Summary of EFSA 2025 evaluation
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.This is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Δ⁸-THC chemistry and formation
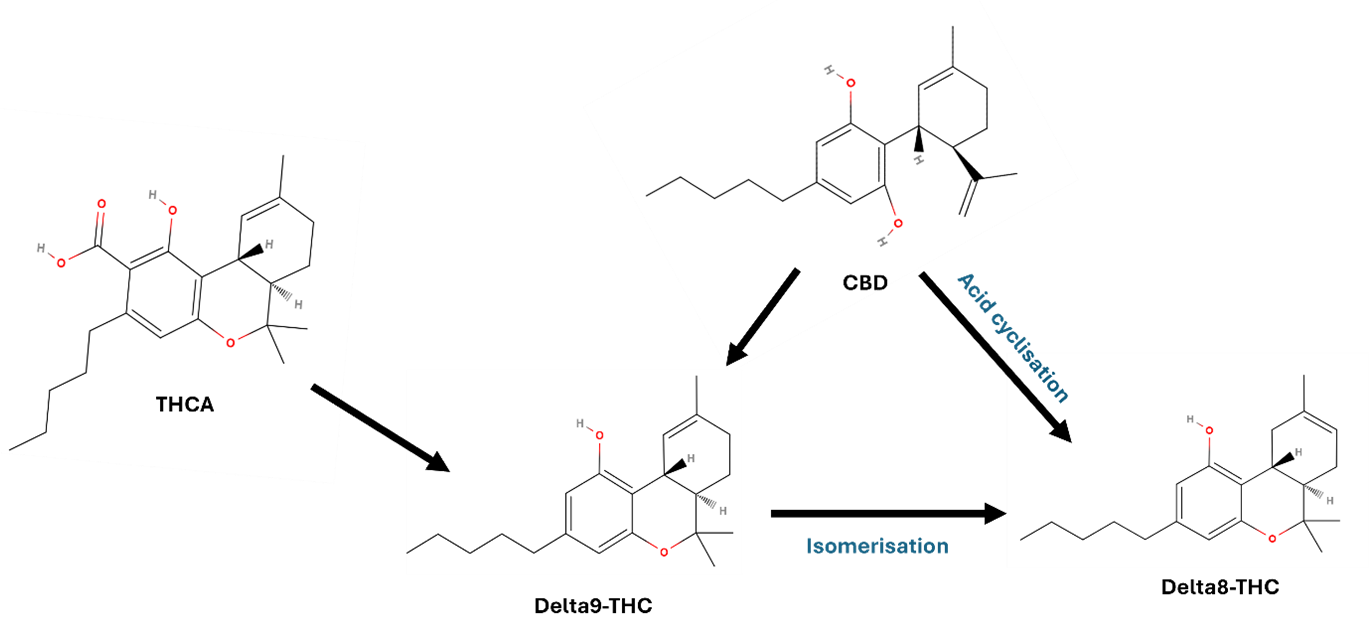
20. Δ⁸-THC is a naturally occurring cannabinoid formed through isomerisation of Δ⁹-THC, the more thermodynamically unstable compound (Figure 1). In hemp, Δ⁹-THC is produced via a biosynthetic pathway that creates Δ⁹-tetrahydrocannabinolic acid (Δ⁹-THCA), which then decarboxylates abiotically to form Δ⁹-THC. Δ⁸-THC arises from this Δ⁹-THC through a structural rearrangement, resulting in the (-) trans isomer, which is the only form found naturally. Unlike Δ⁹-THC, no cis isomers of Δ⁸-THC have been identified in natural hemp.
21. Δ⁸-THC can also be chemically synthesised from other cannabinoids, in particular through the acid cyclisation of CBD (Abdel-Kader et al., 2024).
