EFSA Draft opinion on Δ⁸-THC – Derivation of a Health Based Guidance Value Δ⁸-THC
Introduction
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
1. Following a request from the European Commission (EC), the European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain (CONTAM Panel) were asked to review the existing scientific evidence and all new relevant studies with the aims:
i) To provide scientific opinion on deriving a Health-Based Guidance Value (HBGV) for Δ8-tetrahydrocannabinol (Δ⁸-THC) in food.
ii) To assess the occurrence of Δ⁸-THC food and considering co-occurrence with Δ⁹-THC.
iii) Evaluating toxicological and pharmacological data.
2. This paper provides a summary of the approach used by the EFSA CONTAM Panel to derive a HBGV for Δ⁸-THC in food and a brief summary of the approaches and studies used to reach the conclusion. See link in Annex A.
3. The COT are being asked to review the draft opinion and provide any comments they may have; the Secretariat will then submit the Committee’s comments to EFSA.
4. A document has been provided in the Teams folder for Members to provide comments before and after the Meeting but Members can also send any additional comments directly to the Secretariat. The closing date for the public consultation is the 15th of September 2025. Please provide any comments latest by Wednesday the 10th of September, comments received after this deadline will not be included. Please add the section and/or line number where possible.
5. The background section to this cover paper provides some US Food and Drug Administration (FDA) adverse event reports involving Δ⁸-THC-containing products which may be of interest to COT Members as well as information on the Joint position paper from the Advisory Committee on Novel foods and Processes (ACNFP) & Committee on Toxicity (COT) on establishing a Safe Upper Limit for delta-9-tetrahydrocannabinol (Δ9-THC) and its precursor as including CBD novel foods to provide context.
Background
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Previous evaluations
EFSA
6. In their 2011 opinion, EFSA’s Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) evaluated the safety of hemp (Cannabis genus) derived feed for animals and the potential distribution of psychoactive metabolites, majorly 11-hydroxy- Δ⁹-THC (11-OH-THC), to different tissues and organs of animals, fat and target tissue. Transfer rates of 11-OH-TCH to milk were estimated to be 0.15%. A Provisional Maximum Tolerable Daily Intake (PMTDI) of 0.0004 mg/kg bw was derived from single and repeated dose studies in human volunteers that showed multiple psychotropic effects observed at a lowest observed adverse effect levels (LOAEL) of 0.04 mg/kg body weight and applying an uncertainty factor of 100 (FEEDAP, 2011).
7. Exposure calculations showed both adult and children had exceedances in the PMTDI which occurred via consumption of milk from animals fed whole hemp plant material. However, exposure to milk from hemp seed-derived fed animals was below the PMTDI. The Panel recommended a maximum THC content of 10 mg/kg in hemp seed-derived feed materials, in reference to the Δ9-THC compound only.
8. In its 2015 scientific opinion, the EFSA CONTAM Panel evaluated the risks to human health from the presence of Δ⁹-THC in milk and other animal-derived foods, particularly when animals are fed hemp seed-derived feed. From reported human data in the literature, the Panel identified a LOAEL of 2.5 mg/day (0.036 mg/kg bw) for Δ⁹-THC, primarily due to central nervous system effects. An Acute Reference Dose (ARfD) of 1 μg/kg body weight (bw) was established after applying the uncertainty factor (UF) of 30. Exposure estimates showed that high consumers of milk and dairy products were below this threshold, 3% of the ARfD for adults and 13% for toddlers, and therefore unlikely to pose a health concern.
9. The opinion also discussed Δ⁸-THC. From the limited literature, it was noted that concentrations in Cannabis sativa preparations were typically very low and did not significantly contribute to the psychoactive effects associated with Δ⁹-THC. This reinforced the focus on Δ⁹-THC as the primary compound of concern in food safety and there is limited contribution from Δ⁸-THC within the assessments related to hemp-fed animals.
10. The EFSA Scientific Report (2020) provided an assessment of acute human exposure to Δ⁹-THC through hemp and hemp containing food products. The report found that high adult consumers of most hemp-based products exceeded the ARfD, of 1 μg/kg bw, under both lower bound and upper bound exposure scenarios. The report focused exclusively on Δ⁹-THC and did not include any discussion of Δ⁸-THC.
US FDA
11. The FDA memoranda from 2021 and 2024 concluded that Δ⁸-THC is not Generally Recognised as Safe (GRAS) for use in food. The FDA cited concerns about its potential adverse effects on multiple systems including the nervous, respiratory, reproductive, and endocrine systems as well as risks to neurodevelopment in individuals exposed during pregnancy. In 2024, the FDA reaffirmed this position, stating that new literature up to October 2023 did not change their original safety concerns. As a result, Δ⁸-THC remains not approved for inclusion in food products.
FDA Reported Cases
12. The Secretariat have included the following FDA reports of adverse events in addition to information within the EFSA opinion for Members interest.
13. The FDA received 104 reports of adverse events in patients who consumed Δ⁸-THC products between December 1, 2020, and February 28, 2022 (Ou et al., 2021). Of these 104 adverse event reports:
i) 77% involved adults, 8% involved paediatric patients less than 18 years of age, and 15% did not report age.
ii) 55% required intervention (e.g., evaluation by emergency medical services) or hospital admission.
iii) 66% described adverse events after ingestion of Δ⁸-THC -containing food products (e.g., brownies, gummies).
14. Adverse events included, but were not limited to: hallucinations, vomiting, tremor, anxiety, dizziness, confusion, and loss of consciousness.
15. National poison control centres received 2,362 exposure cases of Δ⁸-THC products between January 1, 2021 (i.e., date that Δ⁸-THC product code was added to database), and February 28, 2022. Of the 2,362 exposure cases:
i) 58% involved adults, 41% involved paediatric patients less than 18 years of age, and 1% did not report age.
ii) 40% involved unintentional exposure to Δ⁸-THC and 82% of these unintentional exposures affected paediatric patients.
iii) 70% required health care facility evaluation, of which 8% resulted in admission to a critical care unit; 45% of patients requiring health care facility evaluation were paediatric patients.
iv) One paediatric case was coded with a medical outcome of death.
2025 Joint ACNFP and COT position paper Δ9-THC paper summary
16. The Secretariat have included the Joint position paper from the (ACNFP) & (COT) on establishing a Safe Upper Limit for delta-9-tetrahydrocannabinol (Δ9-THC) and its precursor as contaminants of hemp-derived products including CBD novel foods summary for information to Members.
