Discussion paper on the potential health effects of Echinacea in the maternal diet
Introduction
In this guide
In this guide1. The Scientific Advisory Committee on Nutrition (SACN) last considered the maternal diet and nutrition in relation to offspring health in its reports on ‘The influence of maternal, foetal and child nutrition on the development of chronic disease in later life’ (SACN, 2011) and on ‘Feeding in the first year of life’ (SACN, 2018). In the latter report, the impact of breastfeeding on maternal health was also considered. In 2019, SACN agreed to conduct a risk assessment on nutrition and maternal health, focusing on maternal outcomes during pregnancy, childbirth and up to 24 months after delivery.
2. SACN agreed that, where appropriate, other expert committees would be consulted and asked to complete relevant risk assessments. A provisional list of chemicals was proposed by SACN Members. However, this was subject to change following discussion by the COT. A scoping paper was presented to the Committee (TOX/2020/45) to define the scope of the work from a toxicological safety perspective and request their input on the selection of candidate chemicals or chemical classes that could be added or removed.
3. As part of this work, the Committee decided it would be useful to consider the use of dietary supplements during pregnancy. A scoping paper (TOX/2020/51) was presented, reviewing the dietary supplements commonly used during pregnancy. These supplements are not officially recommended by relevant health and regulatory authorities but are promoted by anecdotal evidence and unofficial sources as having various purported benefits.
4. The review was confined to herbal dietary supplements which would be regulated under food law, as opposed to traditional herbal medicines, which are overseen by the Medicines and Healthcare Products Regulatory Agency (MHRA). Following this review, the COT suggested that Echinacea required further investigation, noting that both human and animal in vitro and in vivo data were available. The main areas of concern included general toxicity to the mother, effects on the development of the foetus or embryo and possible interactions with drugs.
5. Based on the COT’s recommendations, a more extensive literature search was undertaken to evaluate the safety of Echinacea use during pregnancy and is presented below (for full details of the search method, see Annex A).
Background
In this guide
In this guideUses
6. Echinacea is a genus of herbaceous flowering plants, comprised of ten species and originally native to North America (Ahmadi et al., 2024). Three Echinacea species (Echinacea purpurea, Echinacea pallida, and Echinacea angustifolia) are used medicinally for the prevention and treatment of the common cold, influenza, and upper respiratory tract infections (Ardjomand-Woelkart and Bauer, 2015). E. purpurea is the most widely used and extensively studied of the three. Prior to 1968, Echinacea angustifolia and Echinacea pallida were considered to be different varieties of the same species until a revision of the genus described them as two separate species (WHO, 1999).
7. Echinacea herbal products are often sold as dietary supplements to boost the immune system and to reduce the symptoms of common cold and upper respiratory tract infections. These are popular products in North America and Europe, generating more than 300 million USD annually in the U.S. alone (Ahmadi et al., 2024).
8. Complementary and alternative medicines (CAM), including herbal and traditional preparations, are often perceived as safer than conventional medicines. Women are the highest consumer of CAM and survey data suggests that their use continues during pregnancy as women may turn to ‘natural alternatives’ due to fear of teratogenicity from conventional medicines (Hall et al., 2011). The most commonly used herbal preparations during pregnancy include raspberry leaf, ginger, chamomile, Echinacea and cranberry (Cuzzolin et al., 2010; Hall et al., 2011; Holst et al., 2011; Nordeng et al., 2011).
9. Echinacea extracts are used for respiratory infections (colds and flu, bronchitis, strep throat, toothache), urinary tract infections, skin disorders (Staphylococcus infections, cold sores, ulcers, wounds, burns, insect bites, eczema, allergies) and rheumatoid arthritis (Hudson, 2012). Between 4.3% (Holst et al., 2011) and 9.2% (Cuzzolin et al., 2010) of pregnant women report using Echinacea during pregnancy for the treatment of cold and flu, boosting the immune system and the prevention of common cold (Cuzzolin et al., 2010; Holst et al., 2011).
Constituents and preparations
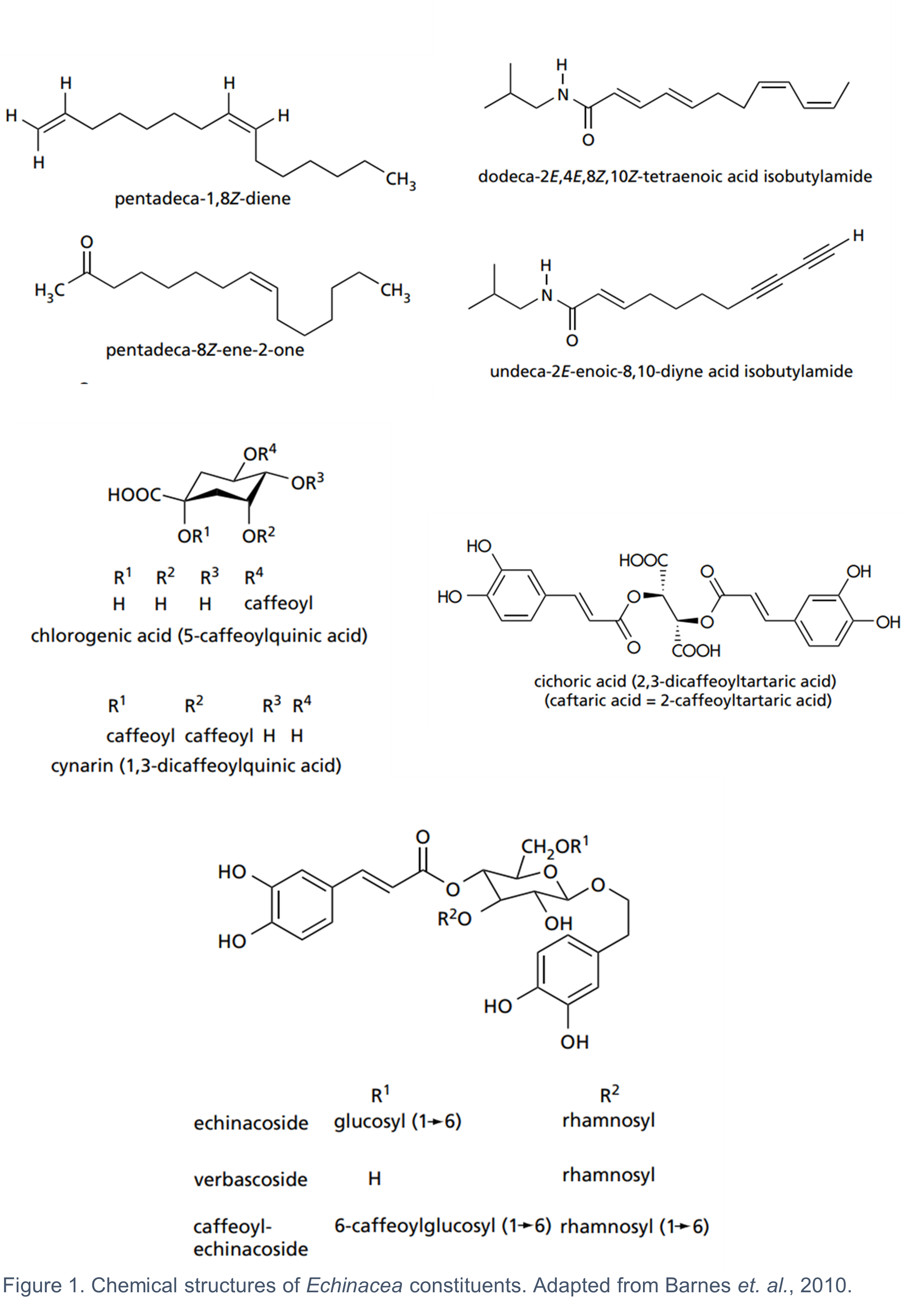
10. The fresh or dried aerial parts and the fresh pressed juice from the flowering tops of E. purpurea, as well as the whole plant, and the dried roots of all three species are used medicinally (Barnes et al., 2010). The composition of bioactive metabolites varies across the three species and their respective plant parts (Table 1). It is generally considered that there is no single constituent or group of constituents responsible for the activity of Echinacea. The combined effects of several groups of bioactive constituents, including the alkylamides, caffeic acid derivatives, polysaccharides and alkenes, contribute to the biological activity of Echinacea (Barnes et al., 2010). It is worth noting that some of the key pharmacological effects of Echinacea such as its antitumor, antioxidant, antimicrobial, antifungal, antiviral, and immunomodulatory activities are mainly attributed to the caffeic acid derivatives and alkylamides (Ahmadi et al., 2024). The chemical structures of some of the most common Echinacea constituents are shown in Figure 1.
Table 1: Major constituents of medicinally used Echinacea species (adapted from (Barnes et al., 2010).
|
Species |
Plant part |
Constituents |
Comment |
|
E. purpurea |
Aerial parts |
Alkylamides, caffeic acid derivatives (mainly chicoric acid), polysaccharides, polyacetylenes. |
Echinacoside is absent. |
|
E. angustifolia |
Roots |
Alkylamides, caffeic acid derivatives (mainly echinacoside), cynarin, polysaccharides, polyacetylenes. |
Cynarin is characteristic of E. angustifolia. |
|
E. pallida |
Roots |
Caffeic acid derivatives (mainly echinacoside), polysaccharides, polyacetylenes. |
Alkylamides are largely absent. |
11. The concentration of the different bioactive constituents in Echinacea preparations is not only affected by the species, plant part, season and growing conditions, but also by the extraction method employed. Different methods of extraction are used for preparing the Echinacea products (Table 2) and the final products can contain powdered plant parts, dry and liquid extracts, pressed and dried pressed juice. There is no consensus of which of the chemical constituent(s) should serve as a standardisation marker (Upton, 2007).
Table 2: Echinacea extraction and preparation methods (Upton, 2007).
|
Preparation type |
Description |
|
Pressed/expressed juice |
Direct pressure is applied to the fresh flowering aerial parts of E. purpurea herb (leaf stem, flowers). The juice is stabilised with 20-22% v/v ethanol. |
|
Dried pressed/expressed juice |
Dried encapsulated form of the pressed juice. |
|
Herb or root extract |
The flowering aerial parts of E. purpurea herb (leaf stem, flowers) or the roots of all three medicinally used species (E. purpurea, E. angustifolia, E. pallida) are macerated and extracted with 60% v/v ethanol with optimal DER (drug extract ratio) 6-8:1. The liquid or dry encapsulated form of the extract is used medicinally. |
|
Powdered root or herb |
Roots or herb are dried and powdered. |
Existing authorisations for Echinacea products in the UK
12. Herbal products containing E. purpurea (L.) Moench. (EMA 2014), E. angustifolia DC, radix (EMA 2012) and E. pallida (Nutt.) Nutt., radix have herbal medicinal licences in EU/EEA member states. In the UK, there are a range of Echinacea products holding a Traditional Herbal Registration (THR) from the MHRA under the THR scheme (for the list of products see Table 10 Annex B). These products have been approved for the relief of the common cold symptoms and influenza type infections, symptomatic relief of minor skin conditions such as spots, pimples, and blemishes and relief of minor urinary complaints associated with cystitis in women based on traditional use only in adults and children over 12 years for maximum duration of 10 days. None of these products are recommended for pregnant or lactating women. Although Echinacea dietary supplements are the focus of this paper, the products holding a THR are worth noting for reference to doses and preparations (for further information on doses and preparations of THR Echinacea products and EMA monographs please see Table 11 Annex B).
13. For a product to be granted a THR, it should meet the required standards relating to its ‘quality, safety, evidence of traditional use and other criteria as set out in the Human Medicines Regulations 2012 (HMR, 2012)’. The evidence of traditional use relates to the product having been in traditional medicinal use for a continuous period of at least 30 years, of which at least 15 years must be within the European Union (Part 7 HMR, 2012). The safety requirements are a bibliographic review of safety data together with an expert report on safety (Schedule 12, HMR, 2012).
14. The MHRA confirmed in recent correspondence with the FSA that Echinacea products with THR are not recommended for use in pregnancy or lactation due to insufficient data available rather than any evidence of adverse effects. MHRA also stated that since there is no established threshold below which side effects are not expected to occur, they would expect any herbal product being marketed as a food supplement to carry the same warnings and precautions on the labelling as stated in the leaflet accompanying THR products. The MHRA have also stated that they don’t actively monitor the market for products which should have a THR but are sold without one. Therefore, it is possible that there are Echinacea products on the market sold as food supplements but would be regarded as medicinal products and require a THR.
EMA assessment reports and conclusions
15. The EMA has published detailed assessment reports on three medicinally used species: E. purpurea (L.) Moench. (EMA, 2014), E. angustifolia DC, radix (EMA, 2012) and E. pallida (Nutt.) Nutt., radix (EMA, 2018). PubMed and Toxline were searched for E. purpurea and E. pallida, whilst the search strategy for E. angustifolia is not specified. There were 1,325 references identified for E. purpurea, screened manually and the relevant ones were included in the assessment report (EMA, 2014). For E. pallida there was a total of 54 references identified and all were included in the assessment report (EMA, 2018).
16. Tests on reproductive toxicity, genotoxicity and carcinogenicity had not been performed for preparations of E. pallida (EMA, 2018) or E. angustifolia (EMA, 2012) at the time the EMA reports were written. In the absence of these data, the use of these species in pregnancy and lactation was not recommended by EMA. Due to the lack of genotoxicity data, the EMA did not recommend the addition of E. pallida (EMA, 2018) and E. angustifolia (EMA, 2012) to the Community list of herbal substances, herbal preparations and combinations thereof for traditional medicinal products. There was also insufficient clinical data to support the criteria for well-established medicinal use of E. angustifolia and E. pallida roots, in accordance with Directive 2001/83/EC. The traditional use of E. angustifolia and E. pallida root extracts for the relief of common cold symptoms was deemed as acceptably safe due to longstanding history of use without reports of serious adverse effects.
17. E. purpurea is on the Community list of herbal substances, herbal preparations and combinations thereof for traditional medicinal products based on traditional topical use for the treatment of small superficial wounds (HMPC, 2007). The benefit-risk assessment, conducted by EMA, concluded that there was sufficient clinical evidence to support the well-established medicinal use, in accordance with Directive 2001/83/EC, of expressed juice preparations from E. purpurea fresh herb for the short-term prevention (maximum 10 days) and treatment of common cold in adults and children over the age of 12 (EMA, 2014).
18. No genotoxic or mutagenic effects have been observed in bacterial reverse mutation tests, human lymphocyte assay and micronucleus assay with lyophilised E. purpurea (EMA, 2014). There was limited epidemiological data suggesting no adverse effects associated with oral E. purpurea use and pregnancy outcomes (EMA, 2014). However, EMA did not recommend its use (both topical and oral) during pregnancy and lactation due to the lack of guideline conforming preclinical data on reproductive and developmental toxicity.
Health-based guidance values (HBGV)
19. There are currently no health-based guidance values (HBGV) with respect to Echinacea or its constituents.
Mechanism of action
In this guide
In this guide20. The exact mechanism by which Echinacea preparations exert their beneficial effect on the treatment and prevention of common cold is not known. Antiviral, immunomodulatory and anti-inflammatory effects of Echinacea were demonstrated in in vitro, in vivo and human studies discussed in more detail below. However, their relevance to clinical efficacy is not known and exact pharmacodynamic mechanism cannot be established (EMA, 2014).
21. The information presented below collates the data available in the EMA reports on E. purpurea (L.) Moench. (EMA, 2014), E. angustifolia DC, radix (EMA, 2012) and E. pallida (Nutt.) Nutt., radix (EMA, 2018), as well as data from a literature search performed by the Secretariat. For further details on the literature search methodology, please see Annex A.
22. Where information was available about the bioactive components present in the particular Echinacea preparation used in the studies, this has been included in this paper.
In vitro and Animal Studies
Antiviral effects
23. The stems, leaves, and flowers of E. purpurea (L.) Moench were fractionated and the fractions evaluated for antiviral activity in vitro against herpes simplex virus (HSV) Type I, influenza virus and rhinovirus (Vimalanathan et al., 2005). Ground dried E. purpurea aerial parts were extracted with 70% ethanol and fractionated 3 times with equal parts n-hexane, resulting in n-hexane and hydroalcoholic fractions. The latter were fractionated twice with ethyl acetate. E. purpurea dried parts were also extracted with water either 40°C or 80°C and the extracts were filtered. H-1 cells, a subclone of HeLa cells that is particularly sensitive to rhinovirus replication, were used for the propagation of the influenza virus and rhinovirus, whilst HSV was propagated in Vero cells. Cytopathic effects (CPE) end point assay was used, with the end point defined as the highest dilution of extract giving complete elimination of viral CPE produced by 100 infectious units of virus (MIC100). Some of the experiments were designed to test for photosensitisers and the MIC100 was calculated in the presence and absence of light exposure. The chemical composition of the fractions was also determined and described below.
24. Echinacoside was absent from all the fractions, whilst cynarin was found in the 80°C water extract from the flower parts. The aqueous fractions from all aerial parts (total herb, flower, stem and leaves) contained caftaric, caffeic and chicoric acid; the concentration of these bioactive components was 3-40 times higher in the 80°C water fractions compared to the 40°C ones. All the aqueous extracts exhibited potent activity against HSV and influenza virus with MIC100 of 1.2-23.5 µg/mL and contained no photosensitiser compounds as determined by measurement of the MIC in the presence and absence of light exposure. The ethanol extracts of the total herb and stems/leaves and their ethyl acetate fractions were also active against HSV and influenza virus (MIC100 of 1.5-31.2 µg/mL) and contained photosensitising compounds. On the other hand, the ethanol extracts of the flower parts had only marginal antiviral activity (MIC100 of 100-125 µg/mL) with no photosensitising compounds. The crude ethanol extracts of the total herb, flower part and stems/leaves contained high levels of chicoric (2,879 – 7,340 µg/mL), caftaric (767-919 µg/mL), caffeic (220-421 µg/mL) and chlorogenic acid (45-208 µg/mL). Vimalanathan et al. (2005) stated that whilst other studies (Binns et al., 2002) attribute the antiviral activity of Echinacea to chicoric acid and other caffeic acid derivatives, there was no clear correlation between phenolic concentrations and relative activity observed, suggesting synergy between bioactive compounds and/or the presence of other antiviral compounds. The authors concluded that the aerial parts of E. purpurea contain multiple antiviral compounds, including more than one water-soluble compound, and at least one caffeic acid derivative, plus at least one ethyl acetate–soluble component that was a photosensitiser and was absent from the flower extract. In addition, none of the extracts tested were active against rhinovirus, which suggested a possible membrane target for the antiviral agents as the rhinovirus does not have a membrane, whilst HSV and influenza do.
25. A study with a similar design by the same authors evaluated the antiviral activity of E. purpurea, E. pallida and E. angustifolia root extracts in vitro (Hudson et al., 2005). The antiviral activity of E. purpurea roots was predominantly in the aqueous fractions with MIC100 of 1.2-2.5 µg/mL against HSV Type I and influenza virus compared to MIC100 of 88-500 µg/mL with the ethanol and ethyl acetate extracts. Again, no activity against rhinovirus was observed. Interestingly, the aqueous fraction had the lowest concentration of chicoric acid, caffeic acid and echinacoside, suggesting that the compounds responsible for the observed potent antiviral effects of the aqueous fraction are not the major phenolic constituents associated with previously reported antiviral activity of Echinacea. It was speculated that the antiviral activity of the E. purpurea root aqueous fraction was attributable to cell wall extractable polysaccharides or glycoproteins. In contrast, the aqueous fractions of E. angustifolia root had no antiviral activity, but the ethanol and ethyl acetate fractions had moderate activity against all three viruses, including rhinovirus. The authors stated that this correlated very well with the presence of alkylamides, but not with echinacoside, the principal phenolic constituent. None of the E. pallida fractions demonstrated any antiviral activity, consistent with the lack of alkylamides in these fractions. The study concludes that based on these results, commercial preparations of Echinacea roots can be expected to differ widely in their antiviral activities.
26. The antiviral effects and mode of action of a Echinaforce were investigated against influenza viruses human Victoria (H3N2) and PR8 (H1N1), avian strains KAN-1 (H5N1) and FPV (H7N7), and the pandemic S-OIV (H1N1) in Madin-Darby canine kidney cells (MDCK) or embryonated chicken eggs (PR8) (Pleschka et al., 2009). Echinaforce is a standardised E. purpurea root and herb preparation derived by ethanol extraction of freshly harvested E. purpurea herb and roots (95:5). The preparation contains caftaric acid (264.4 µg/mL), chlorogenic acid (40.2 µg/mL), chicoric acid (313.8µg/mL), echinacoside (6.9 µg/mL), chlorogenic acid (40.2 µg/mL) and alkylamides (PID 8/9 at 36.3 µg/mL) in 65% ethanol concentration (Sharma et al., 2009). The MIC100 of the Echinacea extract was determined using cytopathic effects (CPE) end point assay, which involves incubating the cells with serial dilution of the herb extract and the virus until CPE are complete in the control wells containing untreated virus. The MIC100 was defined as the maximum dilution of the Echinacea at which CPE were inhibited. Concentrations of Echinacea ranging from the recommended oral daily dose of 1.6 mg/mL to 1:1,000 dilution (1.6 µg/mL) achieved over 99% inactivation of the H3N2 virus with a viral load of 105 PFU/mL. The authors state that similar results were obtained with human H1N1 and avian FPV (H7N7), but the data is not shown. It was noted that direct contact between the virus and the Echinacea extract was required for the inhibitory effect since Echinacea treatment of cells before or after the virus infection resulted in substantially less inhibition than pre-incubation of the virus particles with the extract. The authors conclude that the anti-viral effect of Echinacea manifests at a very early stage in the infection process.
27. This was confirmed by a hemagglutination assays, which demonstrated inhibition of viral hemagglutinin by Echinacea leading to prevention of the entry of virus into the cells (Pleschka et al., 2009). The interaction between viral hemagglutinin and cellular sialic acid containing receptor is the first step of influenza virus entry in the cell. The receptor binding can be measured by the ability of the virus to agglutinate chicken erythrocytes. The assay was performed by pre-incubating 5 concentrations of Echinacea extract with the virus prior to the addition of chicken erythrocyte suspension. Plates were the incubated for a further 1 or 4 hours and the wells were visually inspected for presence or absence of hemagglutination. E. purpurea inhibited the hemagglutination activity of the avian strains KAN-1 (H5N1), FPV (H7N7) and the pandemic S-OIV (H1N1) in a concentration and time-dependent manner.
28. The same study also determined the production and intracellular localisation of viral ribonucleoprotein (RNP) in MDCK cells infected with KAN-1 (H5N1), in the presence and absence of Echinacea treatment (Pleschka et al., 2009). The intracellular production, pattern of migration and localisation of the viral RPN were similar between untreated virally infected cells and cells treated with Echinacea after infection. However, the overall number of cells positive for viral RNP was significantly reduced when cells were infected with Echinacea treated virus. This suggests that Echinacea treatment is effective at a very early stage before the virus enters the cells. Once the viral particles are in the cells, the replication and spread are not affected by the treatment.
29. The antiviral activity of a Echinaforce, standardised ethanol extract of freshly harvested E. purpurea herb and roots, was evaluated in vitro against influenza (strain H3N2, human isolate), HSV Type I, rhinovirus types 1A and 14, adenovirus types 3 and 11, respiratory syncytial virus (RSV) and feline calicivirus (FCV) (Sharma et al., 2009). This preparation has high levels of caftaric and chicoric acid, moderate levels of chlorogenic acid and echinacoside, but no caffeic acid or cynarin (see paragraph 25 for composition of Echinaforce preparation). Antiviral activity was tested using the CPE end-point assay with either pre-incubating the extract with the virus prior to adding it to the cells for calculating virucidal MIC100 or incubating the cells with the extract before adding the virus for calculating the intracellular MIC100. The influenza virus and HSV were very sensitive to Echinacea with virucidal MIC100 < 1.0 μg/mL.and so was the RSV with MIC100 2.5 μg/mL. The non-membrane viruses rhinovirus, adenovirus and feline calicivirus resistant to the highest concentration tested (800 μg/mL), although the rhinoviruses showed partial inhibition of CPE at this concentration. The intracellular MIC100 values were two orders of magnitude higher than the virucidal MIC for HSV, influenza and the RSV indicating that the Echinacea had a direct virucidal effect on membrane containing viruses, rather than inhibition of virus replication.