Figure 1. Formation of Δ⁸-THC. Shows the transformation of CBD through acid-catalysed cyclisation, into Δ⁸-THC or Δ⁹-THC. Δ⁹-THC can isomerise into Δ⁸-THC. Additional formation of Δ⁹-THC can form via decarboxylation of THCA.
Analytical methods
22. The opinion reports contradictory information on the natural occurrence of Δ⁸-THC in hemp. In the majority of studies, Δ⁸-THC was not detected where Δ⁹-THC was present.
23. Detection of Δ⁸-THC in food and hemp products is infrequent and inconsistent, often appearing in only a few samples. This sporadic presence may be due to analytical artifacts, high limits of detection (LOD), co-elution with Δ⁹-THC, or the deliberate or accidental addition of synthetic Δ⁸-THC.
24. Detection of Δ⁸-THC requires highly specific analytical techniques due to its structural similarity to Δ⁹-THC. Liquid chromatography/mass spectrometry (LC-MS/MS) is the preferred method, due to efficient separation and sensitive detection of both neutral and acidic cannabinoids. However, because Δ⁸-THC and Δ⁹-THC share the same mass, they must be chromatographically separated to avoid misidentification or inaccurate quantification. Partial co-elution could lead to Δ⁸-THC being measured for Δ⁹-THC or missed entirely.
25. Gas chromatography (GC) is also usable but requires careful method validation to prevent artefacts from co-elution or unintended isomerisation. In the evaluation, the EFSA Panel prioritised data from samples analysed using reliable LC-MS methods, acknowledging this method to be more reliable.
Toxicokinetics
26. In 2015, the EFSA CONTAM Panel concluded that Δ⁹-THC exhibits low oral bioavailability in humans (~6%) and animals (~26% in monkeys), primarily due to extensive first-pass metabolism and limited absorption influenced by factors such as P-glycoprotein transport. In both species, Δ⁹-THC is highly lipophilic, distributing widely to adipose tissue, brain, and other organs, with transplacental and mammary transfer documented. Metabolism occurs mainly via CYP2C and CYP3A enzymes, producing the active metabolite 11-OH-Δ⁹-THC, followed by oxidation to the inactive 11-nor-9-carboxy-Δ⁹-THC and subsequent glucuronidation. Excretion occurs predominantly via faeces and urine, with enterohepatic recycling and tissue redistribution contributing to prolonged elimination. Sex related metabolic differences were noted in rats, and genetic variability in humans may influence metabolite ratios.
Animal studies
27. Absorption, distribution, metabolism, and excretion (ADME) data was deemed insufficient for the assessment of Δ⁸-THC as most studies use non-oral routes for administration. However, there is a large data set for Δ⁹-THC which was previously evaluated by EFSA CONTAM panel in 2015.
28. In an oral dose study in Sprague-Dawley rats (Moore et al., 2023), Δ⁸-THC was shown to be rapidly absorbed following oral administration in rats, with blood concentrations detectable as early as 0.45 hours post-dose. Peak plasma levels and systemic exposure were shown to be proportional with dose, and repeated daily dosing over 14 days leads to accumulation in the bloodstream, indicating sustained absorption. Δ⁸-THC and Δ9-THC both crossed the placenta.
29. Following oral exposure, the same study showed that there was distribution of Δ⁸-THC to the brain of the highest dosed animals but with no significant difference (SD) between single or 14-day repeated dosing. An additional study using parenteral exposure showed high concentrations were detected in the bile and fat.
30. Cytochrome (CYP)-mediated oxidisation of Δ⁸-THC in the liver produced the same potency of metabolites as those derived from Δ⁹-THC. Overall, no major differences in ADME between Δ8 -THC and Δ⁹-THC have been reported after oral exposure.