17. In July 2025, the ACNFP and COT published a joint position paper on establishing a Safe Upper Limit for Δ⁹-THC and its precursor as contaminants of hemp-derived products including cannabidiol (CBD) novel foods.
18. The Committee conducted a risk assessment on CBD and other minor cannabinoids, including Δ⁹-THC as an accidental contaminant, in hemp (Cannabis sativa Linnaeus) after concerns were raised on physiological and psychoactive effects cause by the isomers (ACNFP and COT, 2025).
19. An oral safe upper limit of 1 μg Δ9-THC/kg bw/day (as the sum of Δ⁹-THC and the precursor Δ⁹-THC A) was established, with consumer protected at intake at or below this value. This was established after considering that 100% of Δ⁹-THCA could be converted to Δ⁹-THC if heated. Considerations of EFSA scientific opinion (EFSA, 2015) and Advisory Council on the Misuse of Drugs (ACMD) were used when considering a safe upper limit.
Summary of EFSA 2025 evaluation
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Δ⁸-THC chemistry and formation
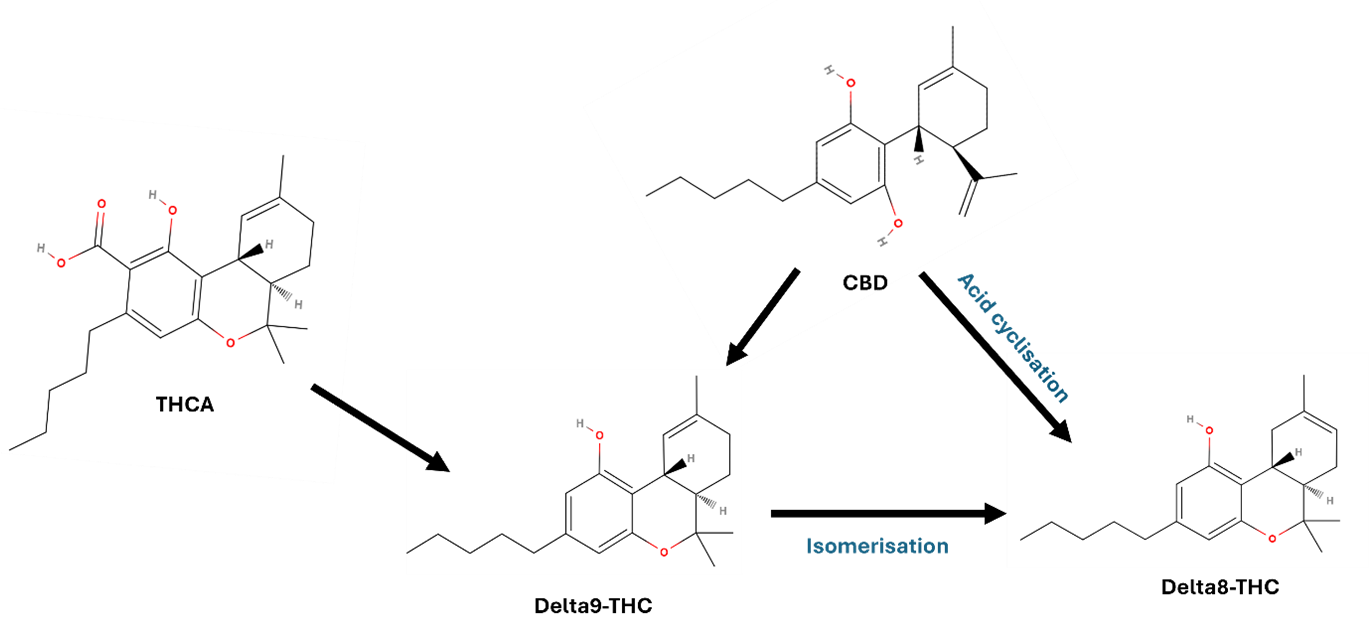
20. Δ⁸-THC is a naturally occurring cannabinoid formed through isomerisation of Δ⁹-THC, the more thermodynamically unstable compound (Figure 1). In hemp, Δ⁹-THC is produced via a biosynthetic pathway that creates Δ⁹-tetrahydrocannabinolic acid (Δ⁹-THCA), which then decarboxylates abiotically to form Δ⁹-THC. Δ⁸-THC arises from this Δ⁹-THC through a structural rearrangement, resulting in the (-) trans isomer, which is the only form found naturally. Unlike Δ⁹-THC, no cis isomers of Δ⁸-THC have been identified in natural hemp.
21. Δ⁸-THC can also be chemically synthesised from other cannabinoids, in particular through the acid cyclisation of CBD (Abdel-Kader et al., 2024).
Figure 1. Formation of Δ⁸-THC. Shows the transformation of CBD through acid-catalysed cyclisation, into Δ⁸-THC or Δ⁹-THC. Δ⁹-THC can isomerise into Δ⁸-THC. Additional formation of Δ⁹-THC can form via decarboxylation of THCA.
Analytical methods
22. The opinion reports contradictory information on the natural occurrence of Δ⁸-THC in hemp. In the majority of studies, Δ⁸-THC was not detected where Δ⁹-THC was present.
23. Detection of Δ⁸-THC in food and hemp products is infrequent and inconsistent, often appearing in only a few samples. This sporadic presence may be due to analytical artifacts, high limits of detection (LOD), co-elution with Δ⁹-THC, or the deliberate or accidental addition of synthetic Δ⁸-THC.
24. Detection of Δ⁸-THC requires highly specific analytical techniques due to its structural similarity to Δ⁹-THC. Liquid chromatography/mass spectrometry (LC-MS/MS) is the preferred method, due to efficient separation and sensitive detection of both neutral and acidic cannabinoids. However, because Δ⁸-THC and Δ⁹-THC share the same mass, they must be chromatographically separated to avoid misidentification or inaccurate quantification. Partial co-elution could lead to Δ⁸-THC being measured for Δ⁹-THC or missed entirely.
25. Gas chromatography (GC) is also usable but requires careful method validation to prevent artefacts from co-elution or unintended isomerisation. In the evaluation, the EFSA Panel prioritised data from samples analysed using reliable LC-MS methods, acknowledging this method to be more reliable.