30. The study also tested the ability of the Echinacea extract to inhibit the induction of cytokines in response to a viral infection in human bronchial epithelial cells (BEAS-2B cells), A549 cells, and primary human skin fibroblasts (Sharma et al., 2009). Viruses were added to the cells at 1 pfu/cell for 1 h, followed by the addition of Echinaforce at 160 μg/mL. Culture supernatants were then harvested at 24 and 48h post infection and the cytokine concentration of IL-6, IL-8 and TNF-α determined by ELISA. A fluorescent antibody array assay was also performed for 20 cytokines and inflammation-related mediators. The Echinacea preparation was effective at inhibiting the induction of IL-6, IL-8 and TNF-α by the virus infection with all viruses tested in the three human cell lines.
31. The antiviral and immunomodulatory properties of E. purpurea aerial parts extracts were evaluated in female C57BL6 mice intranasally infected with 500 PFU influenza A/WSN/33 (H1N1) strain (Fusco et al., 2010). The E. purpurea aerial used in this study contained significant amounts of polysaccharides and little to none of other known immunomodulatory components (alkylamides, chicoric acid and cynarin). In total 59 mice were used in the study and split into 4 groups: infected, untreated (n=24), infected, treated (n=16), uninfected, treated (n=9) and uninfected, untreated (n=10). E. purpurea aerial parts including leaves, stems and flower heads were homogenised, extracted with 20% aqueous ethanol, the extract lyophilised and dissolved in deionised water. The treated groups received 10 mg of this preparation administered daily by oral gavage for 5 consecutive days starting on the day of the infection. The mice were weighed daily, viral titres and cytokine analysis were performed throughout the study.
32. The authors reported that Echinacea treated mice had better clinical outcomes than untreated mice after influenza infection based on the percentage body weight change, which was used as a surrogate marker for disease severity. The weight loss caused by influenza infection was attenuated by Echinacea administration with the mean weight loss being significantly reduced in treated vs untreated mice in repeated measures analysis (p < 0.001). The viral titers in lung homogenates of treated and untreated mice were measured on days 2-7 post-infection. No significant differences in the viral titers were observed between the Echinacea treated and untreated mice. In the absence of influenza infection, mice treated with Echinacea had similar serum and lung cytokine profiles compared to untreated mice. In the influenza infected mice, there was a significant decrease in the serum IL-10 (73%, p = 0.015), serum IFN-γ (74%, p = 0.01) and lung IL-10 (67%, p = 0.003) in Echinacea treated compared to untreated mice on day 7 post-infection. Levels of IL-1β, IL-2, IL-4, and TNFα were similar between treated and untreated mice. The authors concluded that that Echinacea improves the clinical outcomes of influenza infection in mice by modulating the immune response rather than exerting a direct antiviral effect.
33. There have been several recent studies evaluating the antiviral effect of Echinacea against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). The antiviral activity of Echinaforce preparation (see paragraph 25 for composition) was tested in vitro against common cold coronavirus 229E (HCoV-229E) and highly pathogenic coronaviruses (SARS-CoV-1, SARS-CoV-2 and MERS-CoV) (Signer et al., 2020). The virucidal and antiviral effects of Echinaforce against HCoV-229E were determined by pre-treatment of the virus particles or cells with the extract or post-infection treatment of the cells. The residual viral infectivity was determined using a limiting dilution assay to determine tissue culture infectious dose (TCID50), defined as the dilution of a virus required to infect 50% of a given cell culture. The results of the study showed that direct exposure of HCoV-229E to the extract led to a statistically significant permanent inactivation that could not be reverted by extensive washing at all the doses tested (1,10 and 50 μg/mL); complete inactivation of the viral infectivity was observed at 50 μg/mL. In contrast, pre-treatment of cells had no influence on HCoV-229E infectivity or replication. Post-treatment of the cells showed a small reduction in the virus titer at the highest concentration tested (50 μg/mL). The effects observed against MERS-CoV, SARS-CoV-1 and SARS-CoV-2 were comparable to HCoV-229E, with complete inactivation of virus infectivity after pre-treatment of the viral particles with 50 μg/mL Echinacea. This is consistent with previous studies reporting that a direct contact between Echinacea and the virus is required for the antiviral activity of the extract.
Immunomodulatory effects
34. The immunomodulatory properties of Echinacea and its constituents have been extensively studied and reviewed in the literature. Recent review articles report that Echinacea activated macrophages leading to increased cytokine production and phagocytosis, promotes dendritic cell maturation, activates natural killer (NK) cells and leads changes in the percentage of immune cell populations, including T lymphocytes and NK cells (Burlou-Nagy et al., 2022; Rondanelli et al., 2018). Some of the primary studies on the immune system effects of Echinacea are discussed in more detail below.
35. Commercial preparations of E. purpurea fresh and dried juice (EchinaFresh) were tested for their ability to induce cytokine production by human macrophages in vitro (Burger et al., 1997). EchinaFresh is Echinacea extract made from the aerial portion of E purpurea and standardized for a content of 2.4% soluble β-1,2-D-fructofuranosides. Human peripheral blood macrophages were collected from a 50-year-old female. The fresh (0.05-10 µg/mL) and dried (0.01-10 µg/mL) Echinacea juice was mixed with 1 × 106 macrophages and supernatants were collected at 18 h for IL-1 determination and at 36 and 72 h for TNF-α, IL-6 and IL-10 determinations enzyme-linked immunosorbent assay (ELISA). Experiments were performed in triplicate. Cells incubated in media only were used as negative control and cells incubated with 5 µg/mL bacterial lipopolysaccharide (LPS). Production of IL-1, TNF-α, IL-6 and IL-10 by the human macrophages at all concentrations of Echinacea tested was significantly higher than in the media only control (p < 0.05). A dose-response trend in the production of cytokines by Echinacea stimulated macrophages was also observed. The levels of cytokines induced by LPS fell within the range of those induced by Echinacea leading the authors to conclude that the immunostimulatory ability of E. purpurea pressed juice is comparable to the LPS endotoxin.
36. Various Echinacea preparations were tested in vitro for their effects on murine macrophages and human peripheral blood mononuclear cells (PBMCs) (Rininger et al., 2002). Echinacea samples were either dissolved in dimethyl sulfoxide (DMSO) or subjected to a simulated digestion protocol. Simulated digestion was performed with the Echinacea by adding 750 mg raw material to 15 mL simulated gastric fluid, incubating at 37°C in a shaker incubator for 2 h, neutralizing the gastric fluid and incubating with simulated intestinal fluid for a further 2h. Macrophage activation assays were performed by plating the cells (RAW267.7 murine macrophages) at a density of 1 × 106 cells/mL. The test agent was added after 24 hours and the supernatant was assayed for TNF-α, 24 h posttreatment, indicative of macrophage activation. Sample supernatants were taken at 24, 30, and 48 h post-treatment and assayed for cytokine production using an ELISA. Cell viability was routinely monitored by visual inspection and confirmed with a cell proliferation assay kit. Nitric oxide (NO) production was determined by assaying for the presence of nitrites (NO2) in culture medium. Lipopolysaccharide (LPS) from E. coli was also tested as a positive control inducer of cytokine production.
37. DMSO dissolved Echinacea did not stimulate the production of TNF-α and NO. Both the E. purpurea herb processed through simulated digestion and LPS (0.1 µg/mL) produced comparable time- and dose-dependent induction of TNF-α, NO, IL-1α, IL-1β, and IL-6, with TNF-α and NO being the most sensitive biomarkers. At 24 hours, levels of TNF-α and NO induced by Echinacea were significantly higher than the control at doses as low as 5 μg/mL (p < 0.001). The levels of IL-1α and IL-1β were significantly higher at Echinacea doses of 80 μg/mL and above (p < 0.01), whilst with IL-6 the significance was only observed at the highest dose tested (320 μg/mL). The TNF-α levels produced by E. purpurea herb stimulation peaked at approximately 30 h post-stimulation and then declined sharply by 48 h, whereas TNF-α levels from LPS stimulation did not drop dramatically. In contrast, IL-1β, IL-6, and NO continued to rise over the 48-h time-course with both LPS and Echinacea. These immunostimulant effects are in agreement with those reported by Burger et al. (1997), with the exception that Burger et al. (1997) found Echinacea pressed juice (EchinaFresh) to be more potent at stimulating macrophage cytokine synthesis than bacterial endotoxin LPS. Rininger et al. (2002) highlight that the immunostimulatory effects of Echinacea on murine macrophages observed in their study were more transient than with LPS and occurred at 3,200-fold higher concentrations. The authors (Rininger et al., 2002) also comment that it is unlikely for Echinacea preparations to have greater potency at stimulating macrophage cytokine than LPS without resulting in significant toxicities in vivo such as endotoxin shock. It must be noted that Burger et al. (1997) used LPS at 5 µg/mL, whilst Rininger et al. (2002) at a 50 times lower concentration of 0.1 µg/mL.
38. Different E. purpurea root and herb samples were also tested by Rininger et al (2002), but it was found that only 2/7 herb samples and 1/5 root samples activity were similar to the initial herb material, suggesting a high degree of variability between different preparations. Human PBMCs were also treated with the different Echinacea preparations and it was reported that Echinacea materials that stimulated TNF-α production in RAW264.7 macrophage cells significantly enhanced the viability of PBMCs in vitro, whilst preparations that did not stimulate macrophage TNF-α production did not enhance PBMC viability.
39. Echinacea extracts were studied for their ability to stimulate macrophage function in the lung and spleen of male Sprague-Dawley rats (Goel et al., 2002). E. purpurea aerial parts or roots were extracted with aqueous ethanol and four different fractions with different concentrations of chicoric acid, polysaccharide and alkylamides were produced. The authors determined a ‘basal dose level’ of each component through a survey of various products, but further details for the basal dose level determination are not available in the article. One of the fractions contained basal dose level of chicoric acid, polysaccharides and alkylamides, whilst the other fractions contained the three components at levels 3, 20 and 50 times the basal dose level. Male Sprague-Dawley rats were divided into 5 groups (n=6) and administered 100 µL of each fraction, plus a control, twice a day for 4 days via oral gavage. The animals were sacrificed, and alveolar macrophages were obtained from the animals by bronchoalveolar lavage, whilst the immune cells were isolated from the spleen by pressing them through nylon mesh. Phagocytosis assay was performed by adding latex beads to the alveolar macrophages in a ratio of 10 beads/cell and incubating for 1h at 37°C. The cells were washed and the percentage of infected cells and the phagocytic index (number of beads per infected cell) were determined by microscopic examination. Nitric oxide (NO) production by alveolar and spleen cells was determined by measuring nitrite production in colorimetric reaction using Griess reagent. Concentrations of TNF-α, IFN-γ and IL-2 in cell culture supernatant were measured using an ELISA.
40. The weight gain and food intake of the rats were unaffected by the oral administration of Echinacea for 4 days. The percentage of alveolar macrophages
in actively phagocytosis and the phagocytic index increased in a dose dependent manner with increase in the concentration of the three components in the Echinacea fraction. The phagocytic index obtained by treating the macrophages with the fractions containing 20 and 50 times the basal dose levels of chicoric acid, polysaccharides and alkylamides was significantly higher (p < 0.05) than the phagocytic index produced by the fractions containing the basal levels and 3 times the basal levels of these components. Dose related increases in the NO production and by alveolar macrophages were also observed with increasing the concentrations of the 3 components in the Echinacea fractions. The rats administered the fraction with 50 times the basal dose level resulted in an 80% increase (p < 0.05) in the NO production by the lung macrophages compared to the control. A dose-dependent increase in the TNF-α secretion from alveolar macrophages was observed with only the fractions containing 3 and 20 times the basal dose level of the three components as TNF-α produced from the 50 times fraction was not significantly different the basal level and the negative control. Increasing the concentrations of chicoric acid, polysaccharides and alkylamides in the Echinacea fractions led to increases in the release of TNF-α and IFN-γ by the spleen macrophages in a dose-dependent manner, but no effect on the IL-2 production was observed.
41. E. purpurea extracts have been shown to promote phenotypic and functional maturation of murine dendritic cells via modulating the activation of JNK (c-Jun N-terminal kinase), p38-MAPK (mitogen activated protein kinase), and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathways (Li et al., 2017). E. purpurea extract with a defined chemical composition of chicoric acid (3.045%), caftaric acid (1.575%), chlorogenic acid (0.065%), dodeca-2E, 4E, 8Z, 10E/Z-tetraenoic acid isobutylamide (1.635%) was used. Bone marrow-derived dendritic cells (BMDCs) were derived from femur and tibia of 6–8-week-old female C57BL/6 mice. The cells were treated with phosphate buffer saline (PBS), Echinacea extract or bacterial LPS for 48 hours and the expression of cell surface molecules was determined by flow cytometry. A phagocytosis assay was conducted to quantify the uptake of fluorescently labelled dextran by the BMDCs following pre-treatment with 400 μg/mL Echinacea for 48 h. A cytokine assay was also performed by pre-treating the cells with NF-κB, p38-MAPK or JNK inhibitor for 1 hour, followed by an incubation of the cells with 400 μg/mL Echinacea extract for 24 hours and analysing the supernatant for cytokines such as IFN-γ, IL-12p70, IL-10 and TGF-β1 using an ELISA.
42. Li et al. (2017) reported that the percentage of CD40, CD80, CD83 and CD86 markers, associated with antigen presentation and T-cell immunity induction, expression was significantly upregulated (p < 0.05) in the Echinacea treated (400 μg/mL) groups compared to controls. The endocytosis of the fluorescently labelled dextran was markedly reduced in the Echinacea treated cells. Similar results were obtained with LPS treatment of the cells. No significant cytotoxicity was observed in the BMDC following 100-400 μg/mL E. purpurea treatment for up to 48 hours. Echinacea treatment was also reported to increase the phosphorylation levels of the MAPKs JNK and p38 and increase the nuclear translocation of NF-κB subunit p65, suggesting that E. purpurea can activate these pathways in BMDCs. Cytokine assay results showed that Echinacea also increased the secretion of IFN-γ, IL-12, IL-10, and TGF-β1 by BMDCs. Moreover, pre-treating the cells with JNK pathway inhibitor decreased the production of IFN-γ by the Echinacea treated cells, whilst inhibition of p38-MAPK down-regulated IL-10 and TGF-β1 levels. NF-κB inhibition decreased the Echinacea-induced production of IFN-γ, IL-12 and TGF-β1, but not IL-10. The authors conclude that E. purpurea treatment promoted phenotypic maturation of the murine dendritic cells by up regulating the expression of key accessory molecules. Functional maturation was also observed via activation of the JNK, p38-MAPK and NF-κB pathways and subsequent cytokine production by the BMDCs.
43. E. purpurea extracts were evaluated ex vivo for their effects on NK cells from healthy patients and patients with either the chronic fatigue syndrome or the acquired immunodeficiency syndrome (See et al., 1997). PBMC were isolated from 20 healthy patients, 20 patients with chronic fatigue syndrome (CFS) and 20 patients with acquired immunodeficiency syndrome (AIDS). The dried E. purpurea was homogenised in Roswell Park Memorial Institute (RPMI) cell culture medium, filtered and used fresh on the day. The cytotoxicity of the extract was tested by incubating the PBMC with various concentrations of the extract and assessing the cells for viability using trypan blue staining. The NK-cell activity was assessed by adding the PBMC (effector cells) to NK sensitive cell line K562 (target cells) labelled with 51-sodium chromate at an effector: target ratio 40:1, 20:1, 10:1 and 5:1, followed by a 10-fold increase in concentrations of the Echinacea extracts from 0.001 to 1000 pg/mL or medium alone (negative control). After 4 h incubation at 37°C, radioactivity was determined by scintillation counting for 51Cr release and the cytotoxic activity calculated in terms of lytic units (LU), where one LU is defined as the number of effector cells required to achieve 20% specific lysis of 5 x 103 target cells per 107 effector cells. The antibody-dependent cellular cytotoxicity (ADCC) activity was also assessed by incubating 51-sodium chromate labelled target cells (K562) infected with human herpesvirus 6 (HHV-6) virus with high titers of polyclonal antibodies against HHV-6. Effector cells (PBMC) were then added in triplicate atex effector:target ratios of 40:1, 20:1, 10:1 and 5:1 with or without serial 10-fold concentrations of the E. purpurea extracts from 0.001 to 100 µg/mL.
44. Concentrations of E. purpurea of up to 1000 µg/mL were not associated with diminished viability of PBMC after 4 h from either normal controls or patients with CFS or AIDS. The NK cell function was significantly decreased for the 20 patients with CFS (p < 0.01) and AIDS (p < 0.001) compared to healthy controls. The addition of Echinacea at concentrations ≥ 0.1 µg/mL led to a significant increase in the NK cell activity from healthy patients and those with CFS and AIDS in a concentration dependent manner. In addition, the ADCC was significantly increased in all three patient groups when E. purpurea was present at concentrations ≥ 1 µg/mL in a concentration dependent fashion and statistical significance was greatest with the cells of AIDS patients in the presence of the highest concentration of Echinacea tested (100 µg/mL). The authors (See et al., 1997) concluded that increasing concentrations of E. purpurea enhance the immune function of PBMC in normal subjects and in patients with either CFS or AIDS.
45. The effects of E. purpurea extracts on the natural killer (NK) cells present in human PBMCs were examined using flow cytometry (Gan et al., 2003). E. purpurea was dissolved in water and filtered to prepare the Echinacea water soluble extract used in the study. Human PBMCs were treated with the Echinacea extract overnight and analysed for CD16, CD45, CD56, and CD69 markers by flow cytometry. The NK cell-mediated cytotoxicity was assessed by incubating various Echinacea concentrations with peripheral blood lymphocytes (effector cells) for 10 minutes and adding the target cells GL40 cells (2×105/mL), a fluorescently labelled K562 cell line which is sensitive to NK cell cytotoxicity. Effector: target ratios of 20:1, 10:1, 5:1 and 2.5:1 were used. Fluorescence is emitted when the target cells are alive, and the NK induced cytotoxicity was measured by flow cytometry and validated by the standard 51Cr release assay. Four separate experiments, with each experiment performed in triplicate, were conducted. The cytotoxic activity was represented in lytic units LU20/106 cells, where one LU20 represents the concentrations of effector cells that kill 20% of the target cells.
46. The study reports that E. purpurea extract increased the NK-mediated cytotoxic activity in a concentration dependent manner. At the maximum concentration of Echinacea used in the assay (10 µg/ml), there was a 93.3% increase in LU20 compared to the 121% increase observed with IL-2, the control activator of NK cell activity used. Treating the PBMCs with Echinacea extract decreased the frequency and mean fluorescence intensity of CD16 expression by the lymphocytes as a function of the Echinacea concentration used. In the CD16 positive populations, the frequency of CD69 expression increased as a function of the Echinacea concentration used and at 10 µg/ml Echinacea concentration over 90% of the CD16+ cells expressed CD69. The authors (Gan et al., 2003) concluded that water soluble extracts of E. purpurea lead to activation of NK cells marked by decrease in CD16 expression and an increase in CD69. In addition, the study considered Echinacea to be a potent activator of NK cytotoxic function.
47. The immunomodulatory effects of E. purpurea on human T-cells and their cytokine response were evaluated in vitro using human T-cell line Jurkat E6-1 (Fonseca et al., 2014). Fresh E. purpurea aerial parts were ground with water into a slurry, passed through a hydraulic press, the filtrate mixed with 70% ethanol and freeze dried to produce fine powder. The mono and polysaccharide composition of the extract, along with the total amino acid content and the concentrations of bioactive small molecules such as cynarin, chicoric acid, caftaric acid and (2E)-N-isobutylundeca-2-ene-8, 10-diynamide were determined. The human T-cell line Jurkat E6-1 (0.5 × 106 or 5 × 106 cells/mL) was incubated with Echinacea, chicoric or caftaric acids for 40 min at 37 °C, followed by another incubation with/without phorbol 12-myristate 13-acetate (PMA) and/or ionomycin for 24 hours. The cell culture supernatant was then assessed for secretion of IL-2 and IFN-γ using Beadlyte Human Multi-Cytokine Beadmaster Kit. The cells were evaluated for expression of the IL-2 receptor (CD25) using flow cytometry. All experiments were performed in triplicate.
48. The chromatographic analysis of the E. purpurea extract revealed that it contained several phenolic compounds, including chicoric and caftaric acids, as well as cynarin, but not alkylamides. The effects of Echinacea on cytokine secretion by T cells was dependent on the T-cell density. At high cell density, the IL-2 secretion in response to PMA and ionomycin was lower compared to low cell density conditions (p < 0.001). Pre-treatment with Echinacea had a greater impact on IL-2 secretion in high T-cell density than low density conditions. A dose-response relationship was observed with IL-2 secretion by the high-density T-cell group after E. purpurea treatment, with effect of the 250 μg/mL dose being significantly greater than the 100 μg/mL dose (p < 0.01). A similar pattern was observed with IFN-γ secretion, whereby low-density T-cells produced significantly more IFN-γ than high density ones. Treatment with both 100 μg/mL and 250 μg/mL doses of Echinacea caused a five-fold increase in the secretion of IFN-γ in the high-density condition, whilst a small suppression in the IFN-γ secretion was observed in the low-density population. Echinacea treatment had no effect on the expression of the IL-2 receptor CD25 in high density conditions, but in the low density conditions it reduced the percentage of T cells expressing CD25 ( p < 0.001). Neither chicoric nor caftaric acid showed effects on IL-2 or IFN-γ response by the T cells leading the authors to conclude that the principle source of T cell enhancement in the study was the polysaccharide component of the preparation. (Fonseca et al., 2014)
49. Alcohol extracts of E. purpurea, E. angustifolia and E. pallida were investigated for immunomodulating properties in mice (Zhai et al., 2007). E. angustifolia, E. pallida, and E. purpurea root extracts were prepared by extracting the ground powder with ethanol and evaporating to dryness. Eight-week-old male BALB/c mice were split into five groups with groups 1-3 dosed with Echinacea preparations by gavage, group 4 dosed with vehicle control by gavage and group 5 serving as a no gavage group. The Echinacea preparations were given at a dose of 130 mg/kg of body weight once daily for 7 consecutive days and the authors state that this regimen was chosen based on the extrapolation of dose recommended for humans (4 g of powder/day for an average 65 kg human for 1 week). The mice were weighed at the beginning and end of the study, euthanised and the blood was collected for haematological analysis. The spleen was removed, weighed and the splenocytes were enumerated and analysed for CD19+ and CD49+ subsets using flow cytometry. The splenic NK cell cytotoxicity was assessed using the chromium (51Cr) release assay with three effector/target cell ratios, 25:1, 50:1, and 100:1, assessed in triplicate.
50. The study (Zhai et al., 2007) reports differences in the levels of phytochemicals in the three Echinacea preparations. Echinacoside was found to be the main caffeic acid derivative in preparations of both E. angustifolia and E. pallida, whereas no echinacoside was detected in the E. purpurea preparation. Chicoric acid was the main caffeic acid derivative in E. purpurea. Cynarin was detected in the E. angustifolia preparation, chlorogenic acid in the E. angustifolia and E. pallida preparations, and caftaric acid in the E. purpurea and E. pallida preparations. No adverse effects, weight differences or changes in behaviour were observed in the mice with the 7-day administration of the Echinacea preparations. No significant changes in any of the haematological parameters, including leukocyte number, red blood cell number and haemoglobin level, or the spleen parameters such as spleen weight, spleen-to-body weight ratio, and total spleen cell number per mouse, were observed with oral administration of any of the three Echinacea preparations as compared to the vehicle control and the no gavage control. However, the three Echinacea preparations significantly increased the percentage of lymphocytes in peripheral blood over the vehicle control group and the no gavage control (62.7% vs. 58.0%; p = 0.001). No significant differences were observed for other peripheral blood leukocyte populations such as neutrophils, monocytes, eosinophils, and basophils. Echinacea treatment groups also led to a significant increase in the percentage of the splenic lymphocyte subpopulation when compared to the vehicle control plus the no gavage control (83.3% vs. 81.1%; p = 0.004).
