Titanium Dioxide - Background
In this guide
In this guideThis is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
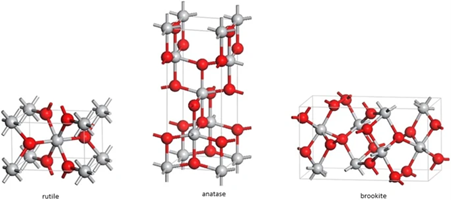
10. Titanium dioxide (TiO2) is an inorganic compound which exists in nature in
different crystalline forms - the anatase and rutile being the two most important (see Fig 1).
Chemical Abstracts Service (CAS) Registry number: 13463-67-7.
European Inventory of Existing Commercial Chemical Substances (EINECS) number: 236-675-5.
Colour Index (C.I.) number: 77891.
11. Titanium dioxide is an authorised Food Additive (E171) in the EU in accordance with Annex II to Regulation (EC) No 1333/2008 in both anatase and rutile forms (Commission Regulation (EU) No 231/2012) and under GB Food Law (retained EU law Regulation No 1333/2008 on food additives).
12. The uses of titanium dioxide include:
- As a colour to make food more visually appealing.
- To give colour to food that would otherwise be colourless.
- To restore the original appearance of food.
It is also widely used in cosmetics and medicines (EFSA, 2016).
Fig 1: Forms of TiO2
Previous Evaluations
Evaluations pre-2016
13. Titanium dioxide has been the subject of numerous evaluations by various scientific bodies.
14. The EU Scientific Committee on Food (SCF) evaluated titanium dioxide on a number of occasions (SCF, 1975 and 1977). In 1975, the SCF did not establish an Acceptable Daily Intake (ADI) for titanium dioxide based on the 1969 JECFA assessment concluding the lack of significant absorption and tissue storage in several species including humans. In 1977, the SCF included titanium dioxide in the category ‘colours for which an ADI was not established but which could be used in food’.
15. The Joint FAO/WHO Expert Committee of Food Additives (JECFA, 1969) - JECFA allocated an ADI ‘not limited except for good manufacturing practice’.
Evaluations from 2016 - to date
16. The use of food additives is regulated under the European Parliament and Council Regulation (EC) No 1333/2008 on food additives. Since titanium dioxide (E 171) was permitted in the EU before 20 January 2009, it belongs to the group of food additives which are subjected to a new risk assessment by the European Food Safety Authority (EFSA), according to Commission Regulation (EU) No 257/2010, and in line with the provision of Regulation (EC) No 1333/2008.
17. The re-evaluation of titanium dioxide (E 171) as food additive was published on 14 September 2016. The EFSA Food Additives and Flavourings (FAF) Panel concluded, on the basis of the available evidence that titanium dioxide could be used as a food additive (E 171). EFSA recommended that additional reproductive toxicity testing could be performed to enable EFSA to establish a health-based guidance value for titanium dioxide (E 171). On the basis of the data available, the Panel concluded that the absorption and oral bioavailability of titanium dioxide was low, independent of size. For endpoints other than genotoxicity, the Panel identified a no-observed adverse effect level (NOAEL) of 2,250 mg/kg bw/d based on a study in rats. Compared to the exposure based on reported use levels and analytical data, the use of E171 was not considered to be of concern.
18. The Panel did not establish an Acceptable Daily Intake (ADI) due to the lack of an extended 90-day toxicity study or a multi-generation or extended one generation reproduction toxicity study with E171. This is because possible adverse effects were identified in the reproductive system in some studies conducted with test substances that were non-food grade or with inadequately characterised nanomaterial.
19. Overall, the Panel concluded that once definitive and reliable data on the reproductive toxicity of E 171 were available, the full dataset would enable the Panel to establish a health-based guidance value (ADI). They further recommended that:
- In order to enable the Panel to establish a health-based guidance value (ADI) for the food additive TiO2 (E 171), additional testing would be required. An extended 90-day study or a multigeneration or extended-one generation reproduction toxicity study according to the current OECD guidelines could be considered. Further studies should be performed with TiO2 (E 171) complying with the EU specifications and additionally including a characterisation of the particle size distribution of the test material. However, in deciding on actual testing, considerations of animal welfare need to be balanced against the improvement in the toxicological database within a tiered testing approach.