Toxicokinetics
26. In 2015, the EFSA CONTAM Panel concluded that Δ⁹-THC exhibits low oral bioavailability in humans (~6%) and animals (~26% in monkeys), primarily due to extensive first-pass metabolism and limited absorption influenced by factors such as P-glycoprotein transport. In both species, Δ⁹-THC is highly lipophilic, distributing widely to adipose tissue, brain, and other organs, with transplacental and mammary transfer documented. Metabolism occurs mainly via CYP2C and CYP3A enzymes, producing the active metabolite 11-OH-Δ⁹-THC, followed by oxidation to the inactive 11-nor-9-carboxy-Δ⁹-THC and subsequent glucuronidation. Excretion occurs predominantly via faeces and urine, with enterohepatic recycling and tissue redistribution contributing to prolonged elimination. Sex related metabolic differences were noted in rats, and genetic variability in humans may influence metabolite ratios.
Animal studies
27. Absorption, distribution, metabolism, and excretion (ADME) data was deemed insufficient for the assessment of Δ⁸-THC as most studies use non-oral routes for administration. However, there is a large data set for Δ⁹-THC which was previously evaluated by EFSA CONTAM panel in 2015.
28. In an oral dose study in Sprague-Dawley rats (Moore et al., 2023), Δ⁸-THC was shown to be rapidly absorbed following oral administration in rats, with blood concentrations detectable as early as 0.45 hours post-dose. Peak plasma levels and systemic exposure were shown to be proportional with dose, and repeated daily dosing over 14 days leads to accumulation in the bloodstream, indicating sustained absorption. Δ⁸-THC and Δ9-THC both crossed the placenta.
29. Following oral exposure, the same study showed that there was distribution of Δ⁸-THC to the brain of the highest dosed animals but with no significant difference (SD) between single or 14-day repeated dosing. An additional study using parenteral exposure showed high concentrations were detected in the bile and fat.
30. Cytochrome (CYP)-mediated oxidisation of Δ⁸-THC in the liver produced the same potency of metabolites as those derived from Δ⁹-THC. Overall, no major differences in ADME between Δ8 -THC and Δ⁹-THC have been reported after oral exposure.
Human studies
31. Human data on the absorption of Δ⁸-THC indicates that it is rapidly taken up following oral administration. In a clinical study conducted by Zamarripa et al. (2025), 19 healthy volunteers were exposed to single oral doses of 0 (placebo) to 40 mg of Δ 8 -THC, and 20 mg of Δ⁹-THC per person (via brownie ingestion). Δ⁸-THC reached peak plasma concentrations (Tmax) between 2.4 and 2.8 hours, but Cmax and the area under the curve (AUC) increased depending on the dose. Oral bioavailability was measured at approximately 9%, 1.5 times higher than that of Δ⁹-THC (6%), which was predicted to be due to metabolic differences.
32. No data is available on the distribution of Δ⁸-THC in humans.
33. Δ⁸-THC is primarily processed by the CYP2C9 enzyme, forming the active metabolite 11-OH-Δ⁸-THC. This is further oxidized to 11-nor-9-carboxy-Δ⁸-THC, an inactive compound. Additional metabolites such as 7α-OH-Δ⁸-THC and 7β-OH-Δ⁸-THC have been identified, though their pharmacological significance remains unclear. No human data are currently available on the distribution or excretion of Δ⁸-THC.
Toxicity studies
Acute and single dose studies
34. Δ⁹-THC toxicity was previously evaluated by the EFSA CONTAM panel in 2015. Comparisons were made between literature studies looking at both the Δ⁹-THC and Δ8 -THC toxicity.
35. Two studies have looked at single dose studies of Δ⁹-THC and Δ8-THC. Chesher et al. (1973) compared the effects of single oral doses of Δ⁹-THC and Δ8-THC, along with cannabinol (CBN) acetate, CBD, cannabis extract and pethidine in male mice.
36. A study by Thompson et al (1973a) completed single dose testing, via intubation with a catheter, to rats, dogs, and monkeys. The study looked at ⁹-THC, Δ8-THC, and crude marijuana extract. Δ⁸-THC and Δ⁹-THC exhibited lethal dose 50% (LD50) values of similar magnitude, with females showing greater sensitivity than males. In Beagle dogs and Rhesus monkeys, single oral doses of up to 3000 mg/kg and 9000 mg/kg body weight, respectively, were non-lethal. Δ⁸-THC induced central nervous system (CNS) and behavioural effects in mice, rats, dogs, and monkeys. Observed effects included hypothermia, bradypnea, weight loss, inactivity, wide stance, ataxia, tremors, prostration, drowsiness, anaesthesia, salivation, emesis, anorexia, hyperreactivity, lethargy, crouched posture, and abnormal eating behaviour.
37. Both Δ⁸-THC and Δ⁹-THC produce cannabimimetic effects, anxiogenic effects, psychoactivity, altered pain tolerance, and signs of physical dependence. Δ⁸-THC produces Δ⁹-THC-like discriminative stimulus effects in both sexes, with greater potency observed in females. Oral acute studies showed CNS effects for both compounds, with a tendency toward lower potency for Δ⁸-THC compared to Δ⁹-THC. The available data did not support a quantitative comparison of potency between the two compounds following oral administration.
Repeat dose studies
38. A repeated oral dose study by Kulpa et al 2023, Sprague Dawley rats were orally administered various cannabinoids including Δ⁸-THC at multiple dose levels over 14 days (not including Δ⁹-THC). Δ⁸-THC was tested at 0.32-10 mg/kg bw. High-dose Δ⁸-THC caused weight loss and reduced body temperature without affecting feed intake. It also enhanced pain tolerance at moderate doses, mirroring the analgesic and thermoregulatory effects known for Δ⁹-THC. Δ⁸-THC intake enhanced pain tolerance at moderate doses, reflecting the analgesic and thermoregulatory effects known for Δ⁹-THC.