51. Seven days of oral administration of E. purpurea resulted in a significant increase in the percentage of both CD49+ and CD19+ splenic cells. The percentage of CD49+ cells, but not of CD19+ cells, was significantly increased in the E. angustifolia group compared with the vehicle control. No significant effect of E. pallida on splenic CD49+ and CD19+ subsets was seen. The authors (Zhai et al., 2007) stated that since Echinacea treatment altered the CD49+ subset in spleen, NK cell cytotoxicity analysis was covaried for the percentage of CD49+ cells measured in each individual. When compared to the vehicle control, only the E. pallida group demonstrated a significant increase in NK cell cytotoxicity (p < .035).
52. Mitogen-induced proliferation assay was also performed by Zhai et al. (2007) using whole spleen cells and whole blood cells using [3H]-thymidine incorporation in the presence or in the absence of bacterial lipopolysaccharide (LPS) or concanavalin A (Con A). The levels of IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12 (p40), IFN-γ and TNF-α were measured using ELISA in the cultures of mitogen-stimulated mouse splenocytes ex vivo. The proliferation of peripheral blood and splenic lymphocytes was not affected by Echinacea in the presence of LPS treatment. Con A stimulation of splenic lymphocytes from Echinacea treated mice led to a significant increase in their proliferation (p < 0.034), particularly for E. angustifolia and E. pallida, but had no effect on peripheral blood lymphocytes. On the other hand, there was a significant stimulation of baseline blood lymphocyte proliferation by the Echinacea treatment in comparison to both controls in non-mitogen stimulated lymphocytes, particularly for the E. angustifolia and E. pallida groups. The levels of TH2 cytokines (IL-4, IL-6, and IL-10) were mainly influenced by E. angustifolia and E. pallida treatment, with E. angustifolia and E. pallida leading to increased IL-4 (p = 0.046) and IL-10 (p = 0.057) production over the vehicle control in Con A stimulated spleen cells. E. angustifolia also significantly (p = 0.13) increased IL-4 production from non-mitogen stimulated spleen cells. The Echinacea treatment significantly increased TH1 cytokines (IFN-γ and IL-2) production in baseline cultures of splenocytes (p < .035) and IFN-γ production by Con A-stimulated splenocytes (p = 0.005), whilst only E. angustifolia produced a significant increase in IL-2 production in Con A-stimulated splenocytes (P = 0.037). The three Echinacea preparations significantly decreased the production of macrophage cytokines IL-1β (p=0.007) and TNF-α (p = 0.004) by LPS stimulated splenocytes. The authors commented that this is suggestive of anti-inflammatory activity and that the reduced production of IL-1β and TNF-α might be associated with the increased production of IL-4, as IL-4 supports differentiation of CD4+ cells into TH2-type cells and supresses the development of macrophage activating TH1 cells. It is worth noting that E. purpurea induced a significant increase in IL-1β production compared to the vehicle control (p = 0.006) in non-mitogen stimulated splenic lymphocytes.
Anti-inflammatory effects
53. Alkylamides from the roots of E. purpurea (L.) Moench were examined for anti-inflammatory activity in an in vitro cyclooxygenase-inhibition model system (Clifford et al., 2002). The E. purpurea roots were extracted using dichloromethane, followed by the addition of methanol with the methanol-soluble fraction concentrated under vacuum and dissolved in chloroform. Hexane was added and the supernatant, containing crude alkylamides, was separated through preparative high-performance liquid chromatography (HPLC). The alkylamides were tested for inhibition of both COX-I and COX-II using arachidonic acid as a substrate and monitoring the oxygen uptake. Aspirin, ibuprofen, naproxen, celecoxib and rofecoxib were used as positive controls. At 100 µg/mL, several E. purpurea alkylamides inhibited COX-I and COX-II enzymes in the range of 36-60% and 15-46%, respectively, as compared to controls. As a comparison, 80% inhibition of COX-1 was observed with aspirin, 70-90% inhibition of COX-2 by celecoxib and rofecoxib and 30-70% inhibition of both isoforms by ibuprofen and naproxen.
54. A study investigated the anti-inflammatory activity of Echinacea by testing 5-lipoxygenase (5-LOX)-inhibiting activity of root extracts of five wild and three commercially used species including E. purpurea, E. pallida and E. angustifolia (Merali et al., 2003). Their roots were extracted in ethanol, filtered and n-hexane added to the filtrate followed by a rotary evaporation of the solvent to 0.5 g/mL by dry root weight. Rat basophilic leukaemia cells (RBL-1) were incubated with the extracts for 15 minutes, stimulated with calcium ionophore and supernatants metabolite levels analysed by HPLC. The percent inhibition of 5-LOX was determined in comparison to leukotriene B4 (LTB4) standard, which is the product of 5-LOX. A vehicle control was run with each sample to determine the baseline level of enzyme activity. The root extracts of three commercial Echinacea species inhibited the 5-LOX activity with IC50 values of 0.444 µg/mL for E. angustifolia, 0.642 µg/mL for E. purpurea and 1.08 µg/mL for E. pallida. The 5-LOX inhibition was attributed to the presence of alkylamides in the Echinacea preparations.
Human Studies
55. A meta-analysis of randomised control trials concluded that Echinacea can potently lower the risk of recurrent respiratory infections and its immunomodulatory, antiviral and anti-inflammatory effects contribute to the observed clinical benefits (Schapowal et al., 2015). Systematic literature search identified 949 potentially relevant publications and after the exclusion of 848 pre-clinical/non-human studies, the remaining 101 abstracts were reviewed. Eighty-nine studies were excluded because they did not include respiratory tract infections as indications, studied pharmacodynamic effects, lacked appropriate placebo control, or had inappropriate endpoints. Twelve clinical studies on the use of Echinacea in the prevention of respiratory tract infections were reviewed and 6 were excluded due to low methodological quality (1), duplication (2) or investigating experimentally induced infections (3). The included studies varied in the Echinacea preparations and doses administered. Four studies employed ethanol/glycerol extractions from E. purpurea/E. angustifolia (500–4,000 mg extract/day), and two used pressed juices from E. purpurea (6,200–10,000 mg/day). Data on recurrent infections were available from all the six clinical trials included and a total of 2,458 participants, who received a variety of Echinacea extracts for up to 4 months. The cold symptoms were initially self-reported by patients during the observation period and then confirmed by physicians or study staff.
56. Pooling all the included clinical studies together yielded an overall relative risk (RR) of recurrent respiratory tract infection in the Echinacea treated groups of 0.649 (95% CI 0.545–0.774, p < 0.0001). The meta-analysis also looked at a sub-group of patients with increased susceptibility to respiratory tract infections with risk factors including exposure to stress (perceived stress score, PSS-10), being an active smoker, poor sleep, with presumed immune weakness due to low helper: suppressor T cells T4/T8 ratio <1.5, and a history of >2 colds/year. The risk for contracting a recurrent respiratory tract infection in these patients was reduced by treatment with Echinacea (RR = 0.501, 95% CI 0.380–0.661; p < 0.0001). Complications including conjunctivitis, sinusitis, otitis media/externa, tonsillitis/pharyngitis, bronchitis, and pneumonia were reported in three of the studies analysed. The overall complication incidence was effectively reduced by 50% with Echinacea (RR 0.503, 95% CI 0.384–0.658; p < 0.0001), with the reduction in pneumonia being the most prominent with 64.9% decrease. The authors of the meta-analysis (Schapowal et al., 2015) conclude that Echinacea is an effective option for the management of recurrent respiratory tract infections and their related complications. They state that people with presumed lower immune function and high susceptibility to infection may benefit most. They attribute the increased resistance to viral infections observed in the human studies to the reported immunomodulatory effects of Echinacea in in vitro and in vivo studies.
57. The results from five placebo-controlled randomised studies investigating the immunomodulatory activity of Echinacea extracts in volunteers are described and discussed (Melchart, 1995). A total of 134 (18 female and 116 male) healthy volunteers between 18 and 40 years of age were studied. Two of the studies used intravenous homeopathic preparation containing E. angustifolia administered once daily for 4-5 days. One study asked volunteers to take liquid ethanolic extracts of E. purpurea roots at doses of 333 mg three times daily (999 mg daily dose) for 5 days, whilst another study administered the ethanolic extracts of E. purpurea or E. pallida roots at 380 mg three times daily (1,140 mg daily dose) for 5 days. The last study used 70% liquid ethanolic extract of E. purpurea (95% herb and 5% root) at 800-900 mg three times daily (2,400 – 2,700 mg daily dose) for 5 days. The primary outcome measure for immunomodulatory activity was phagocytic activity of polymorphonuclear neutrophil granulocytes (PNG) measured using a microscopic method (2 studies) or cytometric methods (3 studies). The secondary outcome measure was the number of leukocytes in peripheral venous blood.
58. Melchart et al. (1995) report that two of the studies found a significantly enhanced phagocytic activity of PNG compared to placebo, whilst the other three studies did not observe a significant effect. The studies with significant findings involved the intravenously administered homeopathic preparation of E. angustifolia and the study with oral ethanolic extracts of E. purpurea root taken at 333 mg three times daily for 5 days, with maximal stimulation of phagocytic activity of 22.7% (95% CI 17.5-27.9%) and 54.0% (95% CI 8.4- 99.6%), respectively. Peripheral blood leukocyte number was not significantly changed in any of the studies. The review authors state that it is difficult to draw a conclusion on the effects of Echinacea on the PNG activity from these five studies due to the use of different methods for measuring phagocytosis, the small sample sizes and the lack of chemically defined standardised Echinacea preparations.
59. A human study with 10 healthy subjects (5 male and 5 female) evaluated the immunomodulatory effect of a standardised E. angustifolia root extract (Polinacea) by measuring the mRNA and protein levels of the cytokines IL-2, IL-8, IL-6 and TNF-α in plasma samples (Dapas et al., 2014). The subjects took 10 mL, equal to 100 mg E. angustifolia root extract, daily for 4 weeks. The syrup contained 4.7 mg/10 mL of echinacoside and 8.0 mg/10 mL of high molecular weight polysaccharides. Blood samples were taken once a week, with the first sample taken a week before the Echinacea supplementation started. Lympho-monocytes were isolated, their total RNA extracted, and quantitative PCR performed for cytokines IL-2, IL-8, and TNF-α gene expression. The protein levels of the cytokines in the blood samples were measured by flow cytometry. The expression levels of IL-2 and IL-8 were upregulated after Echinacea treatment, whilst a downregulation of the pro-inflammatory cytokines TNF-α and IL-6 occurred. The maximal differential gene expression for the cytokines was observed after 14 days of Echinacea treatment. The up-regulation of the IL-2/IL-8 gene expression and the downregulation of IL-6 detected at mRNA level positively correlated with the protein levels detected in the plasma. The authors acknowledge the study limitations such as small sample size and the lack of comparison to other Echinacea preparations.
Drug-herb interaction potential: effects on cytochrome P450 and P-glycoprotein
In this guide
In this guideIn vitro and animal studies
60. The in vitro inhibition potential of E. purpurea against baculovirus-expressed cytochrome P450 (CYP) 3A4, 2D6 and 2C9 enzymes was evaluated (Yale and Glurich, 2005). One capsule of EchinaCare containing 50 mg of 50:1 E. purpurea aerial parts extract was sonicated for 5 minutes in 70% methanol, the supernatant was collected and diluted 1:10. This was designated as 100% extract and used in the in vitro assay. High throughput screening was performed by incubating 1:3 serial dilutions of the 100% extract with the enzymes and substrate for 45 minutes prior to measuring the metabolite fluorescence. The assays were run in duplicate, and the 50% inhibitory concentration (IC50) was determined from the fluorescence values, signifying the percentage inhibition, against the extract concentration plots. The Echinacea extract showed virtually no inhibition against CYP2D6. There was a mild inhibition of CYP3A4 with IC50 equal to 75% extract. Maximum inhibition of CYP2C9 was seen at 33% of full test concentration, although inhibition did not cross the 50% threshold for IC50 determination.
61. Ethanolic extracts from E. purpurea were assessed for their ability to inhibit CYP 2E1 from human liver microsomes and an in vitro system baculovirus expression system (Raner et al., 2007). Fresh E. purpurea root was extracted with either 33% or 95% ethanol and 2µL of the extract was used in each 500 µL reaction. Eleven alkylamides were isolated from the Echinacea extract and individually tested for enzyme inhibition. The oxidation of p-nitrophenol was used to monitor the enzyme activity. The E. purpurea 95% ethanol extract yielded 30% inhibition in both human liver microsome and baculovirus derived CYP2E1. No inhibition was observed with the 33% ethanol extract. The authors noted that Echinacea extracts prepared using solvents with higher ethanol content have higher proportion of alkylamides, whilst the extracts prepared in ethanol/water mixtures have greater quantities of caffeic acid derivatives. The extracts with higher ethanol content exhibited higher inhibitory activity, suggesting that alkylamides rather than caffeic acid derivatives are likely inhibitory compounds in E. purpurea. No CYP2E1 inhibition was observed with two of the main caffeic acid derivatives, caftaric acid and chicoric acid, at concentrations up to 0.4 mM. The 11 individual alkylamides isolated from the 95% ethanol Echinacea extract showed inhibition against CYP2E1, with the four isobutylamides being most effective inhibitors achieving 40-60% inhibition at concentrations as low as 25 µM.
62. E. purpurea (L.) Moench Echinaforce extract was tested for its ability to inhibit baculovirus expressed CYP isoforms 1A2, 2C19, 2D6 and 3A4 in vitro (Modarai et al., 2010). A fluorescent based assay was used in three independent experiments run in duplicate and IC50 values were estimated by non-linear regression modelling (Hill model). Enzyme inhibition was observed at Echinaforce concentrations between 10 and 500 µg/mL, with IC50 values of 22 µg/mL (CYP3A4), 30 µg/mL (CYP1A2), 61 µg/mL (CYP2C19) and 69 µg/mL (CYP2D6). Nine commercially available Echinacea preparations, containing either E. purpurea, E. pallida or E. angustifolia, were screened for CYP3A4 inhibition. Their IC50 values varied by more than 50-fold, with the highest IC50 values of 824-1,812 µg/mL obtained with E. purpurea expressed juice and the lowest IC50 values of 12.7-27.8 µg/mL with tinctures of E. purpurea and E. angustifolia. The authors quantified the alkylamide content of the preparations and reported that the total alkylamide content was positively associated with the ability of the preparations to inhibit CYP3A4.
63. The in vitro effects of E. purpurea on CYP1A2, CYP2D6 and CYP3A4 in human primary hepatocytes was investigated (Hellum et al., 2007). Echinagard commercially available preparation was used at concentrations 4.735, 47.35 and 473.5 µg/mL. The human primary hepatocytes were obtained from a 32-year-old male donor and all serology data was normal. The cultures were exposed to selected inducers positive controls (omeprazole for CYP1A2, rifampicin for CYP2D6 and CYP3A4) or the Echinacea preparation for 48, 72 and 96 hours. Basal enzyme activity in the absence of inducers was also determined. The activities of the enzymes were measured by HPLC analysis for the production of selected metabolites: phenacetin demethylation for CYP2A1, dextromethorphan O-demethylation for CYP2D6 and 6β-hydroxylation of testosterone for CYP3A4. E. purpurea showed general inhibitory activity against CYP1A2, CYP2D6 and CYP3A4 at all concentrations tested when compared to the basal enzyme activity.
64. The same authors also investigated the effects of Echinagard on the inhibitory potential of baculovirus expressed CYP2D6 (Hellum and Nilsen, 2007). The enzyme was incubated with dextromethorphan substrate for 25 minutes at 37°C prior to the addition of the Echinacea extract, positive control inhibitor quinidine or buffer/ethanol negative control. The dextrorphan, dextromethorphan O-demethylated metabolite, was extracted and quantified by HPLC for determination of the enzyme activity. Echinacea showed a maximum of 28% inhibition of CYP2D6 at the highest concentration tested (473.5 µg/mL).
65. The effect of E. purpurea on the P-glycoprotein (P-gp) was studied in human intestinal Caco-2 cells (Hansen and Nilsen, 2009). Commercially available E. purpurea preparation was used at a concentration range 0.0064-6.36 mg/mL, anticipated to cover an in vivo concentration range of the herb, estimated from a daily dose of 265 mg dried E. purpurea juice dissolved in 1 L of gastrointestinal or 56 L of total body fluid (0.27– 0.005 mg/mL). Digoxin (30 nM) was used as a substrate and verapamil as a control inhibitor. A significant linear dose-related decrease in the net digoxin flux was observed at Echinacea concentrations above 0.4 mg/mL. The Vmax (23.7 nmol/cm2 /h) and Km (385 µm) of the net digoxin flux decreased in the presence of E. purpurea in an uncompetitive fashion. The authors conclude that E. purpurea extracts, in compositions equal to those present in commercial products, can interact with the P-gp mediated transport of digoxin in Caco-2 cells. They state that although the in vivo effects on systemic P-gp activity is probably limited, the potential of Echinacea to influence drug bioavailability cannot be excluded.
66. In a recent study, ethanolic extracts of 123 medicinal herbs, including Echinacea, were tested for their ability to induce CYP3A4 and CYP1A2 and their potential to inhibit P-gp (Husain et al., 2023). The Echinacea extract was prepared by extracting the dried plant material of E. angustifolia root in 95% ethanol, drying it and dissolving it in DMSO to prepare the stock solution. The cytochrome P450 inhibition assays were performed against baculovirus expressed human CYP3A4 and CYP2E1 by incubating the extracts with the enzymes and enzyme-specific substrates for 10 minutes and measuring the fluorescence of the product. Ketoconazole and α-naphthoflavone were used as positive controls for CYP3A4, and CYP1A2 isozymes, respectively. IC50 values were obtained from concentration-response curves generated by plotting percent inhibition versus tested concentrations. The P-gp inhibition was determined using a rhodamine-123 uptake assay in MDR1-MDCK cell line overexpressing P-gp. Various concentration of the Echinacea extract (12.5, 25, and 50 μg/mL), positive control (cyclosporin) and negative control (DMSO) were incubated with the cells for 90 minutes, lysed and the rhodamine-123 fluorescence was measured in the cell lysate. The E. angustifolia root extract was classed as a strong inhibitor of CYP3A4 with an IC50 value of 9 μg/mL, whilst no inhibition was observed against CYP1A2 up to the highest concentration tested (50 μg/mL). The extract exhibited a limited inhibition (>120% to <150%) in the rhodamine uptake assay and was therefore deemed to be a mild inhibitor of P-gp.
67. The effects of a standardised E. purpurea 60% ethanolic extract, containing 3.7% polyphenolic compounds expressed as caffeic acid, on the mRNA expression level of CYP1A1/2, CYP2D1/2, CYP3A1/2, CYP2E1, CYP2C6 in a rat model was investigated (Mrozikiewicz et al., 2010). Male Wistar rats were randomly divided into four groups from A to D (n = 10). Group A was treated once a day with 50 mg/kg oral E. purpurea extract for 3 days, and the control group B received a standard diet. Group C was treated with the same extract as group A, but for 10 days, and group D was used as control for group C. Total RNA was isolated from the rat liver tissue 16 hours after the administration of the last dose and level of gene expression in liver tissues was analysed by real-time quantitative PCR. The E. purpurea extract resulted in a potent inhibition of the expression of CYP3A1 (41%, p < 0.05) and CYP3A2 (25%, p = 0.001), and an induction of CYP1A1 (80%, p = 0.01) and CYP2D1 (40%, p = 0.007) after 10 days of treatment. The authors speculate that the inhibition of the expression of CYP3A1/2 may translate to in vivo inhibition of human homologs CYP3A4/5 and influence the efficacy of chemotherapy as CYP3A4 is important in the metabolism of anti-cancer drugs. The conclusions of the study also state that the induction of CYP2D1, the homolog to the human CYP2D6, can lead to loss of pharmacological effect of prescription drugs in humans by reducing their plasma concentration.
68. A congress abstract describes the administration of different preparations of E. purpurea, E. angustifolia and E. pallida to a total of 216 rats assigned to different experimental groups (n=12) with various dosages, positive controls (ketoconazole, quinidine), or pure compounds (dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides; tetraenes) (Ardjomand-Woelkart et al., 2012). After treatment with different Echinacea preparations, probe drugs for CYP enzymes were orally administered before blood sampling: theophylline (CYP1A2), tolbutamide (CYP 2C9), dextromethorphan (CYP2D6) and midazolam (CYP3A4). Significant inhibition of CYP1A2 were observed with some Echinacea preparations, weak inhibition of CYP2D6, but no inhibition of CYP3A4 and CYP2C9.
Human studies
69. The effects on E. purpurea root on CYP1A2, 2C9, 2D6 and 3A were assessed in 12 healthy volunteers (6 men and 6 women) aged 31 ± 6 years in a 2 week open-label study (Gorski, 2004). Participants were non-smokers, had no significant medical conditions and had not used herbal products in the 6 months prior to the start of the study. They were instructed not to consume any alcohol, caffeine, grapefruit products, apple or orange juice, cruciferous vegetables or charbroiled meats for at least one week before and until the end of the study. Single dose of CYP probe drugs were administered before and after a short course of a commercially available E. purpurea root extract (Nature’s Bounty) taken orally at 1,600mg/day for 8 days. This preparation contains greater than 1% phenols (caftaric acid, chlorogenic acid, echinacoside and chicoric acid). The following probes were used: caffeine (CYP1A2), tolbutamide (CYP2C9), dextromethorphan (CYP2D6), and midazolam (hepatic and intestinal CYP3A4) and their plasma-concentration profiles were determined before and after Echinacea supplementation.
70. The administration of Echinacea at 400 mg 4 times a day for 8 days was well tolerated, and no adverse events were reported by the volunteers or observed by the researchers. The Echinacea significantly reduced the oral clearance of caffeine, the CYP1A2 probe, by 27% (p=0.049) from 6.6 ± 3.8 L/h to 4.9 ± 2.3 L/h, indicating inhibition of the CYP1A2 enzyme and increased the maximum serum concentration of caffeine by 30%. Interindividual variability was observed as 2 men showed greater than 50% reduction in caffeine clearance. The authors concluded that the modest change in the clearance of compounds metabolised by CYP1A2 is considered clinically significant as this can lead to increased toxicity of narrow therapeutic window drugs such as theophylline, which is a substrate for CYP1A2. They also speculated that other drugs metabolised by CYP1A2 such as cyclobenzaprine, tacrine, and clozapine can be affected by Echinacea coadministration (Gorski, 2004).
71. The systemic clearance of intravenously administered midazolam, CYP3A4 probe, significantly increased by 34% (from 32 ± 7 L/h to 43 ± 16 L/h, p=0.003) and the AUC decreased by 23% (p=0.24). The administration of Echinacea resulted in a 43% increase (from 0.23 to 0.33, p =0.028) in the oral bioavailability of midazolam despite an observed reduction in its hepatic availability (15%, p =0.006). The authors concluded that this increase in oral bioavailability is mediated by a 2-fold increase (from 0.33 to 0.61, p=0.015) in the intestinal availability of midazolam, suggesting that Echinacea inhibits the intestinal CYP3A4 isoform. The changes in the intestinal and hepatic availability balanced out, resulting in an oral clearance and AUC of midazolam unchanged by the Echinacea dosing.
72. Echinacea administration also significantly reduced the oral clearance of tolbutamide, the CYP2C9 probe, from 0.81 ± 0.18 L/h to 0.72 ± 0.19 L/h (11%, p = 0.001), but had no significant effects on the maximum serum tolbutamide concentration reached. Two individuals (1 man and 1 woman) had a 25% or greater reduction in the oral clearance of tolbutamide. These results indicate an inhibition of CYP2C9 by Echinacea, but this was not considered to be clinically important as the geometric mean and the 90% confidence intervals (CIs) for AUC (area under the curve), oral clearance, and maximum concentration were within the default no-effect boundaries of 80% to 125% as per the criteria established by the Food and Drug Administration (FDA) in its industry guidance concerning in vivo drug interaction studies.
