Executive Summary - 2020 Workshop Report
In this guide
In this guideAdvances in biology, computer science, and other fields are paving the way for major improvements in how we evaluate environmental and public health risks posed by potentially toxic chemicals. The combined advances in discovery and clinical sciences, data science and technology have resulted in toxicity testing which has reached a pivotal transformation point known as part of the 4th industrial revolution (4IR). One of the major recent scientific advancements is the development of alternative toxicity testing and computer modelling strategies for the evaluation of hazard and exposure.
The UK Food Standards Agency (UK FSA) and the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) held an “Exploring Dose Response” workshop in a multidisciplinary setting inviting attendees from regulatory agencies, government bodies, academia and industry. The workshop provided a platform from which to address and enable expert discussions on the latest in silico prediction models, new approach methodologies (NAMs), physiologically based pharmacokinetics (PBPK), future methodologies, integrated approaches to testing and assessment (IATA) as well as methodology validation.
Using a series of presentations from external experts and case study (including plastic particles, polymers, tropane alkaloids, selective androgen receptor modulators) discussions, the workshop outlined and explored an approach that is fit for purpose applied to future human health risk assessment in the context of food safety. Furthermore, we explored possible future research opportunities to establish points of departure (PODs) using non-animal alternative models and to improve the use of exposure metrics in risk assessment.
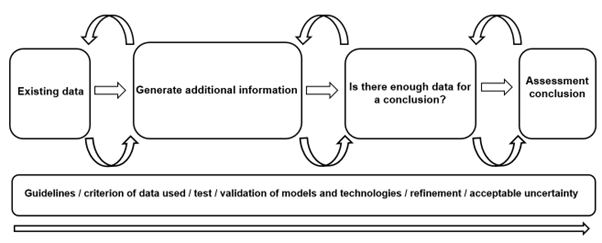
The overall conclusions and recommendations were as follows:
1. The use of pragmatic guidelines/framework for incorporating these models into risk assessment.
2. Case studies, such as those outlined in the workshop, should be used to determine applicability, and provide confidence in the models.
3. Human biomonitoring data will be key to identify a realistic snapshot of exposure scenarios as well as ‘big data’, which need to be linked to human clinical data.
4. Exposure data and exposure science will be key in developing in silico modelling in risk assessment and to explore the use of exposomics.
5. There should be transparency throughout the process i.e., Consumer facing engagement of new approach methods.
Ultimately, it was collectively agreed by attendees, that integration of these new technologies, as part of our risk assessment methodologies with a validation process throughout, will be key in the acceptance of the models (by regulatory bodies) and will be fundamental in the future of human and environmental safety.