39. In a sub chronic study (Thompson et al.,1973b), Fischer rats were treated for 119 days with oral doses ranging from 50- 500mg/kg bw. A two-phase pattern was observed during the test: initial depression after 3 days (marked by bradypnea, hypothermia, and mortality) followed by stimulation (hyperactivity, irritability, fighting, and convulsions) observed after 7 days. Mortality and behavioural effects were more pronounced with Δ⁹-THC than Δ⁸-THC, and convulsions showed a dose-dependent relationship. All substances caused significant weight loss and organ weight changes, suggesting endocrine disruption, with Δ⁹-THC having the strongest impact. Histopathological changes were mild and less frequent with Δ⁸-THC, however ovarian stromal degeneration was observed in Δ⁸-THC-treated females, only. Adverse effects occurred at the lowest dose level of 50 mg/kg bw so a no observed adverse effect level (NOAEL) could not be identified.
Endocrine, developmental, and reproductive toxicity
40. In female Wistar-derived rats, Δ⁹-THC blocked ovulation more effectively than Δ⁸-THC, with an ED₅₀ of 0.98 mg/kg bw compared to 3.76 mg/kg bw (Cordova et al., 1980). In a prolonged oral exposure study in Fischer rats, both Δ⁹-THC and Δ⁸-THC, as well as crude marihuana extract (CME), caused dose-dependent reductions in body weight and significant alterations in reproductive organ weights, including decreased prostate, uterus, and ovary weights, and increased adrenal absolute and relative weights. These effects were more pronounced in females. Histopathological changes included ovarian stromal degeneration with Δ⁸-THC, suggesting hormone imbalance potentially linked to pituitary disruption. No NOAEL was established, as adverse effects were observed at the lowest dose tested (50 mg/kg/day) (Thompson et al., 1973b).
41. A subchronic study by Gupta and Elbracht (1983) using repeated intraperitoneal (i.p.) exposure, found that 4 mg/kg of either Δ⁹-THC or Δ⁸-THC administered from postnatal day 16 to 87 significantly impaired pubertal development in male rats. Δ⁹-THC caused greater reductions in body weight and more pronounced suppression of testosterone, dihydrotestosterone (DHT), luteinising hormone (LH), and follicle-stimulating hormone (FSH) compared to Δ⁸-THC. Growth resumed after treatment stopped, however, testosterone remained suppressed and other hormone levels only partially recovered.
Neurotoxicity effects
42. The EFSA CONTAM Panel reviewed evidence indicating that Δ⁹-tetrahydrocannabinol (Δ⁹-THC) may exert neurotoxic effects, particularly during brain development. Animal studies demonstrated altered locomotor activity, reduced social interaction, impaired learning, and diminished responsiveness to stimulants following parenteral or oral exposure. Developmental exposure in Wistar rats led to long-term cognitive and social deficits, linked to changes in glutamatergic and noradrenergic gene expression (Campolongo et al., 2007). In vitro and in vivo data suggest heightened sensitivity of the developing brain to Δ⁹-THC, with potential synergistic effects when combined with other neuroactive substances such as ethanol and phenobarbital. CONTAM concluded that Δ⁹-THC poses neurodevelopmental risks, especially during critical windows of brain maturation. Only one oral study was available investigating the neurotoxicity of Δ⁸-THC, looking at anxiogenic effects after a single dose. The Panel were unable to perform a quantitative assessment due to lack of dose response data.
43. Evaluation of neurotoxic effects from repeated dose studies showed Δ⁸-THC demonstrated Δ⁹-THC like psychoactive outcomes in both male and females, and suggestive of a qualitatively similar psychoactive outcome. Δ⁸-THC showed greater potency in females compared to males but no sex specific differences were noted for Δ⁹-THC.
Genotoxicity
44. The EFSA CONTAM Panel (2015) reviewed genotoxicity data for Δ⁹-THC, including studies from the US National Toxicology Program and other sources. Standard in vitro assays showed no mutagenic or clastogenic effects, though sister chromatid exchanges were observed at cytotoxic doses in Chinese Hamster Ovary (CHO) cells with metabolic activation. DNA damage assays in human cells and aquatic models showed inconsistent results. A 13-week in vivo study in mice found no increase in micronucleated erythrocytes. It was concluded that Δ⁹-THC was not genotoxic in vivo.
45. No genotoxicity studies were available to assess the potential for Δ⁸-THC induced gene mutations.
46. Read across data from Δ⁹-THC indicates that Δ⁸-THC is not genotoxic in vivo as previously established by the EFSA CONTAM panel in 2015. This is supported by Quantitative Structure Activity Relationship (QSAR) model evidence which indicated negative results for mechanistic and endpoint specific alerts and the Ames bacterial reverse mutation test. Profiler and QSAR predictions are identical for Δ 8 -THC and Δ 9-THC.
Human pharmacological and toxicological data
47. The EFSA CONTAM Panel (2015) assessed human oral exposure to Δ⁹-THC using clinical data and pharmaceutical sources. Studies looked at therapeutic uses of Dronabinol (synthetic Δ⁹-THC) including antiemetic treatment in chemotherapy and appetite stimulation in AIDS-related anorexia. Adverse effects including euphoria, dizziness, cognitive impairment, and cardiovascular changes. These were dose-dependent and variable between individuals. Onset occurs within 30 to 60 minutes, with peak effects at 2 to 4 hours and psychoactivity lasting 4 to 6 hours.
48 A LOAEL of 2.5 mg/day per person was established by the Panel which was in agreement with FDA (2004) medical fact sheets for Dronabinol and a single dose study in health humans (Ballard and de Wit, 2011).
Case studies
49. The assessment of the human pharmacological impact consisted of evaluating Δ8-THC consumption case studies, reports of acute intoxication in children and clinical studies. Adverse effects reported from these studies consisted of bradypnea, lethargy and unresponsiveness in children, however the products consumed were generally unregulated Δ8 -THC product (sweets). These results were seen in children who tested positive for THC in urine.
50. Data reported to the US Drug Administration Adverse Event Reporting System (FAERS) on incidents in people consuming suspected Δ8-THC products was compiled and evaluated by Simon et al., 2023. From the 1st of January to 30th of June 2021, 183 intoxication reports were filed with 22 fatal death and an additional 109 cases classified as serious. Of the adverse effects reported, the majority related to respiratory issues: Dyspnoea (18%) and Respiratory disorders (9%) and the profile of the adverse effect differed to those of Δ⁹-THC. Exposure routes were not specified (Simon et al., 2023).