73. Echinacea dosing did not significantly influence the pharmacokinetic parameters (AUC, clearance, maximum serum concentration and half-life) of the CYP2D6 probe dextromethorphan, suggesting that co-administration of Echinacea is not likely to alter the metabolism of drugs metabolised by CYP2D6 (Gorski, 2004). This aligns with the results of another human study investigating the effects of botanical supplements on the CYP2D6 activity in 16 healthy volunteers, which reported that Echinacea did not have significant inhibitory effect on CYP2D6 (Gurley et al., 2008). All the subjects in that study were confirmed to be extensive CYP2D6 metabolisers, were nonsmokers and were not taking any botanical supplements or prescription medication. E. purpurea (Gaia Herbs, standardised to contain 2.2 mg isobutylamides per capsule) was administered for 14 days at 267 mg, three times daily. The CYP2D6 activity was assessed using 8-hour debrisoquine urinary recovery ratios using fluorescence detection.
74. Another human study with 12 healthy volunteers (6 men, 6 women) investigated the effects of E. purpurea (800 mg, twice daily) for 28 days on CYP1A2, CYP2D6, CYP2E1 and CYP3A4 phenotypes (Gurley et al., 2004). The composition of the Echinacea preparation was analysed using HPLC and it was determined that it contained 13.7 mg chicoric acid per capsule, providing a daily dose of 43.8 mg chicoric acid. All subjects were extensive metabolizers of CYP2D6 as confirmed by debrisoquin urinary recovery screenings. The mean age of the participants was 25 ± 3.0 years, they were nonsmokers, healthy and did not use botanical supplements. All subjects were asked to abstain from alcohol, caffeine, fruit juices, cruciferous vegetables, and charbroiled meat throughout the study. Caffeine and midazolam were used for CYP1A2 and CYP3A4 probes. To avoid potential interference from midazolam and caffeine, CYP2E1 and CYP2D6 phenotypes were assessed 24 hours later by administering oral chlorzoxazone (CYP2E1) and debrisoquin (CYP2D6). The CYP modulatory capability of Echinacea was evaluated by comparing individual differences in metabolite/parent serum ratios before and at the end of 28 days of supplementation.
75. No serious adverse events occurred during the course of the study; one subject experienced a mild rash develop while taking Echinacea. In this study, E. purpurea given over 28 days did not significantly change the activities of CYP3A4, CYP2E1, and CYP2D6 as estimated by comparing the phenotype ratios before and after treatment. Co-administration of E. purpurea caused an approximately 13% decrease in the ratio of paraxanthine/caffeine, suggesting that there was a possible inhibitory effect on CYP1A2 enzyme. However, the difference was not statistically significant and the authors did not think it was clinically relevant (Gurley et al., 2004).
76. The herb-drug interaction between E. purpurea and lopinavir-ritonavir was investigated in 13 healthy volunteers (8 men, 5 women) aged between 18-50 years (Penzak et al., 2010). Patients were healthy, had negative HIV test, were nonsmokers and were not allowed to take any medication within 30 days of study participation. Subjects were given single oral midazolam and fexofenadine to act as CYP3A4 and P-gp probes respectively. Seven to 28 days after midazolam and fexofenadine administration, subjects began taking lopinavir/ritonavir 400 mg/100 mg twice daily with meals. 15 days after the antiretroviral therapy initiation, E. purpurea fresh liquid extract 8:1 softgel capsules from a single batch (Echinamide Natural Factors) was initiated at 500 mg three times daily (1,500 mg daily dose) for 28 days. The extract formulation contained standardized amounts of alkylamides, polysaccharides, and chicoric acid. The patients continued taking the lopinavir/ritonavir for a further 14 days (total 29.5 days) with the last 2 weeks of the study consisting only of Echinacea dosing. At the end of the study, patients returned for repeat fexofenadine and midazolam administration. Neither lopinavir nor ritonavir were significantly influenced by the 2-week Echinacea co-administration. The AUC of midazolam was significantly decreased (p= 0.008), whilst its clearance was significantly increased (p=0.02) after the E. purpurea administration suggesting induction of the CYP3A4-mediated metabolism of midazolam. No effect on the fexofenadine pharmacokinetic parameters was observed. The authors explain the lack of change in the pharmacokinetic parameters of the CYP3A4 substrates lopinavir and ritonavir with ritonavir being a potent intestinal and hepatic CYP3A4 inhibitor, therefore masking any enzyme induction effects of Echinacea.
Toxicity Studies
In this guide
In this guideIn vitro and Animal Studies
Acute toxicity
77. Expressed juice from E. purpurea was administered either orally or intravenously to 8 week old Wistar rats and NMRI mice following the OECD guidelines for Good Laboratory Practice (GLP) and the OECD recommendations for technical methods at the time of the study (Mengs et al., 1991). Eight animals of each sex were given a single oral dose via gastric tube 15,000 mg/kg bw in rats and 30,000 mg/kg bw in mice. The intravenous dose was administered to eight animals of each sex via the tail vein at 5,000 mg/kg bw in rats and 10,000 mg/kg bw in mice. The animals were observed for 14 days and inspected several times daily and at the end of the experiment a necropsy with macroscopic inspection was performed. There were no deaths or any signs of abnormalities or toxicity due to the Echinacea. The authors concluded that a lethal dose cannot be found and LD50 was not calculated. Another study derived an estimated LD50 value 2,500 mg/kg bw from administering a mixture of polysaccharides from E. purpurea herb to 18 mice via intraperitoneal injection (Lenk 1989).
78. Acute toxicity study was performed with E. angustifolia following the OECD-423 criteria (Espinosa-Paredes et al., 2021). E. angustifolia was macerated, extracted with ethyl acetate and the extract was concentrated at low pressure in a rotary evaporator. The preparation contained 11.2 µg/mg echinacoside and 8.18 µg/mg caffeic acid. Three CD-1 male mice were administered 2,000 mg/kg bw of the extract and observed for 14 days to detect changes in behaviour and/or death. No signs of toxicity such as piloerection, mucosal irritation, motor activity alteration or death were recorded after the administration of Echinacea. There were no macroscopic morphological lesions to the lungs, kidneys, heart, stomach, intestines, spleen and liver of the animals. The authors classified the LD50 of the ethyl acetate E. angustifolia extract as Category 5 of the Globally Harmonized Classification System GHS (> 2,000–5,000 mg/kg), which means that it has a very low toxicity and could pose danger only to vulnerable populations.
Subacute toxicity
79. Expressed juice from E. purpurea was administered to groups of 18 Wistar rats per dose per sex at doses of 800, 2,400 or 8,000 mg/kg bw daily for 4 weeks via oral gavage (Mengs et al., 1991). During the study, food consumption was measured, and the rats were weighed once weekly. Biochemical and haematological parameters were determined at the start of the experiment and at weeks 2 and 4. At the end of the study the rats were submitted to an ophthalmological examination followed by necropsy. The organs were weighed, fixed in formalin and the histology was examined.
80. There was a statistically significant fall in plasma alkaline phosphatase in male rats at Echinacea doses 2,400 mg and 8,000 mg/kg compared to controls. A significant increase in prothrombin time was observed in female rats at doses of 2,400 and 8,000 mg/kg compared to controls. The authors concluded that since the alkaline phosphatase and prothrombin time were still in the normal physiological variation range for the rat strain used and there was no dose dependent response, no toxicological point of departure could be derived from the data. The other biochemical and haematological results, body weights, food consumption, ophthalmoscopy, necropsy findings and histology did not show any significant differences between the rat groups receiving the different Echinacea doses.
81. Espinosa-Paredes et al. (2021) conducted a 28-day repeated-dose toxicity study with the ethyl acetate extract of E. angustifolia described in paragraph 78. The extract was administered to five CD-1 mice per dose per sex at 20 mg/kg bw or 200 mg/kg bw. Serum asparate aminotransferase (AST), alanine aminotransferase (ALT) and creatinine levels were determined. Authors reported that there were no statistically significant differences suggesting liver or kidney damage due to the extract administration.
Sub-chronic toxicity
82. The toxicity of E. purpurea extract was evaluated in a 13-week repeated oral dose toxicity test in Sprague Dawley rats (Jeong et al., 2024). The experiments were performed in accordance with GLP Regulation and Test Guidelines of Standards for Toxicity Studies of Drugs issued by the Korean Food and Drug Administration. The protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of BiotoxTech. The dried aerial parts of E. purpurea were powdered and extracted with 60% ethanol, followed by filtration and concentration. The concentrated extract was spray-dried for 1 day and standardised to at least 2% chicoric acid content. The extract was administered at daily doses of 500, 1,000 and 2,000 mg/kg bw to 10 rats per dose for each sex for 13 weeks. Food and water intake, body weight and clinical signs were monitored. Rats were sacrificed at the end of the treatment period and samples were collected for serum biochemical, urinalysis, and histopathological evaluations.
83. During the 13-week repeated oral toxicity study no mortality or abnormal clinical signs were observed in either sex at any of the tested doses (Jeong et al., 2024). There were no significant differences observed in body weight changes and food intake between the treatment and control groups. The ophthalmological examinations revealed no signs of toxicity due to E. purpurea. The absolute organ weights and the organ-to-body-weight ratios at necropsy were not significantly different between the treatment and control groups for both sexes. The organ morphology was unremarkable. There were no significant differences in the haematology and serum biochemistry between the treatment and control groups for either sex. The urinalysis revealed some differences as the mean urine volume in the 1,000 mg/kg male rat group was significantly higher than in the controls. Some individual differences were also observed in the urinalysis, but they were not significantly different when compared to the controls.
Cytotoxicity
84. The cytotoxic effects of Echinacea flower extract and chicoric acid, bioactive constituent from Echinacea, were evaluated using human colorectal cancer cell lines HCT-116 and Caco-2 (Tsai et al., 2012b). The flowers of E. purpurea (L.) Moench were harvested from 6-month-old plants, freeze-dried, ground in a mill and sieved through 0.5 mm sieve. 10g of the powder was extracted with 50% ethanol, centrifuged, filtered, re-extracted with two more portions of 100 mL solvent. The solvent was then evaporated and freeze-dried with a vacuum to produce dry extracts. A previous study by the same authors showed that chicoric acid was the most abundant compound when E. purpurea was extracted with 50% ethanol (Tsai et al., 2012a). The other components of the preparation included caftaric acid, chlorogenic acid, echinacoside (Tsai et al., 2012a).
85. For the cell viability tests, the cell lines were treated with 0-2,000 µg/mL Echinacea extract and 0-200 µg/mL chicoric acid for 24-48 hours. The medium was removed, the cells were incubated for 2 h at 37°C with 5mg/mL MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. The resulting formazan was solubilised with isopropanol, the absorbance was measured at 570 nm and the percent viability was calculated. The DNA fragmentation and telomerase activity of the cells were also determined. (Tsai et al., 2012b)
86. No significant difference in Caco-2 and HCT-116 cell viability was observed when the cells were treated with the different concentrations (0–2,000 μg/mL) of Echinacea extract for 24 hours. At 48 hours, the cell viability of both cell lines reduced in a dose-dependent manner. At 24 hours the chicoric acid showed a significant reduction in cell viability when the concentrations tested increased to 150 and 200 µg/mL, whilst at 48 hours all concentrations tested (50-200 µg/mL) showed a significant reduction in cell viability. Treatment of HCT-116 with 50-150 µg/mL chicoric acid reduced the telomarse activity of the cells determined by telomeric repeat amplification assay. DNA fragmentation was also induced by chicoric acid in a concentration and time-dependent manner. Chicoric acid also led to caspase-9 activation and induced PARP cleavage at higher doses (100-150 µg/mL). The authors concluded that the possible in vitro cytotoxicity mechanism of E. purpurea extract is mediated by repression of telomerase activity, activation of caspase pathway and induction of apoptosis.
87. The cytotoxicity of ethyl acetate extract of E. angustifolia described in paragraph 77 was evaluated against two cancer cell lines (MDA-MB-231 ATCC HTB-26 and MCF-7 ATCC HTB-22) and a healthy breast epithelial cell line (MCF-10 ATCC) using an MTT cell viability assay (Espinosa-Paredes et al., 2021). The three cell lines (8 × 103 cells per well) were exposed to logarithmic concentrations (range 0.3-300 µg/mL) of the Echinacea extract for 24 or 48 hours. The absorbance was measured at 570 nm and the viable cell percentage was calculated. The assay was performed in triplicate in three independent experiments and the 50% inhibitory concentration (IC50) was calculated using GraphPad software. The E. angustifolia was not cytotoxic towards the healthy breast epithelial cell line (MCF-10 ATCC). The calculated IC50 of the extract towards the two cancer cell lines was between 16.3-28.8 µg/mL with no significant differences between the values obtained at 24 vs 48 hours.
Genotoxicity and Carcinogenicity
88. No genotoxic effects were observed in an in vitro bacterial reverse mutation assay, a mouse lymphoma assay, human lymphocyte assay and a micronucleus test performed by Mengs et al. (1991) using lyophilised E. purpurea expressed juice from the commercial product Echinacin Liquidum. The GLP OECD guidelines and the OECD recommendations for technical methods at the time of the study were followed. The details of the tests are described below.
89. E. purpurea was tested at concentrations from 8 to 5,000 µg/plate in an in vitro bacterial reverse mutation test in Salmonella typhimurium strains TA98, TA100, TA1535, TA1537, TA1538 with and without S9 metabolic activation (Mengs et al., 1991). The lyophilised Echinacea expressed juice, the bacteria and S9 mix were added to molten agar, mixed and poured onto minimal agar medium. 2-nitrofluorene 50 µg/plate, sodium azide 2 µg/plate, 9-aminoacridine 50 µg/plate and 2-aminoanthracene 5 µg/plate were used as positive controls, whilst dimethyl sulfoxide (DMSO) was used as the negative control. Plates were incubated at 37°C for 3 days and colonies were counted using an image analyser. The mean counts of 3 replicates were compared with the controls and the presence or absence of a dose response was determined by linear regression analysis. The results were judged significant where reproducible, statistically significant and dose-related increases in revertant numbers were obtained. No significant increase in the number of revertants was observed, with and without metabolic activation, up to the maximum tested concentration of 5,000 µg/plate.
90. Lyophilised Echinacea expressed juice was tested for its ability to induce mutations at the hypoxanthine phosphoribosyltransferase (HPRT) locus of L5178Y mouse lymphoma cells, both in the presence and absence of S9 metabolic activation (Mengs et al., 1991). Lyophilised Echinacea expressed juice was used at concentrations of 50, 158, 500, 1580 and 5000 µg/mL. Two independent experiments were performed with triplicate cultures, each containing at least 1 x 107 cells. The cells were exposed to the test or control solution for 2 hours, washed and resuspended for a 7-day expression period. At the end of the expression period cells were plated in the absence and presence of the 6-thioguanine selective agent and the mutation frequency was determined after 14 days incubation. The induced mutations were considered significant if the lower 95th percentile of the mutant frequency of a treated culture exceeded the upper 95th percentile of the solvent control. Furthermore, the authors considered the overall test results as significant if the induced mutations occurred at consecutive doses in at least one of the experiments, and dose-related increases in mutation frequency could be confirmed by regression analysis. Cytotoxicity was measured by determination of plating efficiency relative to the control, immediately post-treatment with Echinacea. The Echinacea treatment at the tested doses did not result in a statistically significant increase in the mutation frequency, either in the presence or absence of S9 metabolic activation. The Echinacea doses up to the maximum tested (5,000 µg/mL) were also considered virtually non-toxic to the cell line.
91. The lyophilised Echinacin Liquidum was also tested for its ability to induce chromosomal aberrations in an in vitro cytogenetic assay using human lymphocyte cultures from a male donor. The cells were incubated with 2400, 3500 or 5000 µg/mL Echinacea for 20 or 44 hours (in the absence of S9) or 3 hours followed by a 17- or 41-hours recovery period (in the presence of S9). Prior to harvesting the cells, colchicine was added to arrest them in metaphase 1.5 hours before harvest. The cells were fixed and examined microscopically for mitotic index. One hundred metaphases from each treatment were analysed for chromosomal aberrations. The results were considered significant where statistically significant increases in the proportion of structurally abnormal cells occurred at one or more concentrations and exceeded the normal range for this laboratory (0-4 cells with structural aberrations excluding gaps per 100 cells). There was no evidence of mitotic inhibition following the Echinacea treatment of up to 5,000 µg/mL in both the presence and absence of S9 mix at either sampling time (20 or 44 hours). A small, but statistically significant increase in the proportion of cells with structural aberrations was observed at 5,000 µg/mL at the 20 h sampling point in the absence of S9. The authors commented that this was not considered to be of biological significance as the frequency of aberrant cells fell within the range of biological control and it also coincided with an increase in the osmolality of the treatment medium.
92. Mengs et al. (1991) also performed an in vivo micronucleus test using 25,000 mg/kg Echinacin Liquidum administered as a single dose via oral gavage to groups of 5 male and 5 female mice. The animals were sacrificed at either 24, 48 or 72 hours after the dose. The positive control animal groups were given intraperitoneal injection of 100 mg/kg cyclophosphamide, whilst the negative control groups were given 25 mL/kg water via oral gavage. Bone marrow was collected from the femur and two bone marrow smears were prepared from the cells of each animal by fixing and staining. One thousand polychromatic erythrocytes (PCE) were analysed microscopically for the presence of micronuclei. The positive control showed a significant increase in the proportion of micronucleated PCE, whilst the Echinacea preparation did not show any statistically significant differences compared to the negative control.
93. The mutagenicity and the antimutagenic effects of E. purpurea were tested in S. typhimurium TA 98 and TA 100 strains with and without S9 metabolic activation (Tsai et al., 2012a). The E. purpurea extracts were prepared as described in paragraph 82. For the toxicity test each plate was inoculated with 0-5 mg E. purpurea extract and the viability of the bacterial strain was evaluated after 48-hours incubation at 37°C. For the mutagenicity test, the bacteria were mixed with 0.25-5 mg/plate Echinacea extract, incubated on histidine containing media at 37°C for 48 h and the reversion rate was compared to the control plate. The antimutagenic activity of the Echinacea extract was evaluated by calculating the % inhibition of the reversion rate with and without E. purpurea in the presence of mutagenic compounds.
94. The freeze-dried extract of E. purpurea showed no toxicity against S. typhimurium strains TA98 and TA100 concentrations of ≤5.0 mg/plate, with or without S9 metabolic activation (Tsai et al., 2012a). None of the tested concentrations of E. purpurea showed any significant differences in the revertant number with or without S9 mix. The Echinacea extract showed a dose-dependent inhibitory effect on the mutagenicity of 2-aminoanthracene in both S. typhimurium strains.
95. The E. purpurea extract described in paragraph 82 was tested for genotoxicity using an in vitro bacterial reverse mutation test in accordance with OECD Guideline No. 471, in vitro chromosomal aberration test in accordance with OECD Guideline No. 473 and an in vivo micronucleus test in accordance with the OECD guideline No. 474 (Jeong et al., 2024). The paper concluded that the tests did not reveal any evidence of genotoxicity as there was no significant increase in the number of mutant bacterial colonies even at the maximum concentration tested. Furthermore, the chromosomal aberration and micronucleus tests didn’t show any significant differences in chromosomal damage compared to controls. Details of the tests are described below.
96. For the in vitro bacterial reverse mutation test the extract was diluted two-fold in water and the highest concentration tested was 5,000 µg/plate. S. typhimurium strains TA98, TA100, TA1535 and TA1537 and tryptophan auxotroph mutant Escherichia coli strain (WP2uvrA) were used. Sodium azide, 2-nitrofluorene, 2-aminoanthracene, aminoacridine and 4-nitroquinoline N-oxide were used as positive controls and water was used as negative control. The Echinacea extract was incubated with a 109 cells/mL suspension of each strain for 20 minutes at 37°C, with and without S9 mix, poured on agar plates to solidify and incubated for 48 hours prior to checking for revertant colonies. No growth inhibition was observed at any of the doses of E. purpurea tested for the bacterial strains. The authors reported that the number of mutant colonies in the E. purpurea groups at all doses tested, with and without S9 metabolic activation mix, did not exceed twice the negative controls, whilst the positive controls used yielded between 5 to 37 times more colonies than the negative controls. (Jeong et al., 2024)
97. The in vitro chromosome aberration test was performed using mammalian cultured cell lines Chinese Hamster Lung (CHL/IU) cells seeded at a density of 5 × 104 cells/mL in a 60 mm plate and treated with E. purpurea extract (78,1, 156 and 313 µg/mL) for either 6 or 24 hours. Mitomycin C, 0.1 μg/mL and benzo[a]pyrene, 20 μg/mL were used as positive controls, whilst water was used as negative control. Microscopic slides for chromosomal observation were prepared and chromosomal abnormalities were divided into structural and numerical aberrations. No statistically significant difference was observed in the frequency of cells with chromosomal aberrations, in the absence or presence of S9 metabolic activation, in both short-term 6 hour and the 24-hour continuous treatments with E. purpurea extract compared to the negative control. With the positive controls there was significant increase (p>0.01) in the cells with structural abnormalities compared to negative controls. (Jeong et al., 2024)
98. The E. purpurea extract was administered to seven-week-old male Sprague Dawley rats at a two-fold dilution dose range from 1,250-5,000 mg/kg bw with 5 animals per dose for the in vivo micronucleus test. After the second dose, the animals were sacrificed, their femurs were dissected and perfused with phosphate buffer saline (PBS) to collect the bone marrow cells. The bone barrow cells were fixed with 10% formalin and stained with 0.05% acridine orange. Fluorescence microscope was used to count the numbers of micronucleated polychromatic erythrocytes (MNPCE) and polychromatic erythrocytes (PCE) in red blood cells (RBCs). The genotoxicity index was expressed as the average number of MNPCE among 4,000 PCE per rat, and the cytotoxicity index was expressed as the average number of PCE among 500 RBCs. The cytotoxicity index was similar in all groups tested. The genotoxicity index calculated was a 100-fold higher in the positive compared to the negative control, but was not statistically different between the different doses of Echinacea and the negative control. (Jeong et al., 2024)
99. The mutagenicity of E. angustifolia was tested using an in vitro bacterial reverse mutation test in S. typhimurium TA98, TA100 and TA102 in the presence and absence of S9 metabolic activation mix (Espinosa-Paredes et al., 2021). The ethyl acetate extract of E. angustifolia described in paragraph 78 was tested at concentrations between 50 and 200 µg per plate. The plates were incubated at 37°C for 48 hours and the number of mutant colonies were counted. Picrolonic acid (50 μg/plate), 2-amino-anthracene (10 μg/plate), N-methyl-N′-nitro-N-nitrosoguanidine (10 μg/plate) and mitomycin-C (10 ng/plate) were used as a positive control, whilst 0.1% dimethyl sulfoxide (DMSO) was used as negative control. A test was considered positive when the number of spontaneous colonies exceeded twice the number of basal revertants. The authors reported that the tested concentrations of E. angustifolia extract, with or without S9 mix, did not yield a positive test and no genotoxic activity was therefore observed. The positive controls on the either hand yielded between 7 to 55 times more colonies than the negative control.
100. Espinosa-Paredes et al. (2021) also performed an in vivo micronucleus test using male CD-1 mice. The ethyl acetate E. angustifolia extract was administered intragastrically at 1,000 mg/kg bw to 3 mice. 1% DMSO was used as a vehicle control, water as the negative control and cyclophosphamide (50 mg/kg) as the positive control. The animals were euthanised 48 hours after test substance administration, blood samples were collected, fixed and labelled prior to flow cytometry analysis. The number of normochromatic erythrocytes (NCEs) and reticulocytes (RETs), with and without micronuclei (MNs), was determined in order to calculate the percentages of mature normochromatic erythrocytes (% MN-NCEs), micronucleated reticulocytes (% MN-RETs) and the percentage of total reticulocytes (% RETs). The E. angustifolia extract did not lead to a significant increase in the formation of micronuclei yielding a 0.9 % MN-RET compared to 0.3% in the negative control. There was a significant increase in the MN-RET (8.4%) in the positive control compared to the vehicle control (0.4%). There was a decrease in the frequency of RET in the Echinacea extract group compared with the negative control (2.56% vs 5.41%, p < 0.05), but the authors have not commented on that, and its significance is unclear.