Human studies
31. Human data on the absorption of Δ⁸-THC indicates that it is rapidly taken up following oral administration. In a clinical study conducted by Zamarripa et al. (2025), 19 healthy volunteers were exposed to single oral doses of 0 (placebo) to 40 mg of Δ 8 -THC, and 20 mg of Δ⁹-THC per person (via brownie ingestion). Δ⁸-THC reached peak plasma concentrations (Tmax) between 2.4 and 2.8 hours, but Cmax and the area under the curve (AUC) increased depending on the dose. Oral bioavailability was measured at approximately 9%, 1.5 times higher than that of Δ⁹-THC (6%), which was predicted to be due to metabolic differences.
32. No data is available on the distribution of Δ⁸-THC in humans.
33. Δ⁸-THC is primarily processed by the CYP2C9 enzyme, forming the active metabolite 11-OH-Δ⁸-THC. This is further oxidized to 11-nor-9-carboxy-Δ⁸-THC, an inactive compound. Additional metabolites such as 7α-OH-Δ⁸-THC and 7β-OH-Δ⁸-THC have been identified, though their pharmacological significance remains unclear. No human data are currently available on the distribution or excretion of Δ⁸-THC.
Toxicity studies
Acute and single dose studies
34. Δ⁹-THC toxicity was previously evaluated by the EFSA CONTAM panel in 2015. Comparisons were made between literature studies looking at both the Δ⁹-THC and Δ8 -THC toxicity.
35. Two studies have looked at single dose studies of Δ⁹-THC and Δ8-THC. Chesher et al. (1973) compared the effects of single oral doses of Δ⁹-THC and Δ8-THC, along with cannabinol (CBN) acetate, CBD, cannabis extract and pethidine in male mice.
36. A study by Thompson et al (1973a) completed single dose testing, via intubation with a catheter, to rats, dogs, and monkeys. The study looked at ⁹-THC, Δ8-THC, and crude marijuana extract. Δ⁸-THC and Δ⁹-THC exhibited lethal dose 50% (LD50) values of similar magnitude, with females showing greater sensitivity than males. In Beagle dogs and Rhesus monkeys, single oral doses of up to 3000 mg/kg and 9000 mg/kg body weight, respectively, were non-lethal. Δ⁸-THC induced central nervous system (CNS) and behavioural effects in mice, rats, dogs, and monkeys. Observed effects included hypothermia, bradypnea, weight loss, inactivity, wide stance, ataxia, tremors, prostration, drowsiness, anaesthesia, salivation, emesis, anorexia, hyperreactivity, lethargy, crouched posture, and abnormal eating behaviour.
37. Both Δ⁸-THC and Δ⁹-THC produce cannabimimetic effects, anxiogenic effects, psychoactivity, altered pain tolerance, and signs of physical dependence. Δ⁸-THC produces Δ⁹-THC-like discriminative stimulus effects in both sexes, with greater potency observed in females. Oral acute studies showed CNS effects for both compounds, with a tendency toward lower potency for Δ⁸-THC compared to Δ⁹-THC. The available data did not support a quantitative comparison of potency between the two compounds following oral administration.
Repeat dose studies
38. A repeated oral dose study by Kulpa et al 2023, Sprague Dawley rats were orally administered various cannabinoids including Δ⁸-THC at multiple dose levels over 14 days (not including Δ⁹-THC). Δ⁸-THC was tested at 0.32-10 mg/kg bw. High-dose Δ⁸-THC caused weight loss and reduced body temperature without affecting feed intake. It also enhanced pain tolerance at moderate doses, mirroring the analgesic and thermoregulatory effects known for Δ⁹-THC. Δ⁸-THC intake enhanced pain tolerance at moderate doses, reflecting the analgesic and thermoregulatory effects known for Δ⁹-THC.