- The EU specifications for TiO2 (E 171) should include a characterisation of particle size distribution using appropriate statistical descriptors (e.g. range, median, quartiles) as well as the percentage (in number and by mass) of particles in the nanoscale (with at least one dimension < 100 nm), present in TiO2 (E 171) used as a food additive. The measuring methodology applied should comply with the EFSA Guidance document (EFSA Scientific Committee, 2011).
- The maximum limits for the impurities of the toxic elements (arsenic, lead, mercury and cadmium) in the EU specification for TiO2 (E 171) should be revised in order to ensure that TiO2 (E 171) as a food additive will not be a significant source of exposure to those toxic elements in foods.
20. In January 2017, a call for data was published requesting business operators to submit new reproductive toxicity data for titanium dioxide (E 171), as well as data addressing other recommendations concerning the specifications for titanium dioxide (E 171). Data from a new extended one-generation reproduction toxicity (EOGRT) study was submitted.
21. On 4 April 2017, the French Agency for Food, Environment and Occupational Health and Safety (ANSES) published an opinion on dietary exposure to nanoparticles of titanium dioxide assessing, in particular, the study of Bettini et al. (2017) and concluded that the data available do not bring into question the risk assessment performed by EFSA.
22. On 22 March 2018, the European Commission (EC) requested the EFSA Food Additives and Nutrient Sources Added to Food (ANS) Panel to evaluate four new studies describing potential adverse health effect of titanium dioxide used as food additive (E 171). The ANS Panel opinion, published on 4 July 2018, concluded that the outcome of the four studies did not merit re-opening the existing opinion of EFSA related to the safety of titanium dioxide (E 171) as food additive.
23. In that opinion EFSA recommended that biomarkers for putative pre-cancerous lesions in the colon should be examined, as additional parameters, in the reproductive toxicity study recommended by EFSA in 2016. Business operators followed this recommendation and published an EOGRT study to investigate these data gaps.
24. In 2019, ANSES published a review of the risk related to the ingestion of the food additive titanium dioxide (E 171) to include recent scientific studies published after their 2017 opinion. ANSES emphasised the lack of scientific data able to resolve the uncertainties regarding the safety of the additive E171. It reiterated recommendations to obtain data for characterising the different physico-chemical forms of E171 and additional toxicological data on the potential effects associated with their ingestion. Pending a better toxicological characterisation of E171, ANSES restated its previous general conclusions on nanomaterials aimed at limiting the exposure of workers, consumers and the environment as part of a gradual approach, in particular by promoting safe products that are equivalent in terms of function and effectiveness, and that do not contain nanomaterials. In addition to the 2017 opinion. The EC requested EFSA to assess the ANSES review in order to 1) highlight major findings showing that food additive titanium dioxide (E 171) is of safety concern 2) indicate whether it overrules the conclusion of the previous EFSA evaluation and 3) highlight any additional uncertainties that could be addressed in the ongoing follow-up work from the 2016 EFSA opinion.
25. EFSA published a statement on the risk related to the exposure to the food additive titanium dioxide (E 171) performed by ANSES (May 2019). EFSA concluded that the ANSES opinion does not identify any major new finding that would overrule the conclusions made in the previous EFSA scientific opinion on the safety of titanium dioxide (E 171) as a food additive. The ANSES opinion reiterated the previously identified uncertainties and data gaps, which are currently being addressed by recommendations follow-up activities requested after the 2016 EFSA review. EFSA considered that this recommendation should be revisited once the work on the physico-chemical characterisation of the food additive titanium dioxide (E 171) is completed.