101. An in vitro carcinogenicity study was performed using lyophilised E. purpurea extract using Syrian hamster embryo cells (SHE) (Mengs et al., 1991). Six different concentrations of Echinacea in the range 5-55 µg/mL were tested and benzo(a)pyrene at 1.25 and 2.5 µg/mL was used as a positive control. Two independent experiments were performed with 20 replicates per concentration tested. The cells were incubated with the Echinacea or control for 7 days, washed, fixed with methanol and stained with 10% Giemsa. A stereomicroscope was used to evaluate the cells for altered or transformed morphology and average of 40 colonies per dish were examined. There was no significant difference in the frequencies of morphologically transformed colonies between the Echinacea and the negative control. The authors concluded that there was no evidence that Echinacea induces malignant transformation in SHE.
Reproductive and Developmental Toxicity
102. There are no guidelines conforming in vivo studies on the reproductive and developmental toxicity of medicinally used Echinacea species. There is a study looking at the effects of several herbal medicines, including E. purpurea, on human sperm motility (Ondrizek et al., 1999). There are several studies investigating the effects of E. purpurea during pregnancy in mice (Barcz, E. et al., Chow et al., 2006) and pigs (Maass et al., 2005). The reproductive and immune parameters of E. pallida were investigated in pregnant rabbits (Dabbou et al., 2016) and their offspring (Kovitvadhi et al., 2016). No studies were found on the reproductive effects of E. angustifolia.
103. A study investigated the effects of E. purpurea on human sperm motility (Ondrizek et al., 1999). Fresh donor sperm were washed and incubated with either 0.81 mg/mL or 8.1 mg/mL E. purpurea for 48 hours at 37°C. The lower of the tested concentrations was based on one-thousandth of the recommended daily dose dissolved in 1 mL of medium. Sperm aliquots were analysed at 0, 1, 4, 24 and 48 h of incubation. Sperm dimensions and kinematic parameters were measured using the Hamilton Thorn motility analyser. There were no significant differences in the kinematic parameters and the sperm motility between the medium control and lower concentration (0.81mg/mL) of Echinacea. However, inhibition of sperm motility was observed at 48h with the higher E. purpurea concentration (8.1 mg/mL) tested.
104. The possible association between consumption of E. purpurea during pregnancy and spontaneous abortions in mice was investigated by Chow et al. (2006). The authors based their investigation on the implications of natural killer (NK) cells on foetus rejection, which manifests in spontaneous abortions (De Fougerolles and Baines, 1987; Gendron and Baines, 1988). Previous studies have shown that Echinacea significantly increases the number of NK cells in both healthy (Currier, 2000) and leukemic mice (Currier and Miller, 2002). Commercially prepared E. purpurea extract was homogenized into finely ground standard chow that individual mice consumed at 0.45 mg/day. Pregnant mice (n=6) were fed the Echinacea containing chow from pregnancy onset until gestational days 10-14 until females were killed. Half of the mice (n=3) were killed at day 10-11 gestational days (early pregnancy) and the other half (n=3) at 12-14 gestational days (mid pregnancy). Foetuses from 3 pregnancies in both gestational age groups (10–11 days and 12–14 days) were removed and preserved in fixative for subsequent counting. Maternal spleen and bone marrow were taken for enumeration of cells in each of five separate hemopoietic lineages/organ.
105. The study (Chow et al., 2006) reported that the number of spleen lymphocytes and nucleated erythroid cells, normally increased during pregnancy, were decreased in Echinacea-fed mice back to levels not significantly different from those of non-pregnant animals. The bone marrow parameters were not influenced by the Echinacea supplementation. The results also indicated that miscarriages are more likely to happen in the early stages of pregnancy (10–11 days) in the Echinacea-fed mice. At days 10-11, there are no significant differences in the average number of foetuses between Echinacea-fed mice and controls (4.7/pregnancy in controls vs 4.0/pregnancy in treatment group). Foetal loss (resorption) appears to begin early, prior to the first recording (days 10–11) in E. purpurea-consuming pregnant mice, and only 50% of the foetuses from Echinacea-consuming pregnant mice survive by day 12–14 of gestation compared to controls (4.0/pregnancy in controls vs 2.0/pregnancy in treatment group). Based on these results, the study suggests that pregnant women should avoid the consumption of Echinacea in the early stages of pregnancy in women. It is worth bearing in mind that this study investigated only one E. purpurea dose in a small number of animals with supplementation starting after the pregnancy was established.
106. Barcz et al. (2007) investigated the effects of Echinacea on the angiogenic activity and tissue VEGF and bFGF in foetuses from pregnant mice exposed to E. purpurea extracts. Eight female mice were given 0.6 mg E. purpurea extract via an Eppendorf pipette from the 1st day of fertilization until the 18th day of pregnancy. Four mice were given vehicle control. In the experimental group, different Echinacea formulations were used with three mice fed Esberitox, two Immunal and the remaining three Echinapur. On day 18, the females were sacrificed, the foetuses were extracted, counted and weighed. Foetuses from each litter were pooled and homogenised for further angiogenic evaluation. The angiogenic activity of the tissue homogenate was performed by injecting the homogenate in two to three Balb/c mice and counting newly formed blood vessels on the inner skin surface. The concentrations of VEGF and bFGF in the homogenate were determined by ELISA.
107. The study (Barcz et al., 2007) reported that that two of the Echinacea preparations, Echinapur and Esberitox, slightly lowered the mean number of foetuses (n=7.6) per litter when compared to Immunal (n=9.5) and the control (n=10), but results were on the border of statistical significance (0.05<p<0.01). The mean VEGF and bFGF foetal tissue concentrations from mothers exposed to all Echinacea preparations were significantly decreased compared to controls (p <0.0001). The angiogenic activity of the tissue homogenates, expressed as mean number of blood vessels, was significantly increased in the Esberitox group (28.2 ± 6; p<0.0001), but decreased in the Immunal forte group (18.2 ± 5.4 p < 0.01) when compared to standard/control diet (22.5 ± 4.3). No significant difference in the angiogenic activity of the foetal tissue homogenate was observed with Echinapur (21.3 ± 4). The study concluded that E. purpurea preparations may influence foetal angiogenesis and should not be recommended in pregnancy without further studies being carried out (Barcz et al., 2007).
108. In another study (Maass et al., 2005), E. purpurea was introduced in the diet of pregnant sows in the form of dried cobs consisting of the aerial part of the plant. The study was carried out on the total of 36 sows, divided into three groups of 12, from the 85th day of pregnancy to the 28th day of lactation. Echinacea was supplemented in the diet of the three groups during 85-110th day of pregnancy at a dose of 0%, 1.2%, or 3.6%. The Echinacea supplementation was continued until the 28th day of lactation at 0%, 0.5%, or 1.5%. During gestation, the feed amount (2.6 kg/sow/day) was set to match the nutritional requirements of the sows. The lactation diet was offered ad libitum with a maximum of 6 kg/sow/day. Blood analysis was performed on day 85 of pregnancy as well as on day 1 and 28 of lactation. The colostrum was collected manually from 1 to 6 hours after delivery. Body mass, body temperature, health status, crude protein, immunoglobulins, haematological, and clinical chemistry parameters (alkaline phosphatase, alanine, and aspartate, and gamma-glutamyl transferase aminotransferase) were analysed using the acquired samples.
109. No influence of Echinacea on the enzyme results (alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase) was observed. There was no statistical difference in the levels of leukocytes, erythrocytes, lymphocytes, granulocytes, neutrophils, eosinophils, basophils, or monocytes between the Echinacea supplemented and the control groups. The level of crude protein in colostrum was 5% lower in the higher dose Echinacea supplemented group compared to the control, but this difference was not statistically significant (p = 0.11). Daily weight gain of the sows during gestation was 18% higher in the control group than in the Echinacea supplemented groups. During lactation no differences in the average weight loss were found. The piglet body weight at birth in the control group was 4% lower than in the supplemented groups. However, this difference was not significant and the growth performance of the sucking piglets also showed no significant differences between the control and supplemented groups. (Maass et al., 2005)
110. The effects of E. pallida dietary supplementation on the reproductive performance, blood parameters and immune indices were studied in pregnant rabbits (Dabbou et al., 2016). The Echinacea preparation contained caftaric acid, chicoric acid, chlorogenic acid and echinacoside with echinacoside found to be the main caffeic acid derivative. The does were randomly assigned to two groups (50 does/group). The first group was fed a commercial pelleted diet ad libitum, while the second one was fed the same diet supplemented with 3 g/kg E. pallida. Echinacea was given from insemination to kit weaning. The study measured the reproductive performance of the does based on the following parameters: total born; born alive; stillborn; litter size at 21 and 35 days of age; litter weight at 21 and 35 days of age; individual body weights of kits at 21 and 35 days of age; kindling rate (%) = number of kindled does per number of inseminated does × 100; prolificacy = number of born kits per number of does kindled; numerical productivity at birth = number of born alive per inseminated doe; overall productivity at birth = weight of born alive per inseminated doe; perinatal mortality (%) = number of stillborn kits per number of total born × 100; mortality between 0 to 21 and 0 to 35 days of age. Full haematological parameters and blood biochemistry were performed on days 0, 14, and 28 of the experiment. Serum lysozyme was measured as part of the innate immunity evaluation.
111. The body weight of the does at kindling, the kindling rate and the litter size at birth and at days 21 and 35 of age, and the mortality of the kits did not differ between the two groups. The blood morphology parameters of mothers did not significantly differ between treatment and controls: red blood cells, haemoglobin, haematocrit, mean corpuscular volume, mean corpuscular haemoglobin, mean haemoglobin concentration, red blood cell distribution width, platelet count, mean thrombocyte volume, mean platelet volume, platelet distribution width, number of white blood cells, lymphocytes, monocytes, neutrophils and eosinophils. The Echinacea supplemented group showed reduction in basophil cell rate, but it was not significantly different from controls. The concentration of total protein, glutamic oxoloacetic transaminase, blood urea, nitrogen, albumin, urea and cholesterol were also analysed and were not different between treatment and control groups. The serum lysozyme assay didn’t yield significant differences either. Overall, the authors concluded that supplementation with E. pallida did not show any significant effects on the reproductive, haematological, or immune parameters of does.
112. The second part of the above study aimed to evaluate the effects on the performances, bacterial community, blood parameters and immunity of E. pallida dietary supplementation in the offspring of the control and Echinacea supplemented group (Kovitvadhi et al., 2016). Eighty kittens were taken from the control group (C) and Echinacea (E) groups of the previous study (Dabbou et al., 2016). The kittens were weaned at 35-days old, randomly separated into four groups of 20 and were housed in individual wire cages. The growing rabbits were fed a growing commercial basal diet with or without the supplementation (3 g/kg of E. pallida): the CC group (rabbits from the control does fed the control diet), CE group (rabbits from the control does fed the supplemented diet), EC (rabbits from the Echinacea does fed the control diet) and EE group (rabbits from the Echinacea does fed the supplemented diet). The hard faeces of the growing rabbits were collected at 35, 49 and 89 days from five animals per group for microbiome analysis. Blood samples were taken from 10 rabbits per group at 89 days of age for haematological analysis. Renal and liver function and lipid metabolism were also measured. Phagocytic activity was determined in an assay against Streptococcus canis, which looked under immersion light microscope for neutrophils and monocytes that engulfed the Gram-positive cocci. Humoral immune response against vaccination was also studied by measuring antibody production in response to vaccination against rabbit haemorrhagic disease virus. The study reported an increase in phagocytosis in rabbits fed diets supplemented with E. pallida, regardless of the Echinacea supplementation status of maternal diet. There were no significant differences in growth performances, blood parameters, bacterial community, or humoral immune response.
113. A study investigated the ability of levamisole and ethanolic (70%) extracts of E. purpurea dried aerial parts to prevent teratogenic effects such as cleft palate induced by phenytoin (Khaksary Mahabady et al., 2006). Thirty two pregnant NMRI mice of age 6-8 weeks were divided into four groups (n=4) as follows: group 1 received normal saline (10 mL/kg), group 2 received phenytoin (65 mg/kg), group 3 received phenytoin (65 mg/kg) and 12 h later levamisole (10 mg/kg), whilst group 4 received phenytoin (65 mg/kg) and 12 h later extract of E. purpurea (360 mg/kg). All drugs were administered intraperitoneally from the first day of gestation, which was assumed to be upon the discovery of vaginal plug following mating. The mice were sacrificed on gestational day 19, the foetuses were collected (total number of collected foetuses from groups 1-4 were 64, 81, 77 and 61 respectively), stained by Alizarin red–Alcian blue method and investigated by stereomicroscope for cleft palate. In the saline control group the palatal closures of all foetuses were normal, whilst the incidence of phenytoin induced cleft palate was 16%. Levamisole reduced incidence of pheytoin-induced cleft palate to 5.3%, whilst the E. purpurea extract reduced it to 3.2%. Equally, the mean weight and length of the foetuses were significantly decreased in the phenytoin group (p<0.001), whilst the groups that received Echinacea and levamisole had similar parameters to the saline control. The authors concluded that the observed protective activity of levamisole and Echinacea against phenytoin-induced cleft palate was due to immunomodulating and anti-inflammatory effects of these agents.
Human Studies
Exposures in pregnancy
114. A systematic review conducted on the available literature up to 2006 (Perri et al., 2006) concluded that good scientific evidence from a prospective follow up study (Gallo et al., 2000) showed that oral consumption of Echinacea during the first trimester does not increase the risk of major malformations. Low level evidence based on expert opinion from a botanical medicine panel concluded that Echinacea is not teratogenic and oral consumption of Echinacea in recommended doses is safe during pregnancy and lactation. However, the expert panel advised that caution should be exercised until there is stronger evidence on safety of Echinacea during lactation. (Perri et al., 2006).
115. The first prospective controlled study aiming to evaluate the safety of Echinacea use during pregnancy consisted of 206 women who were enrolled and prospectively followed up after contacting the Motherisk Program (in Toronto, Canada) regarding the gestational use of Echinacea (Gallo et al., 2000). There was a disease-matched control group of 206 women to control for maternal age, alcohol consumption and smoking. In this study group, 112 women (54%) used Echinacea in the first trimester, with 17 (8%) exposed in all 3 trimesters. A total of 114 (58%) of 198 respondents used capsule or tablet preparations, or both, of Echinacea (250 to 1000 mg/d); 76 (38%) of the subjects used tinctures (5 to 30 drops per day). The self-reported duration of use was between 5 and 7 days. Different brands of E. purpurea and E. angustifolia were predominantly used, but the number of women using each species is not specified. E. pallida was only used by one woman. No information was provided on the part of the plant used in these preparations.
116. No statistical difference was reported between the Echinacea users and disease-matched controls in terms of pregnancy outcome, delivery method, maternal weight gain, gestational age, birth weight, or foetal distress. There were 195 live births, including 3 sets of twins; 13 spontaneous abortions; and 1 therapeutic abortion in the treatment group. The disease-matched control group reported 198 live births, 7 spontaneous abortions, and 1 therapeutic abortion. (Gallo et al., 2000)
117. Rates of malformations between the treatment and control groups were also not statistically significantly different. There were 6 major malformations including 1 chromosomal abnormality, and 6 minor malformations in the Echinacea exposed group. With first-trimester use of the herb, 4 major and 2 minor malformations were reported. In the control group, 7 major and 7 minor malformations occurred. Overall, the authors concluded that gestational use of Echinacea during organogenesis is not associated with an increased risk for major malformations (Gallo et al., 2000)
118. The Norwegian Institute of Public Health conducted The Norwegian Mother and Child Cohort Study, a prospective population-based pregnancy cohort study (Heitmann et al., 2016). Pregnant women in Norway were recruited through a postal invitation in connection with a routine ultrasound examination offered to all pregnant women around pregnancy week 17. Women filled in 3 questionnaires at week 13-17, week 30 and when the child was 6 months old. Information on Echinacea use was retrieved from the questionnaires. Maternal age, pre-pregnancy BMI, folic acid use, smoking, education, previous miscarriages/stillbirths, the year of delivery were considered as possible confounders and adjusted for. In addition, low birth weight was adjusted for by length of gestation. Women who gave birth to multiples (n=1,291) and children with chromosomal malformations (n=121) were excluded from the study design. The study population consisted of 68,522 pregnancies; amongst these 68,198 (99.5 %) resulted in a live birth, 219 (0.3 %) resulted in a stillbirth, and 104 (0.2 %) resulted in a neonatal death. The mean birth weight and the median gestational age among live-born infants was 3,605 g and 40 weeks, respectively. Any malformation occurred in 3,201 (4.7 %) of the pregnancies.
119. There were 363 (0.5 %) women who reported the use of Echinacea during pregnancy. The most common reasons for Echinacea use were treatment of cold/flu, upper respiratory tract infections (including sinusitis, otitis, tonsillitis, and cough), lower respiratory tract infections (bronchitis and pneumonia), vaginal and oral herpes infections. A total of 206 (0.3 %) and 183 (0.3 %) women had used Echinacea during early and late pregnancy, respectively. Some women had not specified the gestational week of Echinacea use. The dose, preparation and method of administration were not provided. Users of Echinacea were not found to have any increased risk of preterm birth, low birth weight, or small for gestational age. No increased risk of malformations was detected amongst the women who had used Echinacea during early pregnancy compared to controls; adjusted OR (95% CI) = 1.1 (0.6–2.1). There was 1.5% prevalence of major malformations in the women who had used Echinacea compared with 2.6% in the non-exposed group; adjusted OR (95% CI) = 0.6 (0.2–1.8). The three cases of major malformations that were detected among the users of Echinacea were hypospadias, cleft lip, and hypoplastic left heart syndrome. (Heitmann et al., 2016)
120. A study conducted over a 10-month period explored the use of herbal products among Italian women at the maternity wards of Padua and Rovereto hospitals (Cuzzolin et al., 2010). A structured anonymous questionnaire was administered to 392 women during face-to-face interviews in two sessions within 3 days of childbirth. During the first session, details about herbal medicine consumption, including preparation, dose, timing of administration, reasons for consumption, level of satisfaction and adverse reactions were collected. In the second session, data bout pregnancy history (smoking, alcohol consumption, morbidities, medication use) and newborn details (gestational age, birth weight, Apgar score, problems at birth, treatments) were collected.
121. One hundred and nine (27.8%) women reported taking one or more herbal remedies during pregnancy with 37.8% of them throughout the entire pregnancy duration. Oral Echinacea was used by 10 (9.2%) of the interviewed women for common cold, anxiety and strengthening of the immune system. No information about the Echinacea species, plant part, type of preparation, dose, duration of intake/trimester is given in the article. By examining each herb separately, the authors report that in one case there was a possible relationship between prolonged Echinacea intake and intrauterine growth restriction in a 35-week newborn. No further detail is provided for that case. (Cuzzolin et al., 2010)
122. A similar study aiming to investigate the use of herbal medicines in pregnant women in relation to pregnancy outcomes involved the administration of a structured questionnaire to 600 women within five days after delivery at Stavanger University Hospital Norway (Nordeng et al., 2011). The women’s medical charts were reviewed for information on pregnancy outcomes including birthweight, gestational age, presence of neonatal and/or maternal complications. Forty percent of women reported to have used herbal medicines during pregnancy, with Echinacea being used by 45 (7.5%) of those interviewed for cold and flu symptoms. No information about the Echinacea species, plant part, type of preparation, dose, duration of intake/trimester is given in the article. Except for birthweight, there were no significant differences between users and non-users of herbal medicines. Mean birth weight was significantly higher (155 g; p = 0.001) in the users of herbal medicines (3,663 g) compared to non-users (3,508 g). Sub-analysis of the data revealed that this was linked to the use of iron-rich herbs. The association between mean birth weight and Echinacea in particular is not discussed in the article.
123. The UK Teratology Information Service (UKTIS) has 15 reports of maternal exposure to Echinacea in their database for the period between 2004-2017. Only one of these has a record of the pregnancy outcome. A pregnant woman took Echinacea for a cold between gestational week 7-8 along with several other prescription medicines. The outcome was a normal live-born infant born at gestational week 40. Further details regarding the preparation and dose of Echinacea were not provided by UKTIS.
Lactation
124. A case study examined the bioavailability of Echinacea alkylamides in human breast milk in a 35 year old volunteer at six different time points after ingestion of four Echinacea Premium tablets (Matthias et al., 2008). The tablets were prepared from dried ethanolic extracts of two Echinacea species and each tablet contained the equivalent of 675 mg E. purpurea root and 600 mg E. angustifolia root. A total of 13.1 mg of N-isobutyldodeca-2E,4E,8Z,10E/Z-tetraenamide alkylamides were ingested by the volunteer and they were found in the breast milk between 1 and 4 hours after the administration of the Echinacea tablets. Further details were not present in this conference abstract.
Adverse effects in humans
125. A meta-analysis of clinical trials (Schapowal et al., 2015) investigating the use of Echinacea supplements in patients with respiratory tract infections (see paragraph 55 for more details on the study) looked at the adverse events recorded in the 6 clinical trials included in the analysis from a total of 1,440 Echinacea-treated subjects and 1,326 subjects receiving placebo. Overall, 491 adverse events occurred with Echinacea in comparison to 474 with placebo, but there were no significant differences between the groups. Most adverse effects reported were gastrointestinal disturbances and were mild and transient. Only two severe adverse events (stridor) occurred with Echinacea and one (glandular fever, requiring hospitalization) in the placebo group. There were no significant differences in clinical biochemistry associated with Echinacea use.
126. Systematic review summarised evidence of the safety of Echinacea based herbal medicinal products from 36 clinical studies, case reports, and spontaneous reporting programmes from regulatory agencies in Australia, Germany, UK, USA and Sweden (Huntley et al., 2005). Their conclusion was that Echinacea has a good safety profile when taken short-term, with short-term use being defined as ‘days as opposed to weeks’. Adverse effects are mild, transient and reversible with gastrointestinal disturbances and skin-related reactions being most commonly reported. The study emphasises that in rare cases Echinacea use can be associated with allergic reactions, which can be severe and cautions against Echinacea use in atopic or asthmatic individuals.
127. The EMA assessment report on E. purpurea (EMA, 2014) concludes that based on the analysis of pharmacovigilance reports from EU member states, hypersensitivity reactions such as rash, urticaria, itching and swelling are possible and in a case of allergic reaction, Echinacea should not be taken again. There are cases of severe reactions such as Stevens-Johnson Syndrome, angioedema, bronchospasm, asthma and anaphylactic shock with confirmed/probable causality. Cases of autoimmune diseases such as encephalitis disseminata, erythema nodosum, immunothrombocytopenia, Sjögren’s syndrome with renal tubular dysfunction have been reported, but the causality of these is inconclusive. The gastrointestinal side effects reported are unlikely to be linked to Echinacea as their frequency is similar in placebo and treatment groups in clinical trials (EMA, 2014).
128. Analysis of spontaneously reported adverse reactions submitted to the Swedish Medical Products Agency from 2007-15 identified 116 reports related to herbal medicinal products (Svedlund et al., 2017). There were 14 reports concerning Echinacea and the most common adverse drug reactions (ADR) were related to skin and subcutaneous tissue (n=7), which could be the result of hypersensitivity or allergy.
129. A systematic review summarised and critically assessed the available data on adverse effects related to plant food supplements (Di Lorenzo et al., 2015). PubMed/MEDLINE and Embase were searched, and the reports were assessed according to the World Health Organization (WHO) Causality Assessment Criteria. A total of 20 papers reporting adverse effects due to Echinacea were identified, but the causality in 10 out of 20 was defined as ‘unclassifiable’ because there was insufficient evidence of exposure, lack of clear information on the preparation and/or the description of the adverse event. The adverse reactions from papers reporting certain or probable association were either allergy, hepatic or gastrointestinal effects such as diarrhoea, vomiting, headache or drowsiness. Most were associated with preparations containing the ethanolic extracts of herb and root.
130. An Australian study looking at adverse reactions associated with Echinacea identified 51 reports of ADRs in the Australian Adverse Drug Reactions Advisory Committee’s database (Mullins and Heddle, 2002). There were 26 cases which were suggestive of IgE-mediated hypersensitivity reactions (4 anaphylaxis, 12 acute asthma,10 urticaria/angioedema). Seventy eight percent of the affected patients were female, median age was 32 years and over half had a history of asthma, allergic rhinitis or atopic dermatitis. In addition, the authors also personally evaluated five privately referred patients in their practice via skin prick testing (SPT) after exposure to Echinacea.