39. In a sub chronic study (Thompson et al.,1973b), Fischer rats were treated for 119 days with oral doses ranging from 50- 500mg/kg bw. A two-phase pattern was observed during the test: initial depression after 3 days (marked by bradypnea, hypothermia, and mortality) followed by stimulation (hyperactivity, irritability, fighting, and convulsions) observed after 7 days. Mortality and behavioural effects were more pronounced with Δ⁹-THC than Δ⁸-THC, and convulsions showed a dose-dependent relationship. All substances caused significant weight loss and organ weight changes, suggesting endocrine disruption, with Δ⁹-THC having the strongest impact. Histopathological changes were mild and less frequent with Δ⁸-THC, however ovarian stromal degeneration was observed in Δ⁸-THC-treated females, only. Adverse effects occurred at the lowest dose level of 50 mg/kg bw so a no observed adverse effect level (NOAEL) could not be identified.
Endocrine, developmental, and reproductive toxicity
40. In female Wistar-derived rats, Δ⁹-THC blocked ovulation more effectively than Δ⁸-THC, with an ED₅₀ of 0.98 mg/kg bw compared to 3.76 mg/kg bw (Cordova et al., 1980). In a prolonged oral exposure study in Fischer rats, both Δ⁹-THC and Δ⁸-THC, as well as crude marihuana extract (CME), caused dose-dependent reductions in body weight and significant alterations in reproductive organ weights, including decreased prostate, uterus, and ovary weights, and increased adrenal absolute and relative weights. These effects were more pronounced in females. Histopathological changes included ovarian stromal degeneration with Δ⁸-THC, suggesting hormone imbalance potentially linked to pituitary disruption. No NOAEL was established, as adverse effects were observed at the lowest dose tested (50 mg/kg/day) (Thompson et al., 1973b).
41. A subchronic study by Gupta and Elbracht (1983) using repeated intraperitoneal (i.p.) exposure, found that 4 mg/kg of either Δ⁹-THC or Δ⁸-THC administered from postnatal day 16 to 87 significantly impaired pubertal development in male rats. Δ⁹-THC caused greater reductions in body weight and more pronounced suppression of testosterone, dihydrotestosterone (DHT), luteinising hormone (LH), and follicle-stimulating hormone (FSH) compared to Δ⁸-THC. Growth resumed after treatment stopped, however, testosterone remained suppressed and other hormone levels only partially recovered.
Neurotoxicity effects
42. The EFSA CONTAM Panel reviewed evidence indicating that Δ⁹-tetrahydrocannabinol (Δ⁹-THC) may exert neurotoxic effects, particularly during brain development. Animal studies demonstrated altered locomotor activity, reduced social interaction, impaired learning, and diminished responsiveness to stimulants following parenteral or oral exposure. Developmental exposure in Wistar rats led to long-term cognitive and social deficits, linked to changes in glutamatergic and noradrenergic gene expression (Campolongo et al., 2007). In vitro and in vivo data suggest heightened sensitivity of the developing brain to Δ⁹-THC, with potential synergistic effects when combined with other neuroactive substances such as ethanol and phenobarbital. CONTAM concluded that Δ⁹-THC poses neurodevelopmental risks, especially during critical windows of brain maturation. Only one oral study was available investigating the neurotoxicity of Δ⁸-THC, looking at anxiogenic effects after a single dose. The Panel were unable to perform a quantitative assessment due to lack of dose response data.
43. Evaluation of neurotoxic effects from repeated dose studies showed Δ⁸-THC demonstrated Δ⁹-THC like psychoactive outcomes in both male and females, and suggestive of a qualitatively similar psychoactive outcome. Δ⁸-THC showed greater potency in females compared to males but no sex specific differences were noted for Δ⁹-THC.
Genotoxicity
44. The EFSA CONTAM Panel (2015) reviewed genotoxicity data for Δ⁹-THC, including studies from the US National Toxicology Program and other sources. Standard in vitro assays showed no mutagenic or clastogenic effects, though sister chromatid exchanges were observed at cytotoxic doses in Chinese Hamster Ovary (CHO) cells with metabolic activation. DNA damage assays in human cells and aquatic models showed inconsistent results. A 13-week in vivo study in mice found no increase in micronucleated erythrocytes. It was concluded that Δ⁹-THC was not genotoxic in vivo.
