Background
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.Uses
6. Echinacea is a genus of herbaceous flowering plants, comprised of ten species and originally native to North America (Ahmadi et al., 2024). Three Echinacea species (Echinacea purpurea, Echinacea pallida, and Echinacea angustifolia) are used medicinally for the prevention and treatment of the common cold, influenza, and upper respiratory tract infections (Ardjomand-Woelkart and Bauer, 2015). E. purpurea is the most widely used and extensively studied of the three. Prior to 1968, Echinacea angustifolia and Echinacea pallida were considered to be different varieties of the same species until a revision of the genus described them as two separate species (WHO, 1999).
7. Echinacea herbal products are often sold as dietary supplements to boost the immune system and to reduce the symptoms of common cold and upper respiratory tract infections. These are popular products in North America and Europe, generating more than 300 million USD annually in the U.S. alone (Ahmadi et al., 2024).
8. Complementary and alternative medicines (CAM), including herbal and traditional preparations, are often perceived as safer than conventional medicines. Women are the highest consumer of CAM and survey data suggests that their use continues during pregnancy as women may turn to ‘natural alternatives’ due to fear of teratogenicity from conventional medicines (Hall et al., 2011). The most commonly used herbal preparations during pregnancy include raspberry leaf, ginger, chamomile, Echinacea and cranberry (Cuzzolin et al., 2010; Hall et al., 2011; Holst et al., 2011; Nordeng et al., 2011).
9. Echinacea extracts are used for respiratory infections (colds and flu, bronchitis, strep throat, toothache), urinary tract infections, skin disorders (Staphylococcus infections, cold sores, ulcers, wounds, burns, insect bites, eczema, allergies) and rheumatoid arthritis (Hudson, 2012). Between 4.3% (Holst et al., 2011) and 9.2% (Cuzzolin et al., 2010) of pregnant women report using Echinacea during pregnancy for the treatment of cold and flu, boosting the immune system and the prevention of common cold (Cuzzolin et al., 2010; Holst et al., 2011).
Constituents and preparations
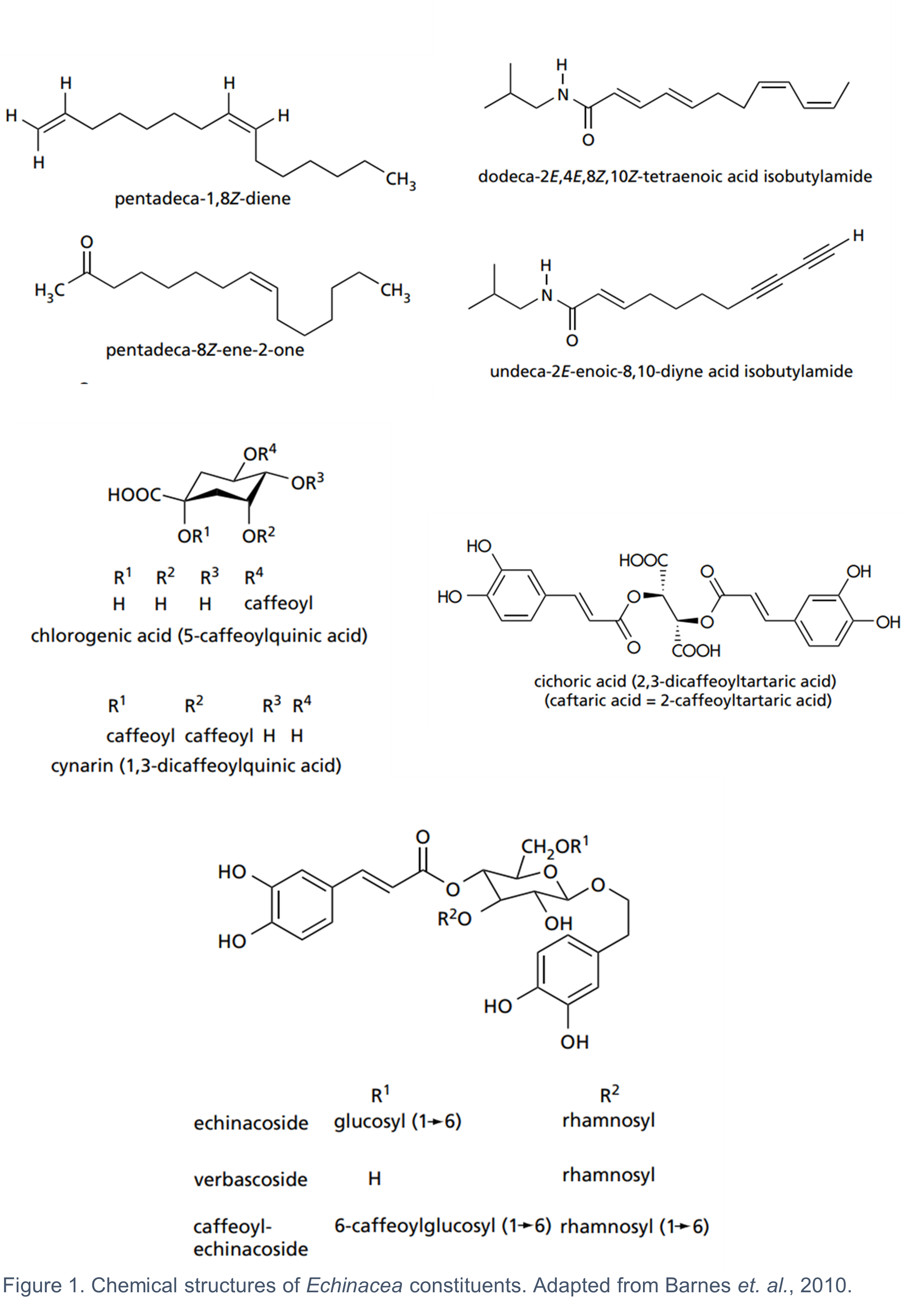
10. The fresh or dried aerial parts and the fresh pressed juice from the flowering tops of E. purpurea, as well as the whole plant, and the dried roots of all three species are used medicinally (Barnes et al., 2010). The composition of bioactive metabolites varies across the three species and their respective plant parts (Table 1). It is generally considered that there is no single constituent or group of constituents responsible for the activity of Echinacea. The combined effects of several groups of bioactive constituents, including the alkylamides, caffeic acid derivatives, polysaccharides and alkenes, contribute to the biological activity of Echinacea (Barnes et al., 2010). It is worth noting that some of the key pharmacological effects of Echinacea such as its antitumor, antioxidant, antimicrobial, antifungal, antiviral, and immunomodulatory activities are mainly attributed to the caffeic acid derivatives and alkylamides (Ahmadi et al., 2024). The chemical structures of some of the most common Echinacea constituents are shown in Figure 1.