51. A study by Burgess et al. (2024) analysed 5,022 cannabinoid-related intoxication cases reported to U.S. poison centres between January 2021 and December 2022, with Δ⁸-THC accounting for 98.1% of these cases. Most exposures were through ingestion (94.2%), and the leading reasons were unintentional use (40.2%) and abuse (33.1%). Among single-substance Δ⁸-THC exposures, the most frequent clinical effects included mild CNS depression (25.0%), tachycardia (23.2%), agitation (15.8%), and neurological symptoms (14.3%). Less common effects included confusion (7.6%), hallucinations/delusions (4.1%), and tremor (4.0%). The authors highlighted limitations in National Poison Data System coding, which may have missed multi-cannabinoid exposures due to product variability, and noted that vaping products were involved in 3.1% of cases, though misclassification of exposure routes could not be ruled out.
52. The CONTAM Panel noted that findings from Simon et al. (2023) and Burgess et al. (2024) are limited in applicability to food products due to the lack of exposure dose data and inclusion of inhalation routes. Additionally, studies involving mixed cannabinoid exposures or insufficient dosing information (Jo et al., 2021; Reid & Banerji, 2021; Miller et al., 2023; Denton et al., 2024; Raghunatha et al., 2024) were excluded from consideration.
53. Acute intoxication case studies were also reviewed looking at accidental Δ⁸-THC intake doses between approximately 15 - 38 mg/kg bw. The 4 studies considered by the CONTAM panel are as follows:
54. Yourish et al. (2021) reported two young children (3 and 5 years old, body weights unknown) who became unresponsive after consuming large quantities of Δ⁸-THC gummies (totalling 900 mg). Both showed minimal responsiveness and required paediatric intensive care unit care, with one child experiencing hypoxia needing high-flow oxygen. Both recovered and were discharged on day 3.
55. Akpunonu et al. (2021) described a 2-year-old girl who consumed approximately 225 mg Δ⁸-THC (estimated to be 15 mg/kg bw), leading to severe CNS depression and intubation. Δ⁸-THC was confirmed in plasma and gummies. She recovered within 34 hours.
56. Bradley et al. (2023) evaluated report of two sisters (2 and 4 years old) who ingested 500 mg and 350 mg Δ⁸-THC, respectively. Both developed bradypnea and agitation with the 2-year-old one requiring intubation due to declining mental status. They were discharged 45 hours post-ingestion.
57. Gibbons and Morris (2024) reported six cases, including a 5-year-old girl (bw unknown) who consumed 324 mg Δ⁸-THC. She presented with lethargy, pallor, and a Glasgow Coma Scale score of 4, with oxygen desaturation to 80%. She recovered after oxygen therapy.
58. Reports from Masilamani et al. (2024) and Vaphiades (2024) were mentioned by the Panel but not considered due to uncertainties in the anecdotal reports.
Clinical studies
59. Clinical studies evaluated the effects of Δ⁸-THC in comparison to Δ⁹-THC. Over 3 different trials, at doses ranging from 10 to 75 mg, Δ⁸-THC causes symptoms such as increased heart rate, altered perception, and mild cognitive impairment, though these effects are less pronounced and shorter-lasting than those seen with 20 mg of Δ⁹-THC. Higher doses of Δ⁸-THC are required to achieve comparable changes in airway conductance and comparable symptoms to Δ⁹-THC. The onset of effects was reported as slower, and the overall symptom severity is lower.
60. No doses below 10 mg/kg bw were tested so it was not possible to establish a low reference point
Mode of action and potency factor
61. The EFSA CONTAM Panel (2015) previously concluded that the primary biological effects of Δ⁹-THC were mediated via cannabinoid receptors (CB1 and CB2), which are widely expressed across mammalian tissues. CB1 receptors are predominantly located in the brain and autonomic nervous system, while CB2 receptors are mainly found in immune and lymphoid organs. Δ⁹-THC interaction with CB1 receptors in the hypothalamus may disrupt the hypothalamic-pituitary-gonadal axis, leading to reduced synthesis of reproductive and growth-related hormones. The Panel also noted Δ⁹-THC may influence epigenetic regulation and modulate neurotransmitter levels (5- 5-hydroxytryptamine, norepinephrine and dopamine) in a dose-dependent manner.
62. For Δ⁸-THC, several studies suggests that Δ⁸-THC primarily acts as an agonist at cannabinoid receptors CB1 and CB2, similar to Δ⁹-THC. It has lower affinity for CB1, which is mainly found in the brain, and comparable affinity for CB2, located in immune and lymphoid tissues. These differences may explain its reduced potency relative to Δ⁹-THC. Both compounds influence neurotransmitter activity in the brain, but no studies were available investigating Δ⁸-THC interaction with non-cannabinoid receptors. Variability in receptor binding results may stem from differences in assay conditions using transfected cell lines, (CHO and Human Embryonic Kidney (HEK) 293 cell lines).
63. The Panel identified the critical effects as cognitive and psychomotor effects as well as increase heart rate in humans, which were observed in the human clinical studies (Zamarripa et al., 2025). These effects are associated with CB1 and CB2 receptors in which Δ⁸-THC and Δ⁹-THC are agonist for in CNS and autonomic nervous system.
64. The panel considered that a relative potency of Δ⁸-THC to Δ⁹-THC is very likely within the range of 1 to 1.6 (90-95% certainty) and alleviates the uncertainties in the hazard characterisation of Δ⁸-THC compared to Δ⁹-THC. This was supported by evidence from quantitative analysis of data from Zamarripa et al. (2025).
Establishing a HBGV
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
65. In the 2025 EFSA opinion on Δ8 -THC, it was established that the ARfD of 1 μg Δ9-THC/kg bw set by CONTAM Panel (CONTAM, 2015) is still valid. No revaluation of Δ9-THC was carried out in this opinion. It was deemed suitable, that due to the similarities between the MOA and effects between Δ9 -THC and Δ8 -THC, the ARfD derived for Δ9 -THC can be considered applicable as a combined group ARfD for both compounds. Therefore, exposure would be calculated as a sum of Δ9 -THC and Δ8 -THC.
Occurrence in food
Δ8 -THC occurrence in food
66. The Panel extracted data from EFSA Scientific Data Warehouse, from 2014 – 2024, included 1,914 analytical results on Δ8 -THC in food reported by 7 European 2035 countries. Following cleaning of duplications with literature reporting and misreporting, this resulted in a dataset of 1,671 samples/analytical results on Δ8 -THC from 18 FoodEx2 (Level1) food categories. 93% of the samples collected were censored and specifically in the category's hemp infusion leaves, hemp seed oil, and hemp seeds, 96%-99% of the samples were censored.