45. No genotoxicity studies were available to assess the potential for Δ⁸-THC induced gene mutations.
46. Read across data from Δ⁹-THC indicates that Δ⁸-THC is not genotoxic in vivo as previously established by the EFSA CONTAM panel in 2015. This is supported by Quantitative Structure Activity Relationship (QSAR) model evidence which indicated negative results for mechanistic and endpoint specific alerts and the Ames bacterial reverse mutation test. Profiler and QSAR predictions are identical for Δ 8 -THC and Δ 9-THC.
Human pharmacological and toxicological data
47. The EFSA CONTAM Panel (2015) assessed human oral exposure to Δ⁹-THC using clinical data and pharmaceutical sources. Studies looked at therapeutic uses of Dronabinol (synthetic Δ⁹-THC) including antiemetic treatment in chemotherapy and appetite stimulation in AIDS-related anorexia. Adverse effects including euphoria, dizziness, cognitive impairment, and cardiovascular changes. These were dose-dependent and variable between individuals. Onset occurs within 30 to 60 minutes, with peak effects at 2 to 4 hours and psychoactivity lasting 4 to 6 hours.
48 A LOAEL of 2.5 mg/day per person was established by the Panel which was in agreement with FDA (2004) medical fact sheets for Dronabinol and a single dose study in health humans (Ballard and de Wit, 2011).
Case studies
49. The assessment of the human pharmacological impact consisted of evaluating Δ8-THC consumption case studies, reports of acute intoxication in children and clinical studies. Adverse effects reported from these studies consisted of bradypnea, lethargy and unresponsiveness in children, however the products consumed were generally unregulated Δ8 -THC product (sweets). These results were seen in children who tested positive for THC in urine.
50. Data reported to the US Drug Administration Adverse Event Reporting System (FAERS) on incidents in people consuming suspected Δ8-THC products was compiled and evaluated by Simon et al., 2023. From the 1st of January to 30th of June 2021, 183 intoxication reports were filed with 22 fatal death and an additional 109 cases classified as serious. Of the adverse effects reported, the majority related to respiratory issues: Dyspnoea (18%) and Respiratory disorders (9%) and the profile of the adverse effect differed to those of Δ⁹-THC. Exposure routes were not specified (Simon et al., 2023).
51. A study by Burgess et al. (2024) analysed 5,022 cannabinoid-related intoxication cases reported to U.S. poison centres between January 2021 and December 2022, with Δ⁸-THC accounting for 98.1% of these cases. Most exposures were through ingestion (94.2%), and the leading reasons were unintentional use (40.2%) and abuse (33.1%). Among single-substance Δ⁸-THC exposures, the most frequent clinical effects included mild CNS depression (25.0%), tachycardia (23.2%), agitation (15.8%), and neurological symptoms (14.3%). Less common effects included confusion (7.6%), hallucinations/delusions (4.1%), and tremor (4.0%). The authors highlighted limitations in National Poison Data System coding, which may have missed multi-cannabinoid exposures due to product variability, and noted that vaping products were involved in 3.1% of cases, though misclassification of exposure routes could not be ruled out.
52. The CONTAM Panel noted that findings from Simon et al. (2023) and Burgess et al. (2024) are limited in applicability to food products due to the lack of exposure dose data and inclusion of inhalation routes. Additionally, studies involving mixed cannabinoid exposures or insufficient dosing information (Jo et al., 2021; Reid & Banerji, 2021; Miller et al., 2023; Denton et al., 2024; Raghunatha et al., 2024) were excluded from consideration.
53. Acute intoxication case studies were also reviewed looking at accidental Δ⁸-THC intake doses between approximately 15 - 38 mg/kg bw. The 4 studies considered by the CONTAM panel are as follows:
54. Yourish et al. (2021) reported two young children (3 and 5 years old, body weights unknown) who became unresponsive after consuming large quantities of Δ⁸-THC gummies (totalling 900 mg). Both showed minimal responsiveness and required paediatric intensive care unit care, with one child experiencing hypoxia needing high-flow oxygen. Both recovered and were discharged on day 3.