131. Two of these patients suffered anaphylaxis and one had an acute asthma attack after their first ever dose of Echinacea. All three patients were female between the ages of 19 and 37 years, had history of atopy including allergic rhinitis or latex allergy and exhibited a positive SPT to aqueous Echinacea solution when tested in the private practice of the authors (Mullins and Heddle, 2002). One of these cases describes the consumption of 5 mL commercially prepared Echinacea (40% alcohol in water) equivalent to 3,825 mg of whole plant extract E. angustifolia and 150 mg of E. purpurea (Mullins, 1998), whilst the other two involved the use of Echinacea tablets or tea (Huntley et al., 2005; Mullins and Heddle, 2002).
132. The fourth patient was a 56-year-old man who had recurrent episodes of mild asthma each time Echinacea tablets were administered, and the symptoms resolved within a few days of stopping. This patient had allergic rhinitis, but no other drug or medical history. The last case involved a 48-year-old woman who developed a maculopapular rash within 2 days of Echinacea tablets ingestion, and this recurred on rechallenge a week later. This patient had history of no-allergic rhinitis and took no regular medication. Both patients had a negative SPT to aqueous Echinacea solution. (Huntley et al., 2005; Mullins and Heddle, 2002)
133. The authors also tested 100 atopic patients who had never taken Echinacea and 20% had positive STP results. The overall conclusion of the study was that there is a possible cross-reactivity between Echinacea and other environmental allergens and atopic patients should be warned accordingly. (Mullins and Heddle, 2002)
134. An autoimmune disease supposedly triggered by Echinacea was described in a 55-year-old man who had a diagnosis of emphigus vulgaris, which was in remission and was managed with low doses of dapsone. The man started taking daily Echinacea supplements for the treatment of a respiratory tract infection and he developed blisters on his body, head and oral mucosa within 1 week of starting the supplement. After the discontinuation of Echinacea, partial disease control was achieved with prednisolone, azathioprine and dapsone, but never a complete remission. (Lee and Werth, 2004)
135. An isolated case of erythema nodosum was reported in a 41-year-old man who experienced four episodes of erythema nodosum after using Echinacea for flu-like symptoms. He had been using Echinacea intermittently for 18 months alongside loratadine and St. John’s wort. The patient responded to prednisolone treatment, discontinued the Echinacea preparation and after 1 year had not experienced further reoccurrences. (Lee Soon and Crawford, 2001)
136. Hypereosinophilia associated Echinacea use was reported in a 58-year-old male patient (Maskatia and Baker, 2010). The patient complained of mild crampy abdominal pain, with occasional nausea and diarrhoea. He had started taking Echinacea supplements several weeks prior to symptom onset. Past medical history included controlled asthma and allergic rhinitis, hyperlipidaemia, resolved hepatitis B infection, cholelithiasis, an inguinal hernia, and a hiatal hernia. The patient had eosinophil count of 17,800/μL (normal <350 eosinophils/μL) and Immunoglobulin E (IgE) level of 1390 IU/ml (normal <114 IU/m). Serum biochemistry, liver function tests and bone marrow cytogenetics were normal. The patient was treated with prednisolone and stopped taking Echinacea. Two weeks after discontinuing the Echinacea supplements, the eosinophil counts and IgE levels had improved to almost normal levels. Prednisolone was tapered off and six months later, the patient continued to have normal IgE and eosinophil levels.
137. A case of leucopenia was reported in a 51-year-old woman who had a blood test at her annual medical appointment and her white blood count (WBC) had decreased from 5,800/µL the preceding year to 3,300/µL (normal range 4,000 – 11,000/µL). Other than that, she appeared healthy. She had been taking 450 mg Echinacea capsules three times daily for the past 2 months for the prevention of respiratory tract infection as well as Gingko biloba, bupropion, vitamins C, E, B and calcium. She stopped taking Echinacea and her white blood cell count increased to 3,700 µL a month later. The following year, the patient resumed the Echinacea supplement and after two months, her WBC had decreased to 2,800 µL. Once again, she discontinued taking Echinacea and her WBC increased to 4,320 µL 7 months later. The authors of the case report attributed the changes in the white blood cell count to the Echinacea supplements. (Kemp and Franco, 2002).
138. Thrombocytopenia was reported in a 32-year-old man who took oral water–alcoholic extract of the E. pallida for about a week for an upper respiratory tract infection (George et al., 2006). The patient was admitted to hospital 20 days later with hypotension, sinus tachycardia, anaemia, thrombocytopenia, microangiopathic type haemolytic anaemia with fragmented red blood cells, increased indirect bilirubin, and markedly elevated lactate dehydrogenase (LDH) level. Bacterial and viral antibodies were negative. The diagnosis was severe thrombotic thrombocytopenic purpura (TTP) and the patient was treated with transfusion of red blood cells and fresh frozen plasma (FFP). The patient had a syncope episode followed by a seizure and was controlled with general anaesthesia and ventilatory assistance. He was treated with plasmapheresis twice a day and FFP for over a month until disease remission.
139. Hepatotoxicity associated with Echinacea were reported in the form of severe acute cholestatic autoimmune hepatitis (ACAH) (Kocaman et al., 2008) and fatal liver necrosis (Jacobsson et al., 2009). The fatal liver necrosis was reported in a 28 year old man who took Echinacea 5 days a week for 6 months (Jacobsson et al., 2009). The acute cholestatic autoimmune hepatitis (ACAH) occurred in a 45-year-old male patient who took Echinacea root at 1,500 mg/day for the treatment of a cold (Kocaman et al., 2008). The patient had fatigue and jaundice with elevated alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total bilirubin and immunoglobulin G (IgG). Anti-smooth muscle antibodies (ASMAs) were positive, whilst anti-nuclear antibodies, anti-liver/kidney microsomes, anti-soluble liver antigen antibodies, and anti-mitochondrial antibodies were negative. Liver biopsy revealed hepatitis, prominent cholestasis, portal lymphoplasmocytic and eosinophilic granulocyte infiltration. Echinacea supplementation was discontinued and a month later all laboratory values were normal except SMA positivity. The authors concluded that this was a case of Echinacea-induced ACAH due to the immunostimulatory effects of Echinacea.
Duration of use
140. EMA recommends that oral Echinacea preparations should be used for a limited duration of up to 10 days (EMA, 2014). The German Commission E monographs on Echinacea recommend that internal and external administration of E. purpurea and E. pallida should not exceed 8 weeks (Blumenthal et al., 1999). No scientific rationale has been provided for the limits on the duration of use. Echinacea preparations have been used for longer durations without any serious adverse effects as described below.
141. The clinical studies involving Echinacea have varying durations from 4-21 days to 4-12 weeks (Ardjomand-Woelkart and Bauer, 2015). The study with the longest duration involved the administration of 800 mg E. purpurea whole plant extract twice a day for 6 months to 50 patients (Vonau et al., 2001). The only side effects reported were nausea (n = 4) and diarrhoea (n = 2). The use of E. purpurea and E. angustifolia root liquid extract for 12 weeks (100 drops daily of a 1:11, 30% ethanolic extract for 5 days a week) was studied in randomized, double-blind, placebo controlled trial involving 289 patients for the prevention of respiratory tract infections (Melchart, 1998). The side effects reported included minor gastrointestinal symptoms, headache/dizziness, allergic reactions and were similar between treatment arm and placebo.
142. The safety and efficacy of Echinaforce was tested in a large randomised, double-blind, placebo-controlled clinical trial for 4 months. A total of 755 subjects were included and the main criteria for inclusion was that they experience ≥2 colds per year. Participants took the equivalent of 2,400 mg of extract a day for illness prevention, but during acute stages of colds the dose was increased to 4,000 mg extract/day. There were no significant differences between the frequencies and the type of adverse effects between treatment and placebo. Haematological and biochemical measures were not significantly different before and after Echinacea treatment and when compared to placebo (Jawad et al., 2012)
Contaminants
In this guide
In this guide143. Very few studies, described below, have investigated the potential contaminants in Echinacea preparations, including heavy metals, moulds and mycotoxins.
144. From 1999-2004, 13,504 adults participated in National Health and Nutrition Examination Survey (NHANES) interviews, examinations and had their blood lead levels assessed (Buettner et al., 2009). The aim was to determine whether there was an association between self-reported herbal supplements use and blood lead levels. The focus was on herbal supplements reported to contain excess lead in previous studies or product testing available to the public, or that had been implicated in heavy metal poisoning. The study also considered prevalence of use of specific herbal supplements in study participants and Echinacea was one of the more commonly used herbs (4.3% of all participants reported the use of specific herbal supplement and 1.1% of the participants used Echinacea). The authors fitted a regression model for women of child-bearing age (16-45 years), which showed that those who used herbal supplements had adjusted blood lead levels 20% (95% CI 5%–34%, p = 0.008) higher than women who did not. However, when broken down by the specific herbal supplement, the difference was not significant for Echinacea supplements (8%, 95% CI -15% – 35%, p = 0.55).
145. Filipiak-Szok et al., (2015) measured the concentrations of heavy metals (Pb, Cd, As, Al, Ni, Ba, Sb) in raw plant material of selected medicinally used herbs and dietary supplements containing them, on the Polish market. The plant samples, purchased from STANLAB (Poland), were dried and ground prior to undergoing microwave digestion with ultrapure nitric acid (65%) for 30 minutes. Samples were analysed using an inductively coupled plasma mass spectrometer (ICP-MS) with quadrupole mass analyser. Three samples were used for each analysis. The authors compared the results against the limits set by WHO (0.3 mg/kg for cadmium, 10 mg/kg for lead and 5.0 mg/kg for arsenic) and by the EU Commission Regulation (EC) No. 1881/2006 (1.0 mg/kg for cadmium and 3.0 mg/kg for lead). The levels found in the dried Echinacea purpurea samples were considerably lower with 0.02 mg/kg cadmium, 0.6 mg/kg lead and 0.16 mg/kg arsenic.
146. Another study analysed popular food supplements, including seven Echinacea containing brands, for presence of heavy metals and microbial contamination (Raman et al., 2004). The supplements analysed were in the forms of tablets, capsules or soft gels. Tablets were powdered, whilst the soft gels and capsules digested individually. Concentrated nitric acid was added to the samples and they were digested using a hot plate 75°C for 16 hours. Analysis was conducted using an ICP-MS. The authors determined the daily dose of each heavy metal that would be ingested if the supplement was taken as recommended by the manufacturer. Depending on the Echinacea brand, the daily doses of heavy metals would be: lead 0.034-2.901 μg/day, cadmium 0.004 – 0.967 μg/day, arsenic 0.027 – 0.908 μg/day, chromium 0.125-8.838 μg/day, thallium 0.002 – 0.383 μg/day. Mercury was not detected in the samples. The authors compared these values to tolerable intake levels at the time of publication and concluded that the supplements do not pose risk to consumers.
147. Alternaria alternata, Aspergillus spp., Fusarium spp., Phoma spp. and yeasts have been detected in Echinacea herbal supplements at 100-1,000 CFU/g with 71% of the Echinacea samples (n=7) harbouring fungi (Tournas, 2009). Twenty one samples were analysed as part of a study investigating the presence of moulds and their secondary metabolites in Echinacea dietary supplements available on the Polish market (Pilarska et al., 2022). It was found that 12 samples were contaminated with Aspergillus spp., whilst Eurotium and Penicillium spp. were detected in 8 of the samples. Mycotoxin contamination was found in 18 of the samples with zearalenone (18/21), deoxynivalenol (5/21) and T-2 (3/21) occurring at the highest frequencies.
Exposure Assessment
In this guide
In this guide148. Echinacea is not used as a food commodity on its own or in recipes for cooking, but there are tea and honey products, found from online sources, supplemented with Echinacea and Echinacea extracts (Table 3), and these could be consumed as part of the general diet. Data from the National Diet and Nutrition Survey (NDNS) (Bates et al., 2014, 2016, 2020; Roberts et al., 2018) on acute herbal and fruit tea consumption and honey among women of childbearing age (16-49 years) may provide an indicator of Echinacea intake from these foods during pregnancy. The NDNS does not provide data for pregnant or lactating women, so while data is based on women of childbearing age, this may not necessarily be representative of the maternal diet. It is also worth noting that some of the Echinacea containing tea products advise pregnant or lactating women to consult a healthcare professional prior to using the product. The Echinacea-containing honey states that it is suitable for pregnant or breastfeeding women, whilst the lozenges contain no warnings. Like tablets and capsules lozenges are solid dosage forms, but they are specifically designed to dissolve or disintegrate slowly in the mouth and are formulated with a flavoured or sweetened base.
Table 3: Food products containing Echinacea.
|
Product name |
Type |
Echinacea species and plant part |
Composition |
Directions for use |
Daily dose of Echinacea (mg) |
Additional information/ warnings |
|
Pukka Herbs | Elderberry and Echinacea Organic Herbal Tea. |
Tea bags. |
Echinacea (species not specified) herb. |
Ginger root, liquorice root, Echinacea herb (11%), beetroot, aniseed, rosehip, peppermint leaf, orange peel, elderflower (5%), elderberry (4%), hibiscus, orange essential oil flavour, blackcurrant flavour. |
Not specified. |
Not specified. |
None. |
|
Yogi Tea | Echinacea Special Formula. |
Tea bags. |
Not specified. |
Cinnamon, Echinacea, ginger, fennel, rooibos, roasted chicory, carob, cardamom, basil, burdock root, black pepper, turmeric root, astragalus, vanilla beans. |
Pour 250 ml of freshly boiled water over the teabag. Allow to infuse for 5 to 6 minutes - or longer for a stronger flavour. |
Not specified. |
None. |
|
Yogi Tea, Echinacea Immune Support, Caffeine Free, 16 Tea Bags, 0.85 oz (24 g). |
Tea bags. |
Echinacea purpurea Plant part not specified. |
Each tea bag contains: |
Bring water to boiling and steep 7 minutes. For a stronger tea, use 2 tea bags. Drink 3-4 cups daily. |
432 - 1,152 mg Echinacea purpurea and |
Consult your healthcare provider prior to use if you are pregnant or nursing, taking any medication or if you have a medical condition. |
|
Traditional Medicinals, Organic Echinacea Plus, Elderberry, Caffeine Free, 16 Wrapped Tea Bags, 0.85 oz (24 g). |
Tea bags. |
Echinacea purpurea herb. |
Each tea bag contains: |
Pour 8 oz (~227 mL) freshly boiled water over 1 tea bag. Cover & Steep for 10-15 min. Enjoy 5-6 cups throughout the day. |
5,025 - 6,030 mg Echinacea purpurea herb. |
Do not use if you are pregnant or breastfeeding unless directed otherwise by your healthcare practitioner. Not recommended for use with children under 12 years of age. |
|
Traditional Medicinals, Organic Immune Zoom®, Lemon Ginger Echinacea, Caffeine Free, 16 Wrapped Tea Bags, 1.13 oz (32 g). |
Tea bags. |
Echinacea purpurea root. |
2,000 mg herb blend (ginger rhizome, Echinacea purpurea herb, lemon myrtle leaf (Backhousia citriodora), lemon peel, liquorice root, peppermint leaf, Echinacea purpurea root dry extract (2-8:1), cardamom seed, Organic liquorice root dry extract (6:1). |
Pour 8 oz (~227 mL) freshly boiled water over 1 tea bag. Cover & Steep for 10-15 min. Enjoy 2 cups throughout the day. |
Not specified. |
Consult your healthcare practitioner prior to use if you are pregnant or breastfeeding; or if you have an autoimmune or other immune system disorder, or if you are taking immunosuppressants; or if you have liver or gallbladder disease. Not recommended for use with children under 12 years of age. |
|
Superblends Defence 20 Tea Bags. |
Tea bags. |
Echinacea (species not specified) root. |
Green tea (26%), Ginger root (15%), White hibiscus, Cinnamon bark, Natural lemon Flavouring with other Natural Flavourings (10%), Echinacea root (9%), Lemon peel (5%), Natural flavouring, Natural lime flavouring (4%), vitamin C (2%). |
At least 1 cup a day. |
Not specified. |
None. |
|
Frontier Co-op, Organic Cut & Sifted Echinacea Angustifolia Root. |
Loose herb for tea. |
Echinacea angustifolia root. |
Half a teaspoon contains 1.1 g cut root. |
To prepare as tea, pour 8 oz. (~227 mL) boiling water over 1/2 teaspoon of root. Cover and steep 20-30 minutes, strain and serve immediately. |
Not specified. |
If pregnant, nursing, suffering from any medical condition, or taking medication, consult a healthcare practitioner before use. |
|
Frontier Co-op, Organic Elderberry Echinacea Wellness Tea, 16 oz. |
Loose herb for tea. |
Echinacea purpurea herb and root. |
Elderberry, Echinacea purpurea herb, peppermint, yarrow, ginger, chamomile flower, Echinacea purpurea root. |
Pour 8 oz. (227 mL) boiling water over 1 tablespoon of tea. Cover and steep 10-15 minutes, strain and serve immediately. |
Not specified. |
If pregnant, nursing, suffering from any medical condition, or taking medication, consult a healthcare practitioner before use. |
|
Frontier Co-op, Cut & Sifted Echinacea Purpurea Herb. |
Loose herb for tea. |
Echinacea purpurea herb. |
One teaspoon contains 820 mg Echinacea purpurea cut herb. |
To prepare as a tea, pour 8 oz. (~227 mL) boiling water over 1 teaspoon of herb. Cover and steep 3-5 minutes, strain and serve immediately. |
Not specified. |
None. |
|
Lemon & Ginger Vitamin Honey. |
Honey. |
Not specified. |
1 teaspoon (7g) contains: 15 mg Echinacea, 3 mcg vitamin D3, 10mg vitamin C, 0.4 mcg vitamin B6, 0.4 mcg vitamin B12. |
2 teaspoons into warm water. |
Not specified. |
This product is suitable for pregnant or breastfeeding women, however we'd always recommend that you consult with a health professional if you are unsure before making a purchase. |
|
Orange Vitamin Honey. |
Honey. |
Not specified. |
1 teaspoon contains: 15 mg Echinacea, 3 mcg vitamin D3, 10mg vitamin C, 0.4 mcg vitamin B6, 0.4 mcg vitamin B12. |
2 teaspoons into warm water. |
Not specified. |
This product is suitable for pregnant or breastfeeding women, however we'd always recommend that you consult with a health professional if you are unsure before making a purchase. |
|
Wedderspoon Natural Manuka Honey and Ginger with Echinacea Drops (20 Drops per box). |
Honey. |
Not specified. |
Organic cane sugar, organic manuka honey (15.5%), organic brown rice syrup, ground ginger (0.6%), Echinacea (0.04%). |
Not specified. |
Not specified. |
None. |
|
A.Vogel Echinacea Lozenges | Extract of Freshly Harvested Echinacea | Blend of Other Herbs | Suitable for Vegetarians | 30g. |
Lozenges. |
Echinacea purpurea herb and root extract. |
Each lozenge (2.2 g) contains: Glucose syrup, raw cane sugar, honey, herb extracts, fresh Echinacea purpurea extract (0.62%), natural flavours, caramel colour, menthol, peppermint essential oil, citric acid. |
As required. |
Not specified. |
None. |
|
Honey Lemon Echinacea Soothe & Clear Drops 75g (Ricola). |
Lozenges. |
Echinacea (species not specified) dry pressed juice. |
Sugar, glucose syrup, Fair Trade honey (5.1%), extract (0.5%) of Ricola's herb mixture, vitamin C, lemon juice concentrate, acid (citric acid, malic acid), natural flavourings, natural Echinacea aroma (Echinacea dry pressed juice), mint oil, peppermint oil, menthol. |
As required. |
Not specified. |
None. |
|
Swanson, Zinc & C with Elderberry & Echinacea, Orange & Lemon, 60 Lozenges. |
Lozenges. |
Echinacea purpurea herb (aerial parts) powder. |
Each lozenge contains: |
As a dietary supplement, dissolve one lozenge in the mouth two times per day. |
40 mg Echinacea purpurea powdered herb. |
149. The NDNS data suggests that women of childbearing age consume a mean of 520 mL/person/day or 1,500 mL/person/day at the 97.5th percentile of herbal and fruit tea at the acute consumption level (Table 4a). This corresponds to a mean of 5.2 g/person/day or 15 g/person/day of fruit and herbal tea at the 97.5th percentile on a dried basis (calculated by applying a conversion factor of 0.01 to convert tea as consumed to dry weight; Table 4b). At the chronic consumption level, women of childbearing age consume a mean of 290 mL/person/day or 1,100 mL/person/day at the 97.5th percentile (Table 4a), corresponding to 2.9 g/person/day or 11 g/person/day respectively on a dried basis (Table 4b).
150. Information from Echinacea tea products available suggests preparing the teacup with 227-250 mL hot water and consumption recommendations vary between 2-6 cups per day (Table 3). Based on the Echinacea content of the tea products (Table 3), that would provide 144 - 1005 mg herb per cup of tea. Taking the NDNS data into consideration and the assumption that a cup of Echinacea tea will be prepared with 250 mL water, this would equate to the consumption of 6 Echinacea cups of tea per day at the 97.5th percentile by women of childbearing age at the acute consumption level, corresponding to 864 – 6,030 mg Echinacea herb. At the chronic consumption level, approximately 4 cups of tea per day will be consumed at the 97.5th percentile, corresponding to 576 – 4,020 mg Echinacea herb.
Table 4a: Acute and chronic consumption of herbal and fruit tea (as consumed) as a proxy for Echinacea tea (without recipes).
|
Consumption |
Consumers (n)^ |
Mean (mL/person/day) |
97.5th percentile (mL/person /day) |
Mean (mL/kg/ bw/day) |
97.5th percentile (mL/kg bw/day) |
Respondents in population group (n) |
|
Acute |
364 |
520 |
1,500 |
8.0 |
23 |
2,556 |
|
Chronic |
364 |
290 |
1,100 |
4.5 |
16 |
2,556 |
*Rounded to 2 significant figures.
^Based on women of childbearing age (16-49 years).
a Conversion factor of 0.99 used to convert tea dry weight to ‘as consumed’.
Table 4b: Acute and chronic consumption of herbal and fruit tea (dry weight) as a proxy for Echinacea tea (without recipes).
|
Consumption |
Consumers (n)^ |
Mean (mL/person/day) |
97.5th percentile (mL/person/day) |
Mean (mL/kg/ bw/day) |
97.5th percentile (mL/kg bw/day) |
Respondents in population group (n) |
|
Acute |
364 |
5.2 |
15 |
0.08 |
0.23 |
2,556 |
|
Chronic |
364 |
2.9 |
11 |
0.045 |
0.16 |
2,556 |
*Rounded to 2 significant figures.
^Based on women of childbearing age (16-49 years).
a Conversion factor of 0.01 used to convert tea from ‘as consumed’ to dry weight.
151. The NDNS data on honey consumption (Table 5) suggests that women of childbearing age have an acute consumption of honey with a mean of 15 g/person/day honey or 48 g/person/day at the 97.5th percentile. Under the chronic consumption scenarios, these figures are a mean of 6 g/person/day honey or 25 g/person/day at the 97.5th percentile. Echinacea honey products contain 0.4-2.1 mg Echinacea per 1 g honey (Table 3). This would equate to the consumption of 19.2-101 mg Echinacea at the 97.5th percentile for acute consumption and 10-52.5 mg Echinacea under the chronic consumption scenario.
Table 5: Acute and chronic consumption of honey as a proxy for Echinacea honey consumption (without recipes).
|
Consumption |
Consumers (n)^ |
Mean (g/person/day) |
97.5th percentile (g/person/day) |
Mean (g/kg/ bw/day) |
97.5th percentile (g/kg bw/day) |
Respondents in population group (n) |
|
Acute |
293 |
15 |
48 |
0.23 |
0.75 |
2,556 |
|
Chronic |
293 |
6.0 |
25 |
0.093 |
0.35 |
2,556 |
*Rounded to 2 significant figures.
^Based on women of childbearing age (16-49 years).
152. Echinacea supplements found online are available as solid dosage forms such as tablets and capsules (Table 6a) and oral liquids such as oral solutions and tinctures (Table 6b). The majority of these supplements advise consultation with a healthcare provider prior to using them if pregnant/breastfeeding or state that they are not suitable for use in pregnancy and lactation. In addition, some of the supplements recommend short-term use only (5 days to several weeks).