67. The LODs and limits of quantification (LOQs), considering the different method types, food categories (at FoodEx2 Level 1) were presented in Annex A1 in the EFSA opinion on Δ8 -THC.
68. The highest reported concentration (P95) was found in the category ‘Sugar and similar, confectionery and water-based sweet desserts’ at 39,100 μg/kg. This was followed by ‘Products for nonstandard diets, food imitates and food supplements,’ ranging from 24,000 to 75,000 μg/kg, and ‘Grains and grain-based products’, with levels between 350 and 1,000 μg/kg.
Cooccurrence with other cannabinoids
69. The panel evaluated further data using occurrence data of Δ8 -THC with other cannabinoids in hemp derive. Selecting CBD data resulted in a further 986 result and Δ9 -THC a further 1145 results. The Panel chose to only evaluate samples with a reliable analysis of LC-MS based methods.
70. Within this data set, Δ⁹-THC was commonly detected alone, while Δ⁸-THC alone appeared in only a few samples. Both cannabinoids were found together in 96 samples, with the Δ⁸/Δ⁹ ratio ranging widely from 0.009 to 17.7 (average 1.37, SD 2.28). Literature suggests that naturally occurring Δ⁸-THC typically has a ratio below 1, whereas many EFSA samples showed ratios above 1.
71. The Panel made observations that the reported values could indicate the possible addition of semi-synthetic Δ⁸-THC, formation during processing, storage, or addition of a natural Δ⁸-THC into the samples. This is most likely possible in product containing hemp extracts (CBD oils) which inherently have higher concentrations of cannabinoids.
72. Very few publications report Δ⁸-THC occurrence in hemp food products. Δ⁸-THC was largely undetected in hemp food products across multiple studies, with only a few samples showing trace levels (0.02 -10 mg/kg). Most findings confirmed its absence even when Δ⁹-THC was present, and the highest concentration reported in food (675 mg/kg) was still below levels found in products intentionally fortified with semi-synthetic Δ⁸-THC for drug use.
Uncertainties in occurrence analysis
73. The Panel acknowledged major uncertainties in the data concerning Δ⁸-THC and its co-occurrence with Δ⁹-THC. Significant uncertainties had arisen from the analytical methods used to detect plant cannabinoids. Although the applicable standards required a relative standard deviation under reproducibility conditions of 25%, corresponding to a measurement uncertainty of 50%, older datasets or those generated by less experienced laboratories may not have met these criteria. This likely led to less reliable concentration estimates. Additionally, analytical artefacts affecting Δ⁸-THC quantification may have caused either underestimation or overestimation of its levels.
74. Another source of uncertainty was the wide range of reported LOQs, which spanned from 2 to 10,000 µg/kg depending on the analytical method and food category. Higher LOQs had an impact on upper bound (UB) exposure estimates, particularly in food categories where cannabinoids were more likely to be present.
75. While some products explicitly recorded the presence of CBN and/or CBD extracts, it was possible that other food samples also contained such extracts without disclosure.
76. Finally, a large proportion of left-censored data had resulted in substantial differences between lower bound (LB) and UB estimates across several food categories. This discrepancy was especially relevant for products such as hemp seed oils and food supplements, where the occurrence of Δ⁸-THC was more likely.
Recommendations from the Panel
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
77. The following recommendations were made by the Panel:
i) According to relevant information from a preliminary screening of the new literature on Δ9-THC, since 2015, the data suggest that a further risk assessment of Δ9-THC is 178 needed. The Panel recommended updating the evaluation of dose-effect relationships especially for the low-dose-range in human and experimental animal studies, and endpoints investigated in developmental and reproductive toxicology studies.
ii) Further studies on the transfer rate of Δ⁸-THC, and its metabolites, into animal products intended for human consumption are needed.
iii) Monitoring/data evaluation of food samples should be performed using suitable and validated analytical methods for Δ⁸-THC.
iv) Investigations into the formation of Δ⁸-THC from naturally present cannabinoids during food processing and storage should be carried out to give more insights into the source of Δ⁸-THC in food.
Discussion
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
78. The Secretariat have noted the following points.
79. The ARfD set by the EFSA panel of 1 μg/kg bw/day is for the sum of Δ⁸-THC and Δ9-THC. Therefore, this is a reduction in the EFSA current standing ARfD for Δ9-THC/Δ9-THCa at 1μg /kg bw. E.g. 0.5 μg of Δ⁸-THC + 0.5 Δ9-THC or 0.6 μg of Δ⁸-THC + 0.4 Δ9-THC.
80. No mention has been made of other precursors or other cannabinoids within this reference value (e.g. THCA).
81. The Secretariat noted the vast limitations in analytical data and sensitivity on detection methods. Therefore, occurrence calculations may not be reflective of actual human exposure to Δ⁸-THC in food.
Questions to the Committee
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Members are invited to consider the following:
i) Do the Committee have any comments to submit to EFSA for the consultation?
ii) Do Members have any other comments?