55. Akpunonu et al. (2021) described a 2-year-old girl who consumed approximately 225 mg Δ⁸-THC (estimated to be 15 mg/kg bw), leading to severe CNS depression and intubation. Δ⁸-THC was confirmed in plasma and gummies. She recovered within 34 hours.
56. Bradley et al. (2023) evaluated report of two sisters (2 and 4 years old) who ingested 500 mg and 350 mg Δ⁸-THC, respectively. Both developed bradypnea and agitation with the 2-year-old one requiring intubation due to declining mental status. They were discharged 45 hours post-ingestion.
57. Gibbons and Morris (2024) reported six cases, including a 5-year-old girl (bw unknown) who consumed 324 mg Δ⁸-THC. She presented with lethargy, pallor, and a Glasgow Coma Scale score of 4, with oxygen desaturation to 80%. She recovered after oxygen therapy.
58. Reports from Masilamani et al. (2024) and Vaphiades (2024) were mentioned by the Panel but not considered due to uncertainties in the anecdotal reports.
Clinical studies
59. Clinical studies evaluated the effects of Δ⁸-THC in comparison to Δ⁹-THC. Over 3 different trials, at doses ranging from 10 to 75 mg, Δ⁸-THC causes symptoms such as increased heart rate, altered perception, and mild cognitive impairment, though these effects are less pronounced and shorter-lasting than those seen with 20 mg of Δ⁹-THC. Higher doses of Δ⁸-THC are required to achieve comparable changes in airway conductance and comparable symptoms to Δ⁹-THC. The onset of effects was reported as slower, and the overall symptom severity is lower.
60. No doses below 10 mg/kg bw were tested so it was not possible to establish a low reference point
Mode of action and potency factor
61. The EFSA CONTAM Panel (2015) previously concluded that the primary biological effects of Δ⁹-THC were mediated via cannabinoid receptors (CB1 and CB2), which are widely expressed across mammalian tissues. CB1 receptors are predominantly located in the brain and autonomic nervous system, while CB2 receptors are mainly found in immune and lymphoid organs. Δ⁹-THC interaction with CB1 receptors in the hypothalamus may disrupt the hypothalamic-pituitary-gonadal axis, leading to reduced synthesis of reproductive and growth-related hormones. The Panel also noted Δ⁹-THC may influence epigenetic regulation and modulate neurotransmitter levels (5- 5-hydroxytryptamine, norepinephrine and dopamine) in a dose-dependent manner.
62. For Δ⁸-THC, several studies suggests that Δ⁸-THC primarily acts as an agonist at cannabinoid receptors CB1 and CB2, similar to Δ⁹-THC. It has lower affinity for CB1, which is mainly found in the brain, and comparable affinity for CB2, located in immune and lymphoid tissues. These differences may explain its reduced potency relative to Δ⁹-THC. Both compounds influence neurotransmitter activity in the brain, but no studies were available investigating Δ⁸-THC interaction with non-cannabinoid receptors. Variability in receptor binding results may stem from differences in assay conditions using transfected cell lines, (CHO and Human Embryonic Kidney (HEK) 293 cell lines).
63. The Panel identified the critical effects as cognitive and psychomotor effects as well as increase heart rate in humans, which were observed in the human clinical studies (Zamarripa et al., 2025). These effects are associated with CB1 and CB2 receptors in which Δ⁸-THC and Δ⁹-THC are agonist for in CNS and autonomic nervous system.
64. The panel considered that a relative potency (ratio Δ9 -THC / Δ8 -THC) was in the range between 1 to 1.4, with 95% confidence between 0.97 and 1.63. This was supported by evidence from quantitative analysis of data from Zamarripa et al. (2025).