26. The European Commission requested EFSA to assess new data addressing the uncertainties identified with respect to the characterisation of this food additive, including its particle size and particle size distribution provided by interested food business operators in response to the call for data published as a follow-up of the re-evaluation of titanium dioxide (E 171) (August 2018).
27. The new data assessment resulted in a scientific opinion on the proposed amendment of the specifications of titanium dioxide (E 171) with respect to the inclusion of the additional parameters related to its particle size distribution which was published in July 2019. EFSA indicated that the conclusions made, and the uncertainties identified, in the previous EFSA assessment (2016) of the food additive titanium dioxide (E 171) remain valid.
28. EFSA concluded that based on the proposed change in the specifications, the toxicological database on titanium dioxide (E 171) as a food additive should be revisited in line with the data requirements specified in the 2018 EFSA “Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain”.
Particle Size Considerations for TiO2 – 2018 review
29. One of the largest uncertainties in the 2016 EFSA evaluation was related to the composition of titanium dioxide. EFSA considered that E 171 mainly consisted of micro-sized titanium dioxide particles, with a nano-sized (< 100 nm) fraction less than 3.2% by mass. Uncertainties around the identity and characterisation of E 171 were however highlighted, noting that no limits for the particle size of E 171 were set in the EU specifications (EFSA, 2016).
30. Subsequently, in 2019, and following the evaluation of data submitted by interested operators, the Panel recommended that “the EU specifications for E 171 include the parameter of median minimum external dimension by particle number >100 nm (measured by electron microscopy), which is equivalent to less than 50% of constituent particles by number with a minimum external dimension <100 nm.”
31. The EFSA Scientific Committee published ‘Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health’ (EFSA Scientific Committee, 2018a) updating the 2011 Guidance Document on nanomaterials (EFSA Scientific Committee, 2011a), and clarifying that conventional materials containing a fraction of nanoparticles require specific risk assessment considerations.
32. A re-evaluation of E 171 was completed by the EFSA ANS Panel in 2018. The EC requested that EFSA assess a proposal for an amendment of the EU specifications for the food additive E 171 based on the data on particle size and particle size distribution that had been provided by the interested business operators in response to the first part of the European Commission call for data. This scientific opinion was adopted and published in June 2019. The ANS Panel recommended the inclusion of additional parameters related to the particle size distribution in the EU specifications for E 171 and concluded that the toxicological database should be revisited. The scope of the document covers engineered nanomaterials and materials containing a fraction of particles less than 50% in the number–size distribution, with one or more external dimensions in the size range 1–100 nm, a definition which could be applicable to the case of the food additive titanium dioxide (E 171).
Additional Evaluations of Titanium Dioxide
33. Before expanding on the 2021 EFSA evaluation of Titanium dioxide following the 2019 recommendation, additional evaluations by other scientific bodies that were published prior to 2021 are discussed in the section below.
ANSES and ECHA (European Chemicals Agency)
34. Following a report by the French Authorities in 2016, and a proposal for evaluation of titanium dioxide the Committee for Risk Assessment (RAC) of the European Chemicals Agency (ECHA) concluded in June 2017 that titanium dioxide met the criteria to be classified as a substance suspected of causing cancer (category 2) if inhaled.
35. The main mechanism to explain the effects induced by titanium dioxide, in common with effects seen with other substances, was inflammation and an indirect genotoxic effect through production of reactive oxygen species (ROS) arising from the biopersistence and insolubility of all forms of titanium dioxide particles. However, a direct interaction with DNA could not be excluded, since titanium dioxide was found in the cell nucleus in various in vitro and in vivo studies.
36. This was in line with the International Agency for Research on Cancer (IARC) evaluation which concluded that “titanium dioxide is possible carcinogenic to humans (Group 2B) based on sufficient evidence in experimental animals and inadequate evidence from epidemiological studies. ” This was with relation to exposure via inhalation. However, in the same report by the French Authorities, ANSES concluded that there was no carcinogenic concern after oral or dermal administration.