Secretariat
August 2025
List of Abbreviations
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
|
Abbreviation |
Definition |
|
ACMD |
Advisory Council on the Misuse of Drugs |
|
ADME |
Absorption, distribution, metabolism, and excretion |
|
ARfD |
Acute reference dose |
|
AUC |
Area under the curve |
|
CBD |
Cannabidiol |
|
CBN |
Cannabinol |
|
CME |
crude marihuana extract |
|
CNS |
Central nervous system |
|
CONTAM |
The Panel on Contaminants in the Food Chain |
|
CYP |
Cytochrome |
|
DHT |
Dihydrotestosterone |
|
EC |
European commission |
|
ED50 |
Effective dose 50% |
|
EFSA |
European food safety authority |
|
FDA |
USA Food and drug administration |
|
FEEDAP |
EFSA’s Panel on Additives and Products or Substances used in Animal Feed |
|
FSH |
Follicle-stimulating hormone |
|
GC |
Gas chromatography |
|
GRAS |
Generally Recognised as Safe |
|
HBGV |
Health-based guidance value |
|
HEK |
Human Embryonic Kidney |
|
i.p. |
Intraperitoneal |
|
LB |
Lower bound |
|
LC-MS/MS |
Liquid chromatography/mass spectrometry |
|
LD50 |
Lethal dose 50% |
|
LH |
Luteinising hormone |
|
LOAEL |
Lowest observed adverse effect level |
|
LOD |
Limit of detection |
|
LOQ |
Limit of quantification |
|
NOAEL |
No observed adverse effect level |
|
PMTDI |
Provisional Maximum Tolerable Daily Intake |
|
QSAR |
Quantitative structure–activity relationship |
|
SD |
Significant difference |
|
THC |
Tetrahydrocannabinol |
|
UB |
Upper bound |
|
UF |
Uncertainty factor |
|
THCA |
Tetrahydrocannabinolic acid |
Technical terms
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
|
Term |
Definition |
|
Anxiogenic effects |
Physiological or psychological responses that provoke or exacerbate anxiety. These effects may be induced by certain drugs, stressors, or neurochemical imbalances, particularly involving the serotonergic or noradrenergic systems. |
|
Bradypnea |
A clinical term for abnormally slow respiratory rate, typically fewer than 12 breaths per minute in adults. |
|
Cannabinol |
A mildly psychoactive cannabinoid found in aged cannabis, formed through the oxidation of THC. Cannabinol is a Class B drug under Part 2 of Schedule 2 to the MDA 197. |
|
Dyspnoea |
A subjective experience of breathing discomfort, often described as shortness of breath. It can result from respiratory, cardiac, or metabolic. |
|
Glasgow Coma scale |
A standardized neurological scale used to assess consciousness in trauma or critical care settings. It scores eye opening, verbal response, and motor response, with a total score ranging from 3 (deep coma) to 15 (fully alert). |
|
Glucuronidation |
A phase II metabolic process in the liver where glucuronic acid is conjugated to drugs or endogenous compounds, increasing their solubility and facilitating renal or biliary excretion. It’s a key detoxification pathway. |
|
GRAS (Generally Recognised as Safe) |
GRAS is an FDA designation for substances added to food that are considered safe by qualified experts, based on scientific evidence or a history of safe use, and therefore do not require formal FDA approval. |
|
Pallor |
A noticeable paleness of the skin and mucous membranes, often due to reduced blood flow or haemoglobin levels. |
|
Psychotropic |
Refers to any substance that affects brain function, altering mood, perception, cognition, or behaviour. These include antidepressants, antipsychotics, and anxiolytics, and are commonly used in psychiatric treatment. |
References
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Abdel-Kader, M.S., Radwan, M.M., Metwaly, A.M., Eissa, I.H., Hazekamp, A. & ElSohly, M.A., 2024. Chemistry and pharmacology of delta-8-tetrahydrocannabinol. Molecules, 29(6), p.1249. doi:10.3390/molecules29061249. Chemistry and Pharmacology of Delta-8-Tetrahydrocannabinol - PubMed
ACNFP (Advisory Committee on Novel Foods and Processes) & COT (Committee on Toxicity), 2025. Joint position paper on establishing a Safe Upper Limit for delta-9-tetrahydrocannabinol (Δ9-THC) and its precursor as contaminants of hemp-derived products including CBD novel foods. [online] Joint position paper from the (ACNFP) & (COT) on establishing a Safe Upper Limit for delta-9-tetrahydrocannabinol (Δ9-THC) and its precursor as contaminants of hemp-derived products including CBD novel foods | Advisory Committee on Novel Foods and Processes
Akpunonu, P., Baum, R.A., Reckers, A., Davidson, B., Ellison, R., Riley, M., Trecki, J. & Gerona, R., 2021. Sedation and acute encephalopathy in a pediatric patient following ingestion of delta-8-tetrahydrocannabinol gummies. The American Journal of Case Reports, 22, p.e933488. doi:10.12659/AJCR.933488. Sedation and Acute Encephalopathy in a Pediatric Patient Following Ingestion of Delta-8-Tetrahydrocannabinol Gummies - PubMed
Ballard, M.E. and de Wit, H., 2011. Combined effects of acute, very-low-dose ethanol and delta(9)-tetrahydrocannabinol in healthy human volunteers. Pharmacology, Biochemistry and Behavior, 97(4), pp.627–631. https://doi.org/10.1016/j.pbb.2010.11.013. Combined effects of acute, very-low-dose ethanol and delta(9)-tetrahydrocannabinol in healthy human volunteers - ScienceDirect
Bradley, E.K., Hoots, B.E., Bradley, E.S. & Roehler, D.R., 2023. Unintentional ingestion of putative delta-8 tetrahydrocannabinol by two youth requiring critical care: a case report. Journal of Cannabis Research, 5, p.9. doi:10.1186/s42238-023-00176-x. Unintentional ingestion of putative delta-8 tetrahydrocannabinol by two youth requiring critical care: a case report | Journal of Cannabis Research | Full Text
Burgess, A., Hays, H.L., Badeti, J., Spiller, H.A., Rine, N.I., Gaw, C.E., Ding, K. & Smith, G.A., 2024. Delta-8 tetrahydrocannabinol, delta-10 tetrahydrocannabinol, and tetrahydrocannabinol-O acetate exposures reported to America’s Poison Centers. Clinical Toxicology, 62(4), pp.256–266. doi:10.1080/15563650.2024.2340115. Delta-8 tetrahydrocannabinol, delta-10 tetrahydrocannabinol, and tetrahydrocannabinol-O acetate exposures reported to America's Poison Centers - PubMed
Cordova, T., Ayalon, D., Lander, N., Mechoulam, R., Nir, I., Puder, M. & Lindner, H.R., 1980. The ovulation blocking effect of cannabinoids: structure-activity relationships. Psychoneuroendocrinology, 5(1), pp.53–62. doi:10.1016/0306-4530(80)90009-8. The ovulation blocking effect of cannabinoids: structure-activity relationships - PubMed