Table 1: Major constituents of medicinally used Echinacea species (adapted from (Barnes et al., 2010).
|
Species |
Plant part |
Constituents |
Comment |
|
E. purpurea |
Aerial parts |
Alkylamides, caffeic acid derivatives (mainly chicoric acid), polysaccharides, polyacetylenes. |
Echinacoside is absent. |
|
E. angustifolia |
Roots |
Alkylamides, caffeic acid derivatives (mainly echinacoside), cynarin, polysaccharides, polyacetylenes. |
Cynarin is characteristic of E. angustifolia. |
|
E. pallida |
Roots |
Caffeic acid derivatives (mainly echinacoside), polysaccharides, polyacetylenes. |
Alkylamides are largely absent. |
11. The concentration of the different bioactive constituents in Echinacea preparations is not only affected by the species, plant part, season and growing conditions, but also by the extraction method employed. Different methods of extraction are used for preparing the Echinacea products (Table 2) and the final products can contain powdered plant parts, dry and liquid extracts, pressed and dried pressed juice. There is no consensus of which of the chemical constituent(s) should serve as a standardisation marker (Upton, 2007).
Table 2: Echinacea extraction and preparation methods (Upton, 2007).
|
Preparation type |
Description |
|
Pressed/expressed juice |
Direct pressure is applied to the fresh flowering aerial parts of E. purpurea herb (leaf stem, flowers). The juice is stabilised with 20-22% v/v ethanol. |
|
Dried pressed/expressed juice |
Dried encapsulated form of the pressed juice. |
|
Herb or root extract |
The flowering aerial parts of E. purpurea herb (leaf stem, flowers) or the roots of all three medicinally used species (E. purpurea, E. angustifolia, E. pallida) are macerated and extracted with 60% v/v ethanol with optimal DER (drug extract ratio) 6-8:1. The liquid or dry encapsulated form of the extract is used medicinally. |
|
Powdered root or herb |
Roots or herb are dried and powdered. |
Existing authorisations for Echinacea products in the UK
12. Herbal products containing E. purpurea (L.) Moench. (EMA 2014), E. angustifolia DC, radix (EMA 2012) and E. pallida (Nutt.) Nutt., radix have herbal medicinal licences in EU/EEA member states. In the UK, there are a range of Echinacea products holding a Traditional Herbal Registration (THR) from the MHRA under the THR scheme (for the list of products see Table 10 Annex B). These products have been approved for the relief of the common cold symptoms and influenza type infections, symptomatic relief of minor skin conditions such as spots, pimples, and blemishes and relief of minor urinary complaints associated with cystitis in women based on traditional use only in adults and children over 12 years for maximum duration of 10 days. None of these products are recommended for pregnant or lactating women. Although Echinacea dietary supplements are the focus of this paper, the products holding a THR are worth noting for reference to doses and preparations (for further information on doses and preparations of THR Echinacea products and EMA monographs please see Table 11 Annex B).