Dutch Office for Risk Assessment
37. In 2018, the Dutch Office for Risk Assessment and Research held a workshop on the “potential health effects of the food additive titanium dioxide (E171)”, the results of which were published in 2019 , where overall the need for further studies to further investigate the effects of titanium dioxide exposure- particularly for the endpoints of colon tumours and immunotoxicology based on the data gaps and study limitations of the available database at the time was highlighted. Furthermore the need to better characterise the composition of E171 was noted.
38. In 2020, a review was published that summarised the outcomes of this workshop and additionally aimed to identify and evaluate recent toxicological studies on food-grade titanium dioxide and nano-sized titanium dioxide in ex-vivo, in-vitro, and in-vivo experiments along the gastrointestinal route, and to postulate an Adverse Outcome Pathway (AOP) following ingestion.
39. Adverse effects were identified including the generation of ROS, alterations of the gut microbiota, persistent inflammation, and other effects on the immune system. It was noted that findings were inconsistent between the different species and independent research groups.
40. With regards to the animal studies which reported positive effects with respect to precancerous lesions/tumour formation, it was noted that those were mainly used as research models and a proper investigation of a dose-response relationship was not performed. Based on the available information, it was not possible to carry out a risk assessment.
41. When considering the mode of action, it was postulated that it was closely related to the ability of titanium dioxide to induce ROS formation and promote inflammation. The potential key events were considered to be persistent inflammation and ROS generation that can result in oxidative stress as well as persistent epithelial cell injury and potentially lead to DNA damage and exert a tumour-promoting effect of E171 seen in some of the studies.
42. Finally, it was noted that it is generally assumed that the round and spherical crystal forms of TiO2 contribute to a lower extent to the induction of adverse effects, when ingested and similarly that titanium dioxide nanoparticles are suspected to induce more adverse effects than other particle sizes. However, a study by Proquin et al. (2017) was also mentioned, that demonstrated that a mixture of nano- and micro-sized TiO2 particles, as they are present in E171, induce more adverse effects than the single fractions alone.
43. The authors further expanded on possible interactions of E171 with its direct environment as well as other factors that could potentially affect agglomeration for example and discussed how these could directly affect the properties of titanium dioxide.
44. Therefore, they considered that “it is important to carefully examine and analyze the physicochemical characteristics of TiO2 particles in its vehicle, as well as in its surrounding matrix as their final milieu, to guarantee a profound assessment of potential adverse health effects of E171 and to adequately compare different studies in the process of risk assessment.” (Bischoff et al.,2020)
Scientific Committee on Consumer Safety (SCCS)
45. The EU Scientific Committee on Consumer Safety (SCCS) assessed titanium dioxide used in cosmetic products that lead to exposure by inhalation. With regards to mutagenicity and genotoxicity, the SCCS noted that in the 2010 evaluation, IARC concluded that that most of the in vitro genotoxicity studies with titanium dioxide exposure were negative despite the high rate of false positives and that the EFSA Panel in 2016 considered that the positive genotoxicity results may have been due to experimental conditions associated with the induction of oxidative stress.
46. The SCCS also noted that studies showing a positive association between the so-called group of Poorly Soluble Low Toxicity (PSLT) particles exposures and genotoxicity are generally consistent with the mechanism that sub-toxic concentrations of PSLT particles can cause inflammation and oxidative stress, which may lead to mutations.
47. Oxidative stress is considered to be the underlying mechanism of the proliferation and genotoxic responses to PSLT particles including titanium dioxide and thus there is a large body of evidence that titanium dioxide has no direct genotoxic potential.
48. The SCCS was of the opinion that “The genotoxic effects of titanium dioxide most probably manifest through an indirect mechanism (oxidative stress), or secondary mechanisms (e.g., oxidative stress and inflammation caused by immune cells).
49. The SCCS therefore considered it plausible that there is a practical threshold for this mode of action and therefore a risk assessment could be carried out for its use in cosmetic products.” They concluded that when used in cosmetic products titanium dioxide does not pose a genotoxic risk. (SCCS, 2020). Genotoxicity is not considered further in this paper.