EFSA (European Food Safety Authority), Arcella, D., Cascio, C. & Mackay, K., 2020. Acute human exposure assessment to tetrahydrocannabinol (Δ9-THC). EFSA Journal, 18(1), p.5953. doi:10.2903/j.efsa.2020.5953. Acute human exposure assessment to tetrahydrocannabinol (Δ9‐THC) | EFSA
EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2015. Scientific Opinion on the risks for human health related to the presence of tetrahydrocannabinol (THC) in milk and other food of animal origin. EFSA Journal, 13(6), p.4141. doi:10.2903/j.efsa.2015.4141. Scientific Opinion on the risks for human health related to the presence of tetrahydrocannabinol (THC) in milk and other food of animal origin - - 2015 - EFSA Journal - Wiley Online Library
EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), 2011. Scientific Opinion on the safety of hemp (Cannabis genus) for use as animal feed. EFSA Journal, 9(3), p.2011. doi:10.2903/j.efsa.2011.201. Hemp for use as animal feed | EFSA
FDA (Food and Drug Administration), 2004. Marinol (Dronabinol) Capsules, NDA 18-651/S-021 500012 Rev Sep 2004. [online] 018651s021lbl.pdf
FDA (Food and Drug Administration), 2021. Use of delta(8) tetrahydrocannabinol as a food ingredient. [online] Joint position paper from the (ACNFP) & (COT) on establishing a Safe Upper Limit for delta-9-tetrahydrocannabinol (Δ9-THC) and its precursor as contaminants of hemp-derived products including CBD novel foods | Advisory Committee on Novel Foods and Processes
FDA (Food and Drug Administration), 2024. Use of delta(8) tetrahydrocannabinol as a food ingredient. [online] Joint position paper from the (ACNFP) & (COT) on establishing a Safe Upper Limit for delta-9-tetrahydrocannabinol (Δ9-THC) and its precursor as contaminants of hemp-derived products including CBD novel foods | Advisory Committee on Novel Foods and Processes
Gibbons, K. & Morris, J., 2024. Delta-8 tetrahydrocannabinol in the emergency department: a case series. WMJ: Official Publication of the State Medical Society of Wisconsin, 123(4), pp.300–303. Wisconsin Medical Journal 114no5
Gupta, D. & Elbracht, C., 1983. Effect of tetrahydrocannabinols on pubertal body weight spurt and sex hormones in developing male rats. Research in Experimental Medicine (Berlin), 182(2), pp.95–104. doi:10.1007/BF01851115. Effect of tetrahydrocannabinols on pubertal body weight spurt and sex hormones in developing male rats - PubMed
Kulpa, J., Henderson, R.G., Schwotzer, D., Dye, W., Trexler, K.R., McDonald, J., Lefever, T.W. & Bonn-Miller, M.O., 2023. Toxicological evaluation and pain assessment of four minor cannabinoids following 14-day oral administration in rats. Cannabis and Cannabinoid Research, 8(S1), pp.S25–S41. doi:10.1089/can.2023.0049. Toxicological Evaluation and Pain Assessment of Four Minor Cannabinoids Following 14-Day Oral Administration in Rats | Cannabis and Cannabinoid Research
Masilamani, M.S., Leff, R. & Kawai, Y., 2024. Asystole in a young child with tetrahydrocannabinol overdose: a case report and review of literature. Frontiers in Toxicology, 6, p.1371651, Asystole in a young child with tetrahydrocannabinol overdose: a case report and review of literature - PubMed
Moore, C.F., Weerts, E.M., Kulpa, J., Schwotzer, D., Dye, W., Jantzi, J., McDonald, J.D., Lefever, T.W. & Bonn-Miller, M.O., 2023. Pharmacokinetics of oral minor cannabinoids in blood and brain. Cannabis and Cannabinoid Research, 8(S1), pp.S51–S61. doi:10.1089/can.2023.0066. Pharmacokinetics of Oral Minor Cannabinoids in Blood and Brain | Cannabis and Cannabinoid Research
Ou, O., Atoigue, A., Kenez, S., Midura, J., Smith, E. & Nolan, N., 2021. Adverse event reports involving delta-8 tetrahydrocannabinol (THC) products from the FDA CFSAN Adverse Event Reporting System (CAERS), 2021. [Poster]. Office of Analytics and Outreach, Center for Food Safety and Applied Nutrition, Food and Drug Administration, College Park, MD, USA. Adverse Event Reports Involving Delta-8 Tetrahydrocannabinol (THC) Products from the FDA CFSAN Adverse Event Reporting System (CAERS), 2021
Simon, T.A., Simon, J.H., Heaning, E.G., Gomez-Caminero, A. & Marcu, J.P., 2023. Delta-8, a cannabis derived tetrahydrocannabinol isomer: evaluating case report data in the Food and Drug Administration Adverse Event Reporting System (FAERS) database. Drug, Healthcare and Patient Safety, 31 December, pp.25–38. Delta-8, a Cannabis-Derived Tetrahydrocannabinol Isomer: Evaluating Case Report Data in the Food and Drug Administration Adverse Event Reporting System (FAERS) Database - PMC
Thompson, G.R., Mason, M.M., Rosenkrantz, H. & Braude, M.C., 1973b. Chronic oral toxicity of cannabinoids in rats. Toxicology and Applied Pharmacology, 25, pp.373–390. Chronic oral toxicity of cannabinoids in rats - PubMed
Thompson, G.R., Rosenkrantz, H., Schaeppi, U.H. & Braude, M.C., 1973a. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicology and Applied Pharmacology, 25, pp.363–372. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys - PubMed
Vaphiades, M.S., 2024. Delta-8 gummies causing visual snow: a case report. Frontiers in Ophthalmology, 3, p.1349525, 29 January. Frontiers | Delta-8 gummies causing visual snow: a case report
Yourish, H., Micciche, A., Westover, R., Scanlon, M., Malley, C. & Lynch, M., 2021. Toxicity from accidental consumption of delta-8-tetrahydrocannabinol gummies in two pediatric patients. Clinical Toxicology, 59(11), p.1178. North American Congress of Clinical Toxicology (NACCT) Abstracts 2021: Clinical Toxicology: Vol 59 , No 11 - Get Access
Zamarripa, C.A., Spindle, T.R., Schriefer, D., Cone, E.J., Winecker, R.E., Flegel, R., Hayes, E., Davis, L.S., Kuntz, D. & Vandrey, R., 2025. A within-subject cross-over trial comparing the acute effects of oral delta-8-tetrahydrocannabinol and delta-9-tetrahydrocannabinol in healthy adults. Drug and Alcohol Dependence, 272, p.112676. doi:10.1016/j.drugalcdep.2025.112676. A within-subject cross-over trial comparing the acute effects of oral delta-8-tetrahydrocannabinol and delta-9-tetrahydrocannabinol in healthy adults - PubMed