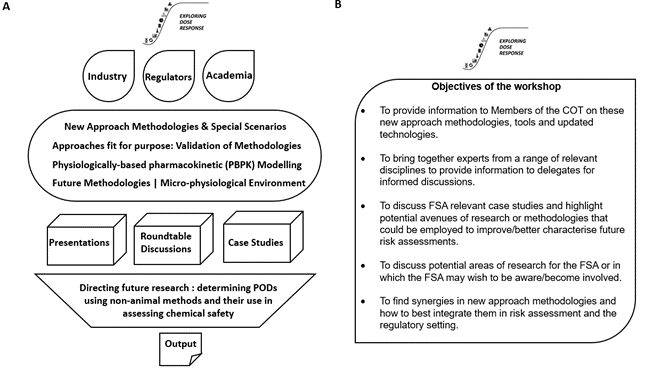
The workshop provided a platform (Figure 2A) from which to address the latest in silico prediction models and PBPK modelling techniques. It provided speakers with the opportunity to share their knowledge and experience through case studies and roundtable discussions on approaches fit for purpose, including consideration of their validation and integration into current health risk assessment practices (Figure 2B).
Outline of the workshop
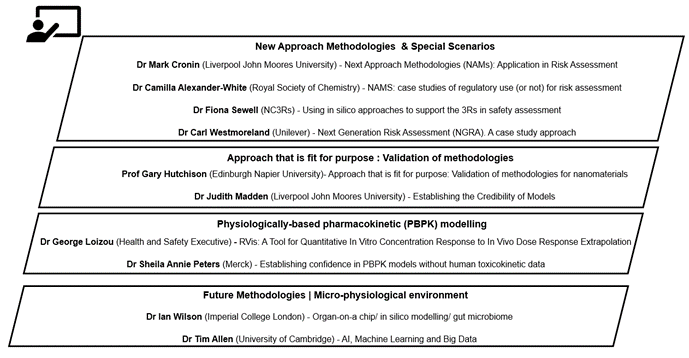
The workshop was divided into different area sessions; New Approach Methodologies & Special Scenarios, Approaches fit for purpose: Validation of methodologies, PBPK modelling, Future Methodologies (micro-physiological environment) and consisted of presentations accompanied by roundtable discussions of case studies with feedback and a discussion about future research needs. The presentations were delivered by invited experts (Figure 3) and had been designed to provide relevant information to inform the later discussions and case studies.
Figure 2. Diagrams representing outline and objectives of workshop. Overview and outline (A) and objectives (B) of the Exploring Dose Response Workshop.
Figure 3. Diagram overview of sessions and presentations of the Exploring Dose Response Workshop.
Brief overview of case studies
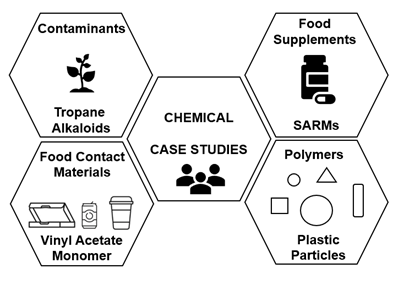
The case studies (Figure 4) were based on a range of chemical areas (man-made and environmental) relevant to the FSA, where limited information was available for the purposes of risk assessment: contaminants (tropane alkaloids (TAs), food supplements (selective androgen receptor modulators (SARMs), food contact materials (vinyl acetate monomer (VAM) and polymers (plastic particles). Background on the chemical groups was provided and questions were asked to prompt discussion. Each of the case study discussion groups included invited experts, Members of the COT, COT Secretariat and other attendees to try and ensure consistent expertise across groups.
Figure 4. Case studies overview. The case studies were based on a range of chemical areas (man-made and environmental) relevant to the FSA, where limited information was available for the purposes of risk assessment: contaminants (tropane alkaloids (TAs), food supplements (selective androgen receptor modulators (SARMs), food contact materials (vinyl acetate monomer (VAM) and polymers (plastic particles).