Table 6a: Echinacea food supplements (solid dosage forms).
|
Product name |
Dosage form |
Echinacea species and plant part |
Composition |
Directions for use |
Daily dose Echinacea (mg) |
Additional information |
|
NOW Foods Echinacea 400 mg 100 Veg Capsules. |
Capsules. |
Echinacea purpurea root. |
400 mg root. |
Take 2 capsules 1 to 4 times daily as needed. |
800 - 3,200 mg root. |
For adults only. Consult physician if pregnant/nursing, taking medication, or have a medical condition. |
|
Grape Tree Echinacea Root 500mg. |
Tablets. |
Echinacea (species not specified) root. |
500 mg root. |
1 tablet daily. |
500 mg root. |
Linked to many health benefits including reduced inflammation, improved immunity and lower blood sugar levels. |
|
Swanson Echinacea, 400mg herbal supplement. |
Capsules. |
Echinacea purpurea herb (aerial parts). |
400 mg herb. |
1 capsule up to 3 times per day. Limit use to eight consecutive weeks. Use periodically for a few weeks at a time (for maintenance purposes). |
400-1,200 mg herb. |
For adults only. Do not take this product if you are pregnant or nursing. Consult your healthcare provider before using this or any product if you are taking medication or have a medical condition, especially an autoimmune condition. |
|
Echinacea, 1300 mg (per serving), 180 Vegetarian Capsules. |
Capsules. |
Echinacea purpurea herb (aerial parts). |
65 mg herb extract (DER 10:1) equivalent to 650 mg herb. |
Take 2 vegetarian capsules per day preferably with a meal. |
130 mg herb extract equivalent to 1,300 mg herb. |
If you are pregnant, nursing, taking any medications or have any medical conditions, consult your doctor before use. |
|
Life Extension, Echinacea Elite, 60 Vegetarian Capsules. |
Capsules. |
Echinacea purpurea herb and Echinacea angustifolia root. |
Echinacea purpurea (aerial parts) extract 125 mg [standardised to 4% phenolic compounds]. Echinacea angustifolia (root) extract 125 mg [standardised to 4% echinacosides] |
Take 1 capsule twice daily. |
250 mg Echinacea purpurea (aerial parts) extract and 250 mg Echinacea angustifolia (root) extract. |
Consult with your physician if you are undergoing treatment for a medical condition or if you are pregnant or lactating. |
|
Specialist Herbal Supplies (SHS) Echinacea Capsules. |
Capsules. |
Echinacea angustifolia. |
325 mg. Preparation not specified.. |
1 capsule, 3 times a day, taken with food or a drink. If desired, up to four times this amount can safely be taken. |
975-1,300 mg Echinacea angustifolia. |
If you are pregnant, breast-feeding, have a medical condition or are under medical supervision, please consult a doctor before use. |
|
Nuke Nutrition Echinacea Tablets High Strength x180 - Immune Support Echinacea Herbal Supplements. |
Tablets. |
Echinacea. Species and part of plant not specified. |
200 mg extract (DER 10:1) equivalent to 2,000 mg Echinacea. |
Take 1 tablet with your first meal of the day. |
200 mg extract equivalent to 2,000 mg Echinacea. |
Consult your physician if you are taking medication or are under medical supervision or if you are pregnant and breastfeeding. |
|
Echinacea Extract Capsules 3500mg (High Strength) Echinacea purpurea. |
Capsules. |
Echinacea purpurea. Plant part not specified. |
350 mg extract (DER 10:1) equivalent to 3,500 mg Echinacea pupurea. |
Take 1 capsule per day with water. |
350 mg extract equivalent to 3,500 mg Echinacea purpurea. |
None. |
|
Nature's Way, Echinacea Goldenseal, 450 mg, 100 Vegan Capsules. |
Capsules. |
Echinacea purpurea herb (aerial parts) and Echinacea angustifolia root. |
450 mg Echinacea 7 Herb Blend: |
Take 2 capsules twice daily, preferably with food. |
1,800 mg Echinacea 7 herb blend. |
Do not use if you are pregnant, nursing, have stomach or duodenal ulcers, stomach irritation or inflammation. Not recommended for individuals with autoimmune conditions. If you have diabetes, or are taking any medications, consult a healthcare professional before use. |
|
Nature's Way, Echinacea Purpurea Herb, 1,200 mg, 180 Vegan Capsules (400 mg per Capsule). |
Capsules. |
Echinacea purpurea herb (aerial parts). |
400 mg herb. |
Adults take 3 capsules three times daily, preferably with food. Only take this supplement if they are suffering severe illness to stimulate the immune system and to not take for longer than 5 days. |
3,600 mg herb. |
If pregnant, nursing, or taking any medications, consult a healthcare professional before use. |
|
California Gold Nutrition, EuroHerbs, Echinacea Herb Extract, Euromed Quality, 80 mg, 180 Veggie Capsules. |
Capsules. |
Echinacea purpurea herb (aerial parts). |
80 mg Echinacea purpurea (aerial parts) extract (DER 5:1) equivalent to 400 mg dried herb. |
Take 1 capsule daily, with food. |
80 mg extract equivalent to 400 mg dried herb. |
Pregnant or lactating women should consult with a physician, pharmacist, naturopath or other qualified healthcare professional prior to taking dietary supplements. |
|
21st Century, Echinacea Complex, 250 mg, 60 Vegetarian Capsules (125 mg per Capsule). |
Capsules. |
Echinacea purpurea herb and Echinacea angustifolia root. |
125 mg Echinacea blend (Echinacea purpurea herb extract and Echinacea angustifolia root powder). |
Adults take two (2) capsules daily with any meal or as directed by a healthcare provider. |
250 mg Echinacea blend (Echinacea purpurea herb extract & Echinacea angustifolia root powder). |
Consult a healthcare provider prior to use if pregnant, nursing, on medications, have a medical condition or are planning a medical procedure. |
|
Sundown Naturals, Whole Herb Echinacea, 400 mg, capsules. |
Capsules. |
Echinacea purpurea herb (aerial parts). |
400 mg Echinacea purpurea herb. |
Take (1) capsule seven times daily, preferably with meals. Capsules may be opened and prepared as a tea. |
2,800 mg herb. |
If you are pregnant, nursing, taking any medications or have any medical condition, consult your doctor before. |
|
Gaia Herbs, Echinacea Goldenseal, 60 Vegan Liquid Phyto-Caps. |
Capsules. |
Echinacea purpurea root, aerial parts and seed and Echinacea angustifolia root. |
800 mg Proprietary Extract Blend: |
Adults take 2 capsules 3 times daily between meals. |
4,800 mg Proprietary Extract Blend. |
Not for use during pregnancy or lactation. |
|
Specialist Herbal Supplies (Shs) Echinacea Compound. |
Capsules. |
Echinacea angustifolia. |
Echinacea angustifolia 92mg, Garlic 92mg, Myrrh 92mg, Wild Indigo 46mg. Echinacea preparation not specified. |
1 capsule, 3 times a day, taken with food or a drink. |
276 mg Echinacea angustifolia. |
Do not take alongside blood thinning drugs such as warfarin. Not to be used in pregnancy and breastfeeding or for children under 12 years old. |
Table 6b: Echinacea food supplements (oral liquids).
|
Product name |
Dosage form |
Echinacea species and plant part |
Composition |
Directions for use |
Daily dose Echinacea (mg) |
Additional information |
|
Nature's Way, Echinacea, 500 mg, 1 fl oz (30 mL). |
Oral solution. |
Echinacea purpurea herb (aerial parts). |
250 mg herb extract per 1 mL. |
Adults: Take 2 mL 3 times daily. |
Adults: 500-1,500 mg herb extract |
If pregnant, nursing, or taking any medications, consult a healthcare professional before use. Not recommended for individuals with auto-immune conditions. |
|
Echinacea Single Herbal Tincture 150mL. |
Tincture. |
Echinacea (species not specified). |
Dried herb to liquid ratio W/V 1:5 or fresh herb to liquid ratio W/V 1:3. |
Dosage is normally between 1mL and 5 mL added to a little water up to three times a day. |
3 - 15 mL herb extract daily equivalent to 600 -3,000 mg dried herb or 1,000 - 5,000 mg fresh herb. |
None. |
|
Baldwins Echinacea (angustifolia) Herbal Tincture. |
Tincture. |
Echinacea angustifolia. |
Herb:Liquid 1:3. |
No guidance. |
No guidance. |
None. |
|
Napiers the Herbalists Napiers Organic Echinacea Drops. |
Oral drops |
Echinacea purpurea herb (aerial parts) |
Not stated |
Take 15-20 drops 2-3 times a day. 15 drops = 0.5 mL |
30 - 60 drops daily. Equivalent to 1-2 mL solution. |
Not suitable for children under 12 years. Do not take if pregnant or breastfeeding. |
|
100% Organic Echinacea Tincture Viridian 50ml. |
Tincture. |
Echinacea purpurea whole plant. |
1 mL = 480 mg whole fresh plant. |
Take 15 – 30 drops, 2-3 times daily in a little fruit juice or water. |
31 - 60 drops daily. Equivalent to 1-2 mL solution or 480 - 960 mg fresh plant. |
Not to be used during pregnancy or lactation unless recommended by a healthcare practitioner. |
|
NOW Foods, Echinacea Extract, 2 fl oz (59 ml). |
Oral solution. |
Echinacea angustifolia and Echinacea purpurea root. |
Root extract. 1.6 mL per 2 droppersfuls. |
Take 1 to 2 droppersful in tea or water 1 to 3 times daily as needed. Continuous high-level consumption of this product for more than 2 weeks of each month is not recommended. |
0.8 - 4.8 mL. |
Not recommended for pregnant or nursing women. |
|
Cytoplan Organic Echinacea. |
Oral solution. |
Echinacea angustifolia. |
1:3 extract. |
Take 20 drops mixed into water or liquid of choice 2-3 times daily. 20 drops = 2mL. |
40-60 drops daily. Equivalent to 4-6 mL extract daily. |
Not suitable for children under 12 years of age. Not suitable for use whilst pregnant or breastfeeding. |
153. If the supplements are composed of herbal blends or don’t specify directions for use or preparation type, the recommended daily dose of Echinacea is difficult to derive. Where extracts are used in preparations, the daily dose of Echinacea from capsules/tablets (Table 6a) is 130 – 700 mg dry herb extract (equivalent to 1,300 – 7,000 mg herb). For tablets/capsules containing dried plant parts (herb or root), the dose of Echinacea is 400 – 3,600 mg (herb) or 500 – 3,200 mg (root). Fewer oral liquid products were found from online sources and the directions for use, or the exact composition was lacking for some (Table 6b). From the liquid products available, the daily Echinacea dose is 500 – 1,500 mg herb extract or 600 – 3,000 mg dried herb.
154. The Echinacea products for oral use with THR from the MHRA are available as tablets, capsules, oral solutions, tinctures and oromucosal spray (see Table 10 Annex B). The most common products with THR in the UK are tablets and capsules based on dry extract from E. purpurea root, with daily doses of 143-429 mg dry root extract (equivalent to 858 – 3,000 mg root). If THR preparations (available in both solid and liquid dosage forms) made with dried pressed juice from E. purpurea herb are consumed, the daily dose would be 176-352 mg dried pressed juice (equivalent to 3.5-9.8 g fresh herb). A comparison between the THR products and the EMA monographs in terms of species used, preparations and doses can be found in Table 11, Annex B. There is no evidence to suggest that THR products and Echinacea food supplements are taken together, and the assumption is that this is unlikely, especially since the THR products advise against use in pregnancy in their patient information leaflets. In addition, the regulation of THR is a remit of the MHRA and the Echinacea exposure from THR is therefore not considered in the combined consumption scenarios discussed below.
155. Pregnant women may use a combination of Echinacea containing foods such as tea, honey and lozenges in addition to food supplements in the form of tablets, capsules and oral liquids during pregnancy. The FSA’s Exposure Team calculated consumption values for Echinacea during pregnancy, assuming different combinations of tea, honey, lozenges, tablets/capsules and oral liquids in order to cover different worst-case exposure scenarios. These combined consumption values displayed in Tables 8a-8c Annex B (acute consumption) and Tables 9a-9a Annex B (chronic consumption) are based on supplement dose recommendations for the food supplements and NDNS consumption data for the tea, honey and lozenges combined with information on their Echinacea content from the product ingredient listing. Tables 7a and 7b show the estimated minimum and maximum consumption levels of Echinacea for different numbers of Echinacea containing products consumed under the acute and chronic consumption scenarios respectively. It is apparent that the consumption level resulting from combined use of food products and food supplements may reach up to 13,000 mg Echinacea (as dried herb/root) for acute consumption and up to 11,000 mg for the chronic consumption scenario, especially if 3 or more Echinacea containing products are consumed together.
Table 7a: Estimated minimum and maximum acute consumption of Echinacea (as dried root/herb), based on number of products consumed per day, during pregnancy.
|
Number of Echinacea products consumed per day |
Minimum estimated consumption Echinacea (mg/day) |
Maximum estimated consumption Echinacea (mg/day) |
|
2 |
60 |
9,600 |
|
3 |
460 |
13,000 |
|
4 |
1,100 |
13,000 |
|
5 |
1,900 |
13,000 |
*Rounded to 2 significant figures.
Table 7b: Estimated minimum and maximum chronic consumption of Echinacea (as dried root/herb), based on number of products consumed per day, during pregnancy.
|
Number of Echinacea products consumed per day |
Minimum estimated consumption Echinacea (mg/day) |
Maximum estimated consumption Echinacea (mg/day) |
|
2 |
50 |
7,600 |
|
3 |
450 |
11,000 |
|
4 |
1,000 |
11,000 |
|
5 |
1,600 |
11,000 |
*Rounded to 2 significant figures.
Risk Characterization
In this guide
In this guide156. There are several layers of uncertainty regarding the safety of Echinacea supplements consumption during pregnancy and lactation. There are three different Echinacea species in medicinal use, E. purpurea, E. pallida and E. angustifolia, with different parts of the plant (root, herb, flower or whole plant) utilised and different methods of extraction used (powdered plant parts, dry and liquid extracts, pressed and dried pressed juice). The composition of bioactive components varies depending on the preparation and there is currently no consensus on how the Echinacea preparations should be standardised. The impact of differences in composition on the toxicological potential between the available products is therefore unknown. In addition, some of the supplements and food products do not state the Echinacea species, part of plant or preparation type, rendering comparison between products challenging.
157. The Echinacea doses used in clinical studies vary between 100-4,000 mg/day extract and 6,200-10,000 mg/day pressed juice with duration from 5 days to 4 months with E. purpurea and E. angustifolia being most commonly used. These doses are comparable to those estimated by the FSA Exposure Assessment Team (EAT) for Echinacea consumption from different supplements and foods during pregnancy. One caveat is that the doses estimated by the EAT team are based on dried Echinacea root/herb rather than extracts/pressed juice as many of the supplements and food products either list the Echinacea content as dried plant parts or do not specify the nature of the preparation. Thus, a direct comparison is challenging as generally extracts are more concentrated and potent than the dried plant equivalents.
158. There are limited data from animal and human studies on the safety of Echinacea use during pregnancy and lactation. None of the animal studies available on the reproductive and developmental effects of Echinacea conform to the OCED guidelines. Two mice studies (Chow et al., 2006 and Barcz et al., 2007) investigated the effects of Echinacea during pregnancy with one focused on spontaneous abortions and the other on foetal angiogenesis. Chow et al. (2006) reported increased foetal loss in the Echinacea treated mice by 12-14 days of gestation and warned against the consumption of Echinacea in the early stages of pregnancy. Barcz et al (2007) reported a significant decrease in angiogenic factors VEGF and bFGF with the three different Echinacea preparations tested and some conflicting results on angiogenic activity, whereby one of the preparations increased angiogenic activity, another decreased it and the third one had no effect. The study concluded that Echinacea may influence foetal angiogenesis and should be avoided during pregnancy as a precaution. Small numbers of animals were used in both studies and only one dose of Echinacea was tested. A mice study by Khaksary Mahabady et al. (2006) suggested that E. purpurea extracts can reduce the incidence of phenytoin induced cleft palate.
159. A study examining the effects of E. purpurea in pregnant pigs (Maass et al., 2005) found no significant differences in the biochemical and immunological parameters of the sows and reported no adverse effects in the piglets. Another study looking at E. pallida supplementation in pregnant rabbits found no significant effects on the reproductive, haematological or immune parameters of the does (Dabbou et al., 2016). The second part of the study (Kovitvadhi et al., 2016) investigated the effects of Echinacea supplementation on the offspring and reported no significant differences in growth performance, blood biochemistry or humoral immune response between rabbits born to Echinacea treated and control group does.
160. There are no prospective interventional clinical trials on Echinacea use in pregnant or lactating women (EMA, 2014). There is a prospective controlled study (Gallo et al., 2000) and a cohort study (Heitmann et al., 2016) looking at pregnancy outcomes associated with Echinacea use. There are two studies administered through self-reported surveys aiming to investigate the use of herbal remedies, including Echinacea, in pregnant women and link them to pregnancy outcomes. (Cuzzolin et al., 2010; Nordeng et al., 2011). The limited data from the human studies did not reveal any adverse effects on the mother or the infant that are specifically linked to Echinacea use during pregnancy. The two observational studies by Gallo et al. (2000) and Heitmann et al. (2016) reported no significant differences in the rates of malformations, birth weight and pregnancy outcomes between treatment and control groups.
161. The human studies demonstrate that Echinacea is consumed during pregnancy for similar indications as in the general population including the treatment and prevention of cold and flu and respiratory tract infections such as sinusitis, tonsilitis, cough, bronchitis and pneumonia. Overall, the human studies available lack information about the specific Echinacea species, plant part, type of preparation used, administered dose, the duration of intake and the trimester during which Echinacea was used. It is therefore not possible to directly compare doses used during pregnancy in ‘real life’ situations to those estimated by the FSA EAT team.
162. No evidence of genotoxicity has been observed with E. purpurea and E. angustifolia preparations in in vitro bacterial reverse mutation assays, in vitro chromosomal aberration tests as well as in vivo micronucleus test conducted by several OECD guideline conforming studies. The animal data from studies investigating the acute, subacute and sub-chronic toxicity of Echinacea suggest that overall Echinacea has low toxicity and is well tolerated. Upon reviewing the data from human studies on Echinacea purpurea, EMA (2014) concluded that oral preparations are well tolerated and have an acceptable safety profile with mild, transient and reversible adverse effects, with gastrointestinal disturbances and allergic skin reactions being the most commonly reported adverse effects. A systematic review on the adverse effects of Echinacea found that they were most frequently associated with ethanolic herb and root extracts (Di Lorenzo et al., 2015).
163. There are case reports in the literature and pharmacovigilance databases suggesting that Echinacea can be associated with serious allergic reactions, including anaphylaxis, particularly in patients with history of atopy. A study by Mullins and Heddle (2002) looked at potential IgE-mediated hypersensitivity reactions identified from the Australian Adverse Drug Reactions Advisory Committee’s database and evaluated five of the patients, who had suffered anaphylaxis, asthma attacks or macular rash after Echinacea exposure. The study concluded that patients with history of atopic diseases should be cautioned against Echinacea use due to possible cross-reactivity between Echinacea and other allergens. EMA (2014) also recommends that atopic patients should consult their doctor before using Echinacea.
164. There are isolated case reports of Echinacea triggering autoimmune diseases such as erythema nodosum, hyperoesinophilia, leucopenia, thrombocytopenia, severe acute cholestatic autoimmune hepatitis and fatal liver necrosis. EMA has reviewed these case reports and deemed that the causality of adverse events in pharmacovigilance cases concerning autoimmune diseases is not known or inconclusive, but association with autoimmune diseases cannot be excluded (EMA, 2014). EMA also highlights that infectious agents and inflammatory processes present in common cold can promote autoimmune diseases.
165. The Echinacea products with THR recommend a duration of use no longer than 10 days. This is in line with the EMA monographs on E. purpurea, E. angustifolia and E. pallida. The monographs don’t provide a scientific rationale for the short duration of use recommended. Echinacea has been used in clinical studies for durations up to 6 months at doses of 1,800 mg/day with minimal side effects such as nausea and diarrhoea (Vonau et al., 2001). Doses of 2,400-4,000 mg daily were also well tolerated in a 4 month long study with 755 participants (Jawad et al., 2012). Given the indications for Echinacea use and the warnings on most products to avoid prolonged use, it can be assumed that if used during pregnancy, Echinacea products will be consumed short term for the treatment and relief of common cold symptoms.
166. Most commercially available Echinacea preparations, both food supplements and products with THR, warn against use in people with autoimmune conditions. EMA also states that based on the presumption that Echinacea has immunomodulatory properties, it is not recommended in progressive systemic disorders, autoimmune diseases, immunodeficiencies, immunosuppression and diseases of the white blood cell system (EMA, 2014). There is a body of evidence from in vitro, in vivo and human studies that Echinacea has immunomodulatory properties, which are in part responsible for its effects on lowering the risk of recurrent respiratory tract infections, their complications and alleviating the symptoms of influenza infections. An in vivo mice study by Fusco et al. (2010) suggested that treatment with Echinacea improved the clinical outcomes of influenza infected mice due to immune system modulation and decrease in serum IFN-γ and IL-10 brought by Echinacea. The effects of Echinacea on cytokine production by the immune system are complex. Echinacea was effective at inhibiting the induction of IL-6, IL-8 and TNF-α in human cell lines infected with influenza, rhinovirus, adenovirus and respiratory syncytial virus (Sharma et al., 2009). On the other hand, Echinacea has been shown to stimulate TNF-α, IL-1α, IL-1β production from macrophages in a similar way to bacterial LPS in vitro (Burger et al., 1997; Rininger et al., 2002). A study in rats (Goel et al., 2002) showed that Echinacea stimulates phagocytosis in alveolar macrophages and release of TNF-α and IFN-γ release by spleen macrophages, whilst the results of an in vivo mice study suggested that Echinacea treatment decreases the production of IL-1β and TNF-α by LPS stimulated macrophages (Zhai et al., 2007). Zhai et al. (2007) also reports that treatment with E. angustifolia and E. pallida increases the production of TH2 cytokines (IL-4, IL-6, and IL-10) by splenic lymphocytes, suggestive of anti-inflammatory activity.
167. Studies have also demonstrated that Echinacea can activate NK mediated cytotoxicity in vitro (Gan et al., 2003; See et al., 1997) and in vivo (Zhai et al., 2007). The study by Chow et al. (2006), investigating the link between maternal Echinacea consumption and spontaneous abortions in mice, was based on results from previous studies, showed that Echinacea increases the number of NK cells and that NK cells have a role in foetus rejection and in spontaneous abortions (De Fougerolles and Baines, 1987; Gendron and Baines, 1988). There is evidence from human studies suggesting that abnormalities in uterine NK cell numbers of function can lead to pregnancy complications such as recurrent miscarriage, preeclampsia or infertility (Mahajan et al., 2022). However, there are currently no studies linking the effects of Echinacea on NK and pregnancy complications.
168. Studies have demonstrated that Echinacea and its extracts can inhibit recombinant human cytochrome P450 (CYP) enzymes 3A4, 2E1, 1A2, 2C19 and 2C9 enzymes in vitro to various degrees (Husain et al., 2023; Modarai et al., 2010; Raner et al., 2007; Yale and Glurich, 2005). The total alkylamide content of the Echinacea preparations has been positively associated with its ability to inhibit the enzymes, in particular CYP3A4 (Modarai et al., 2010) and CYP2E1 (Raner et al., 2007). In addition, E. purpurea has demonstrated mild inhibitory activity towards P-glycoprotein in vitro (Hansen and Nilsen, 2009; Husain et al., 2023). Penzak et al. (2010) investigated the effects of E. purpurea 1,500 mg/day for 28 days on the pharmacokinetics of lopinavir-ritonavir using midazolam and fexofenadine as CYP3A4 and P-glycoprotein probes, respectively. They reported that the pharmacokinetics of lopinavir/ritonavir and fexofenadine were not influenced by Echinacea co-administration, whilst the clearance of midazolam increased, suggesting CYP3A4 induction. A study by Gorski (2004) confirmed the inhibitory effect of commercially available E. purpurea root extract preparation, taken at 1,600 mg daily for 8 days, on CYP2C9 and CYP1A2 in 12 healthy human volunteers. The authors only considered the effects on CYP1A2 as clinically significant with the potential to increase the toxicity of narrow therapeutic window drugs such as theophylline. However, there are no reports in the literature of an interaction between Echinacea and theophylline. The results from the same study indicate that Echinacea could also inhibit the intestinal CYP3A4 isoform, but not the hepatic form. Another human study (Gurley et al., 2004) using E. purpurea 1,600 mg/day for 28 days in 12 healthy volunteers reported no significant changes in the activities of CYP3A4, CYP2E1, and CYP2D6. There was a mild inhibition on CYP1A2, but it was not statistically significant. These results suggest that Echinacea may have the potential to interact with prescription medication during pregnancy, but the exact effects are unclear due limited clinical data on these interactions.
