PFAS/2023/02 Annex 1
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.Reliability scoring
Should papers undergo reliability scoring or quality assessment to assess reliability prior to inclusion into the narrative/table? The subgroup may wish to consider providing specific guidance on epidemiology, in vivo and in vitro studies, respectively?
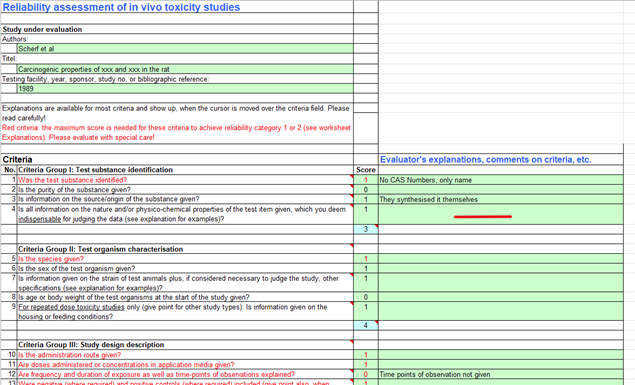
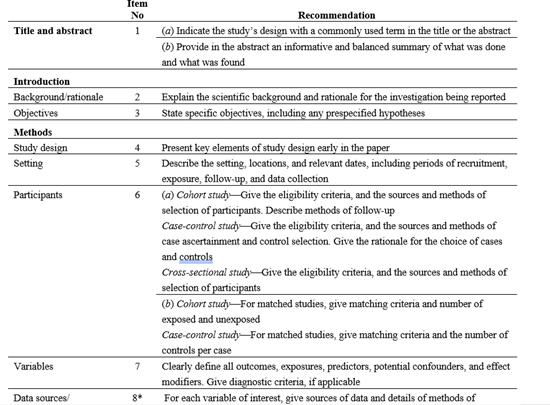
To ensure data used in all reports are of adequate quality all in vivo and in vitro papers could undergo Klimisch scoring using the ToxRTool (Figure 1). Similarly, epidemiology data could undergo quality assessment e.g. using Newcastle Ottowa Score (NOS) (Figure 2), or Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) assessment (Figure 3), or based on Annex 1 of the SETE report..
Screening papers for reliability could reduce the number of papers used in the assessments and may impact on the subgroup decision-making on presentation of data i.e. if data are presented in a narrative, tabular or graphical format.
Figure 1. Example of ToxRTool.
Figure 2. Example of NOS
Figure 3. Example of STROBE