13. For a product to be granted a THR, it should meet the required standards relating to its ‘quality, safety, evidence of traditional use and other criteria as set out in the Human Medicines Regulations 2012 (HMR, 2012)’. The evidence of traditional use relates to the product having been in traditional medicinal use for a continuous period of at least 30 years, of which at least 15 years must be within the European Union (Part 7 HMR, 2012). The safety requirements are a bibliographic review of safety data together with an expert report on safety (Schedule 12, HMR, 2012).
14. The MHRA confirmed in recent correspondence with the FSA that Echinacea products with THR are not recommended for use in pregnancy or lactation due to insufficient data available rather than any evidence of adverse effects. MHRA also stated that since there is no established threshold below which side effects are not expected to occur, they would expect any herbal product being marketed as a food supplement to carry the same warnings and precautions on the labelling as stated in the leaflet accompanying THR products. The MHRA have also stated that they don’t actively monitor the market for products which should have a THR but are sold without one. Therefore, it is possible that there are Echinacea products on the market sold as food supplements but would be regarded as medicinal products and require a THR.
EMA assessment reports and conclusions
15. The EMA has published detailed assessment reports on three medicinally used species: E. purpurea (L.) Moench. (EMA, 2014), E. angustifolia DC, radix (EMA, 2012) and E. pallida (Nutt.) Nutt., radix (EMA, 2018). PubMed and Toxline were searched for E. purpurea and E. pallida, whilst the search strategy for E. angustifolia is not specified. There were 1,325 references identified for E. purpurea, screened manually and the relevant ones were included in the assessment report (EMA, 2014). For E. pallida there was a total of 54 references identified and all were included in the assessment report (EMA, 2018).
16. Tests on reproductive toxicity, genotoxicity and carcinogenicity had not been performed for preparations of E. pallida (EMA, 2018) or E. angustifolia (EMA, 2012) at the time the EMA reports were written. In the absence of these data, the use of these species in pregnancy and lactation was not recommended by EMA. Due to the lack of genotoxicity data, the EMA did not recommend the addition of E. pallida (EMA, 2018) and E. angustifolia (EMA, 2012) to the Community list of herbal substances, herbal preparations and combinations thereof for traditional medicinal products. There was also insufficient clinical data to support the criteria for well-established medicinal use of E. angustifolia and E. pallida roots, in accordance with Directive 2001/83/EC. The traditional use of E. angustifolia and E. pallida root extracts for the relief of common cold symptoms was deemed as acceptably safe due to longstanding history of use without reports of serious adverse effects.
17. E. purpurea is on the Community list of herbal substances, herbal preparations and combinations thereof for traditional medicinal products based on traditional topical use for the treatment of small superficial wounds (HMPC, 2007). The benefit-risk assessment, conducted by EMA, concluded that there was sufficient clinical evidence to support the well-established medicinal use, in accordance with Directive 2001/83/EC, of expressed juice preparations from E. purpurea fresh herb for the short-term prevention (maximum 10 days) and treatment of common cold in adults and children over the age of 12 (EMA, 2014).
18. No genotoxic or mutagenic effects have been observed in bacterial reverse mutation tests, human lymphocyte assay and micronucleus assay with lyophilised E. purpurea (EMA, 2014). There was limited epidemiological data suggesting no adverse effects associated with oral E. purpurea use and pregnancy outcomes (EMA, 2014). However, EMA did not recommend its use (both topical and oral) during pregnancy and lactation due to the lack of guideline conforming preclinical data on reproductive and developmental toxicity.
Health-based guidance values (HBGV)
19. There are currently no health-based guidance values (HBGV) with respect to Echinacea or its constituents.