169. There is additional uncertainty surrounding the health risk posed by potential contaminants in Echinacea preparations. There are very few studies looking at the presence of contaminants such as heavy metals, fungi, bacteria and mycotoxins in Echinacea products. Alternaria alternata, Aspergillus spp., Fusarium spp., Phoma spp., yeasts and mycotoxins have been detected in Echinacea herbal supplements available on the Polish market (Tournas, 2009). Whilst cadmium, arsenic and lead have been detected in commercial Echinacea products, their levels have been considerably lower than the limits set by WHO and they are not considered to pose a health risk to the public (Filipiak-Szok et al., 2015; Raman et al., 2004).
Conclusions and Questions
In this guide
In this guideConclusions
170. Three Echinacea species – E. purpurea, E. angustifolia and E. pallida have been used medicinally to relieve the symptoms and shorten the duration of cold and flu infections. Echinacea preparations can be made from the dried roots of all three species, the fresh or dried aerial parts and the pressed juice from E. purpurea. Ethanolic extracts are also often used in many Echinacea products. The effects of Echinacea are due to the combination of bioactive metabolites including alkylamides, caffeic acid derivatives and polysaccharides. The composition of these compounds varies across the species, the plant parts, season, growing conditions and extraction methods used.
172. There is evidence from in vitro and in vivo studies that Echinacea preparations can inhibit the entry of influenza virus in the cells and modulate the immune response after a viral infection. Clinical studies suggest that Echinacea can lower the risk of recurrent respiratory tract infections and complications that arise from them. Herbal products containing E. purpurea, E. angustifolia and E. pallida have herbal medicinal licences in EU/EEA member states and THR licenses from the MHRA based on traditional use for the relief of common cold symptoms. These products are licensed for adults and children over 12 years of age and not recommended for pregnant or lactating women due to insufficient safety data available. Nevertheless, data from surveys on the use of herbal medicines during pregnancy suggests that 4-10% of pregnant women use Echinacea for the treatment and prevention of cold/flu and immune system support.
173. In addition to products with a THR license, there is a range of foods and food supplements containing Echinacea and its extracts. The most common food supplements are tablets and capsules, and the majority of these products carry a warning against their use in pregnancy/lactation and a recommendation for short term use only. There are also food products such as tea and honey which contain Echinacea. Whilst products with THR are acknowledged in this paper, the focus in the conducted exposure assessment has been the consumption of Echinacea foods and food supplements.
174. There is a lot of uncertainty around the safety of using Echinacea products during pregnancy or lactation due to limited data from in vitro, in vivo and clinical studies. In vitro and in vivo OECD guideline conforming studies suggested that Echinacea is not genotoxic. There are two studies in mice, one in pigs and two studies in rabbits looking at the effects of Echinacea supplementation during pregnancy. Whilst the two mice studies highlighted potential increase in foetal loss and altered angiogenesis with Echinacea, the sample sizes were small and some of the results reported on foetal angiogenesis were conflicting. The pig and rabbit studies did not report any significant differences in relation to birth weight, pregnancy outcomes and frequency of malformations between Echinacea and control groups. There are two human studies investigating the effects of Echinacea on pregnancy outcomes and they did not highlight any adverse effects associated with gestational use of Echinacea. These studies are observational and rely on self-reported use of Echinacea during pregnancy. The dose, preparation or duration of use were not reported.
175. The doses used in clinical studies on the efficacy of Echinacea are comparable to the estimated consumption of Echinacea of women of child-bearing age calculated by the FSA Exposure Assessment Team. Echinacea was well-tolerated in these clinical studies, but they did not include pregnant or lactating women. In addition, an exact comparison between different Echinacea products is challenging due to products containing different combinations of the three medicinally used species, their dried plant parts and extracts. Some food products such as tea and honey often lack information on the exact species, plant parts or extracts used.
176. The in vivo toxicological studies on Echinacea suggested that it has low toxicity. Clinical studies reported that Echinacea products are well tolerated with minor and reversible side effects including gastrointestinal disturbances and allergic skin reactions. There are isolated case reports of Echinacea causing erythema nodosum, hyperoesinophilia, leucopenia, thrombocytopenia and hepatotoxicity, but the causality has not been confirmed. Pharmacovigilance cases and follow up investigation of selected patients also suggested that Echinacea can trigger allergic reactions, as serious as anaphylaxis in some cases, in patients with pre-existing atopic diseases. EMA (2014) recommends Echinacea preparations should be used with caution in patients with asthma or history of atopy. Due to its potential for immune system modulation, Echinacea is also not recommended for people with autoimmune diseases, immunodeficiencies, immunosupression and diseases of the white blood cell system appears in the section.
177. There is an uncertainty around the potential of Echinacea to interact with prescription medicines during pregnancy. In vitro and in vivo studies demonstrated that Echinacea can affect the activity of CYP enzymes leading to inhibition of CYP1A2 and CYP3A4. One of the studies warned of potential toxicity if narrow therapeutic window drug substrates for CYP1A2, such as theophylline, are co-administered with Echinacea. This putative interaction has not been reported in the literature and its clinical relevance remains unclear.
178. Contaminants such as heavy metals, fungi, bacteria, mycotoxins and pesticides are sometimes found in herbal preparations. There is an uncertainty of how much risk the potential contaminants in Echinacea preparations pose to pregnant consumers due to lack of research. Whilst studies have reported that cadmium and lead levels detected in Echinacea preparations have been lower than the WHO limits, the presence of fungal contaminants and mycotoxins found in some Echinacea products can pose an additional risk during pregnancy.
Questions for the committee
I. Is the Committee able to determine the risk to maternal health associated with Echinacea consumption?
II. Is it possible to derive a point of departure to be used in the risk assessment of Echinacea, based on the information presented in this discussion paper?
III. Does the Committee have any other comments on this paper?
List of Abbreviations
In this guide
In this guide|
ACAH |
Acute cholestatic autoimmune hepatitis |
|
ADCC |
Antibody-dependent cellular cytotoxicity |
|
ADR |
Adverse drug reactions |
|
AIDS |
Acquired immunodeficiency syndrome |
|
ALT |
Alanine aminotransferase |
|
AST |
Aspartate aminotransferase |
|
AUC |
Area under the curve |
|
bFGF |
Basic fibroblast growth factor |
|
BMDCs |
Bone marrow derived dendritic cells |
|
bw |
Body weight |
|
CAM |
Complementary and alternative medicines |
|
CFS |
Chronic fatigue syndrome |
|
CFU |
Colony forming units |
|
CI |
Confidence intervals |
|
CYP |
Cytochrome P450 |
|
DER |
Drug extract ratio |
|
EFSA |
European Food Standards Agency |
|
ELISA |
Enzyme-linked immunosorbent assay |
|
EMA |
European Medicine Agency |
|
FCV |
Feline calicivirus |
|
FDA |
Food and drug administration |
|
GLP |
Good laboratory practice |
|
GHS |
Globally Harmonized Classification System |
|
HBGV |
Health-based guidance values |
|
HMR |
Human Medicines Regulation |
|
HMPC |
Committee on Herbal Medicinal Products |
|
HPLC |
High performance liquid chromatography |
|
HPRT |
Hypoxanthine phosphoribosyltransferase |
|
HSV |
Herpes simplex virus |
|
ICP-MS |
Inductively-coupled plasma mass spectrometer |
|
IgE |
Immunoglobulin E |
|
IgG |
Immunoglobulin G |
|
IL |
Interleukin |
|
INF |
Inteferon |
|
IV |
Intravenous |
|
LDH |
Lactate dehydrogenase |
|
LU |
Lytic units |
|
MAPK |
Mitogen-activated protein kinase |
|
MDCK |
Madin-Darby canine kidney cells |
|
MHRA |
Medicines and Healthcare Products Regulatory Agency |
|
MN |
Micronuclei |
|
MNPCE |
Micronucleated polychromatic erythrocytes |
|
NHANES |
National Health and Nutrition Examination Survey |
|
NCE |
Normochromatic erythrocytes |
|
NDNS |
National Diet and Nutrition Survey |
|
NF-κB |
Nuclear factor kappa-light-chain-enhancer of activated B cells |
|
NK cells |
Natural killer cells |
|
OECD |
Organisation for Economic Co-operation and Development |
|
PBMC |
Peripheral blood mononuclear cells |
|
PCE |
Polychromatic erythrocytes |
|
PFU |
Plaque-forming unit |
|
P-gp |
P glycoprotein |
|
PMN |
Polymorphonuclear leukocytes |
|
PNG |
Polymorphonuclear neutrophil granulocytes |
|
RBCs |
Red blood cells |
|
RET |
Reticulocytes |
|
RPMI |
Roswell Park Memorial Institute |
|
RSV |
Respiratory syncytial virus |
|
SARS-CoV-2 |
Severe Acute Respiratory Syndrome Coronavirus-2 |
|
SHE |
Syrian hamster embryo cells |
|
SMA |
Smooth muscle antibodies |
|
STP |
Skin prick testing |
|
TGF-β1 |
Transforming growth factor beta |
|
THR |
Traditional herbal registration |
|
TNF |
Tumour necrosis factor |
|
TTP |
Thrombotic thrombocytopenic purpura |
|
UKTIS |
UK Teratology Information Service |
|
VEGF |
Vascular endothelial growth factor |
|
WBC |
White blood count |
|
WHO |
World Health Organization |
References
In this guide
In this guideAhmadi, F., Kariman, K., Mousavi, M., Rengel, Z. (2024). Echinacea: Bioactive Compounds and Agronomy. Plants 13, 1235. Echinacea: Bioactive Compounds and Agronomy
Ardjomand-Woelkart, K., Bauer, R. (2015). Review and Assessment of Medicinal Safety Data of Orally Used Echinacea Preparations. Planta Med. 82, 17–31. Thieme E-Journals - Planta Medica / Abstract
Ardjomand-Woelkart, K., Kollroser, M., Derendorf, H., Bauer, R., Butterweck, V. (2012). Herb-Drug Interactions: Effects of Echinacea preparations on cytochrome P450 activities in rats. Planta Med. 78.
Barcz, E., Sommer, E., Nartowska, J., Balan, B.J., Chorostowska-Wynimko, J., Ewa, Skopińska-Różewska, E. (2007). Influence of Echinacea purpurea intake during pregnancy on fetal growth and tissue angiogenic activity. Folia Histochem Cytobiol. ;45 Suppl 1:S35-9.
Barnes, J., Anderson, L.A., Gibbons, S., Phillipson, J.D. (2010). Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 57, 929–954. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): a review of their chemistry, pharmacology and clinical properties† | Journal of Pharmacy and Pharmacology | Oxford Academic
Binns, S.E., Hudson, J., Merali, S., Arnason, J.T. (2002). Antiviral Activity of Characterized Extracts from Echinacea spp. (Heliantheae: Asteraceae) against Herpes simplex Virus (HSV-I). Planta Med. 68, 780–783. Thieme E-Journals - Planta Medica / Abstract
Blumenthal, M., Busse, W.R., Bundesinstitut für Arzneimittel und Medizinprodukte (Eds.), (1999). The Complete German Commission E monographs: therapeutic guide to herbal medicines, Reprint. ed. American Botanical Council [u.a.], Austin, Texas.
Buettner, C., Mukamal, K.J., Gardiner, P., Davis, R.B., Phillips, R.S., Mittleman, M.A., (2009). Herbal Supplement Use and Blood Lead Levels of United States Adults. J. Gen. Intern. Med. 24, 1175–1182. Herbal Supplement Use and Blood Lead Levels of United States Adults | Journal of General Internal Medicine
Burger, R.A., Torres, A.R., Warren, R.P., Caldwell, V.D., Hughes, B.G. (1997). Echinacea-induced cytokine production by human macrophages. Int. J. Immunopharmacol. 19, 371–379. https://doi.org/10.1016/S0192-0561(97)00061-1
Burlou-Nagy, C., Bănică, F., Jurca, T., Vicaș, L.G., Marian, E., Muresan, M.E., Bácskay, I., Kiss, R., Fehér, P., Pallag, A. (2022). Echinacea purpurea (L.) Moench: Biological and Pharmacological Properties. A Review. Plants 11, 1244. https://doi.org/10.3390/plants11091244
Chow, G., Johns, T., Miller, S.C. (2006). Dietary Echinacea purpurea during Murine Pregnancy: Effect on Maternal Hemopoiesis and Fetal Growth. Neonatology 89, 133–138. https://doi.org/10.1159/000088795
Clifford, L.J., Nair, M.G., Rana, J., Dewitt, D.L. (2002). Bioactivity of alkylamides isolated from Echinacea purpurea (L.) Moench. Phytomedicine 9, 249–253. https://doi.org/10.1078/0944-7113-00105
Committee on herbal medicinal products (HMPC) (2007). Community list entry on Echinacea purpurea (L.) Moench, herba recens. Community list entry Echinaceae purpureae (L.) Moench, herba recens (europa.eu)
Currier, N., 2000. Natural killer cells from aging mice treated with extracts from Echinacea purpurea are quantitatively and functionally rejuvenated. Exp. Gerontol. 35, 627–639. https://doi.org/10.1016/S0531-5565(00)00106-6
Currier, N.L., Miller, S.C. (2002). The Effect of Immunization with Killed Tumor Cells, with/Without Feeding of Echinacea purpurea in an Erythroleukemic Mouse Model. J. Altern. Complement. Med. 8, 49–58. https://doi.org/10.1089/107555302753507177
Cuzzolin, L., Francini‐Pesenti, F., Verlato, G., Joppi, M., Baldelli, P., Benoni, G. (2010). Use of herbal products among 392 Italian pregnant women: focus on pregnancy outcome. Pharmacoepidemiol. Drug Saf. 19, 1151–1158. https://doi.org/10.1002/pds.2040
Dabbou, S., Rotolo, L., Kovitvadhi, A., Bergagna, S., Dezzutto, D., Barbero, R., Rubiolo, P., Schiavone, A., De Marco, M., Helal, A.N., Zoccarato, I., Gasco, L. (2016). Rabbit dietary supplementation with pale purple coneflower. 1. Effects on the reproductive performance and immune parameters of does. Animal 10, 1101–1109. https://doi.org/10.1017/S1751731115002979
Dapas, B., Dall’Acqua, S., Bulla, R., Agostinis, C., Perissutti, B., Invernizzi, S., Grassi, G., Voinovich, D. (2014). Immunomodulation mediated by a herbal syrup containing a standardized Echinacea root extract: A pilot study in healthy human subjects on cytokine gene expression. Phytomedicine 21, 1406–1410. https://doi.org/10.1016/j.phymed.2014.04.034
De Fougerolles, A.R., Baines, M.G. (1987). Modulation of the natural killer cell activity in pregnant mice alters the spontaneous abortion rate. J. Reprod. Immunol. 11, 147–153. https://doi.org/10.1016/0165-0378(87)90018-0
Di Lorenzo, C., Ceschi, A., Kupferschmidt, H., Lüde, S., De Souza Nascimento, E., Dos Santos, A., Colombo, F., Frigerio, G., Nørby, K., Plumb, J., Finglas, P., Restani, P. (2015). Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality. Br. J. Clin. Pharmacol. 79, 578–592. https://doi.org/10.1111/bcp.12519
EMA (2012): Assessment report on Echinacea angustifolia DC., radix. EMA/HMPC/688212/2008. Assessment report on Echinacea angustifolia DC., radix (europa.eu)
EMA (2014): Assessment report on Echinacea purpurea (L.) Moench., herba recens. EMA/HMPC/557979/2013. Assessment report on Echinacea purpurea (L.) Moench., herba recens (europa.eu)
EMA (2018): Assessment report on Echinacea pallida (Nutt.) Nutt., radix. EMA/HMPC/737379/2017. Assessment report on Echinacea pallida (Nutt.) Nutt., radix (europa.eu)
EMA monograph (2012): Community herbal monograph on Echinacea angustifolia DC., radix. EMA/HMPC/688216/2008. Community herbal monograph on Echinacea angustifolia DC., radix (europa.eu)
EMA monograph (2014): European Union herbal monograph on Echinacea purpurea (L.) Moench, herba recens. (EMEA/HMPC/104945/2006. European Union herbal monograph on Echinacea purpurea (L.) Moench, herba recens (europa.eu)
EMA monograph (2017): European Union herbal monograph on Echinacea purpurea (L.) Moench, radix. EMA/HMPC/577784/2008. European Union herbal monograph on Echinacea purpurea (L.) Moench, radix (europa.eu)
EMA monograph (2018): European Union herbal monograph on Echinacea pallida (Nutt.) Nutt., radix. (EMEA/HMPC/332350/2008. European Union herbal monograph on Echinacea pallida (Nutt.) Nutt., radix (europa.eu)
Espinosa-Paredes, D.A., Cornejo-Garrido, J., Moreno-Eutimio, M.A., Martínez-Rodríguez, O.P., Jaramillo-Flores, M.E., Ordaz-Pichardo, C. (2021). Echinacea Angustifolia DC Extract Induces Apoptosis and Cell Cycle Arrest and Synergizes with Paclitaxel in the MDA-MB-231 and MCF-7 Human Breast Cancer Cell Lines. Nutr. Cancer 73, 2287–2305. https://doi.org/10.1080/01635581.2020.1817956
Filipiak-Szok, A., Kurzawa, M., Szłyk, E. (2015). Determination of toxic metals by ICP-MS in Asiatic and European medicinal plants and dietary supplements. J. Trace Elem. Med. Biol. 30, 54–58. https://doi.org/10.1016/j.jtemb.2014.10.008
Fonseca, F.N., Papanicolaou, G., Lin, H., Lau, C.B.S., Kennelly, E.J., Cassileth, B.R., Cunningham-Rundles, S. (2014). Echinacea purpurea (L.) Moench modulates human T-cell cytokine response. Int. Immunopharmacol. 19, 94–102. https://doi.org/10.1016/j.intimp.2013.12.019
Fusco, D., Liu, X., Savage, C., Taur, Y., Xiao, W., Kennelly, E., Yuan, J., Cassileth, B., Salvatore, M., Papanicolaou, G.A. (2010). Echinacea purpurea aerial extract alters course of influenza infection in mice. Vaccine 28, 3956–3962. https://doi.org/10.1016/j.vaccine.2010.03.047
Gallo, M., Sarkar, M., Au, W., Pietrzak, K., Comas, B., Smith, M., Jaeger, T.V., Einarson, A., Koren, G. (2000). Pregnancy Outcome Following Gestational Exposure to Echinacea: A Prospective Controlled Study. Arch. Intern. Med. 160, 3141.: Pregnancy Outcome Following Gestational Exposure to Echinacea: A Prospective Controlled Study | Complementary and Alternative Medicine | JAMA Internal Medicine | JAMA Network
Gan, X.-H., Zhang, L., Heber, D., Bonavida, B. (2003). Mechanism of activation of human peripheral blood NK cells at the single cell level by Echinacea water soluble extracts: recruitment of lymphocyte–target conjugates and killer cells and activation of programming for lysis. Int. Immunopharmacol. 3, 811–824. https://doi.org/10.1016/S1567-5769(02)00298-9
Gendron, R.L., Baines, M.G. (1988). Infiltrating decidual natural killer cells are associated with spontaneous abortion in mice. Cell. Immunol. 113, 261–267. https://doi.org/10.1016/0008-8749(88)90025-1
George, L., Ioannis, E., Radostina, T., Antonios, M. (2006). Severe thrombotic thrombocytopenic purpura (TTP) induced or exacerbated by the immunostimulatory herb Echinacea. Am. J. Hematol. 81, 224–224. https://doi.org/10.1002/ajh.20531
Goel, V., Chang, C., Slama, J.V., Barton, R., Bauer, R., Gahler, R., Basu, T.K. (2002). Echinacea stimulates macrophage function in the lung and spleen of normal rats. J. Nutr. Biochem. 13, 487–492. https://doi.org/10.1016/S0955-2863(02)00190-0
Gorski, J. (2004). The effect of echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin. Pharmacol. Ther. 75, 89–100. https://doi.org/10.1016/j.clpt.2003.09.013
Gurley, B., Gardner, S., Hubbard, M., Williams, D., Gentry, W., Carrier, J., Khan, I., Edwards, D., Shah, A. (2004). In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin. Pharmacol. Ther. 76, 428–440. https://doi.org/10.1016/j.clpt.2004.07.007
Gurley, B.J., Swain, A., Hubbard, M.A., Williams, D.K., Barone, G., Hartsfield, F., Tong, Y., Carrier, D.J., Cheboyina, S., Battu, S.K. (2008). Clinical assessment of CYP2D6‐mediated herb–drug interactions in humans: Effects of milk thistle, black cohosh, goldenseal, kava kava, St. John’s wort, and Echinacea. Mol. Nutr. Food Res. 52, 755–763. https://doi.org/10.1002/mnfr.200600300
Hall, H.G., Griffiths, D.L., McKenna, L.G. (2011). The use of complementary and alternative medicine by pregnant women: A literature review. Midwifery 27, 817–824. https://doi.org/10.1016/j.midw.2010.08.007
Hansen, T.S., Nilsen, O.G. (2009). Echinacea purpurea and P‐glycoprotein drug transport in Caco‐2 cells. Phytother. Res. 23, 86–91. https://doi.org/10.1002/ptr.2563
Heitmann, K., Havnen, G.C., Holst, L., Nordeng, H. (2016). Pregnancy outcomes after prenatal exposure to Echinacea: the Norwegian Mother and Child Cohort Study. Eur. J. Clin. Pharmacol. 72, 623–630. Pregnancy outcomes after prenatal exposure to echinacea: the Norwegian Mother and Child Cohort Study | European Journal of Clinical Pharmacology
Hellum, B.H., Hu, Z., Nilsen, O.G. (2007). The Induction of CYP1A2, CYP2D6 and CYP3A4 by Six Trade Herbal Products in Cultured Primary Human Hepatocytes. Basic Clin. Pharmacol. Toxicol. 100, 23–30. https://doi.org/10.1111/j.1742-7843.2007.00011.x
Hellum, B.H., Nilsen, O.G. (2007). The in vitro Inhibitory Potential of Trade Herbal Products on Human CYP2D6‐Mediated Metabolism and the Influence of Ethanol. Basic Clin. Pharmacol. Toxicol. 101, 350–358. https://doi.org/10.1111/j.1742-7843.2007.00121.x
HMR 2012: The Human Medicines Regulations 2012. Available at: The Human Medicines Regulations 2012 (legislation.gov.uk)
Holst, L., Wright, D., Haavik, S., Nordeng, H. (2011). Safety and efficacy of herbal remedies in obstetrics—review and clinical implications. Midwifery 27, 80–86. https://doi.org/10.1016/j.midw.2009.05.010
Hudson, J., Vimalanathan, S., Kang, L., Amiguet, V.T., Livesey, J., Arnason, J.T. (2005). Characterization of Antiviral Activities in Echinacea Root Preparations. Pharm. Biol. 43, 790–796. https://doi.org/10.1080/13880200500408491
Hudson, J.B. (2012). Applications of the Phytomedicine Echinacea purpurea (Purple Coneflower) in Infectious Diseases. J. Biomed. Biotechnol. 1–16. https://doi.org/10.1155/2012/769896
Huntley, A.L., Thompson Coon, J., Ernst, E. (2005). The Safety of Herbal Medicinal Products Derived from Echinacea Species: A Systematic Review. Drug Saf. 28, 387–400. The Safety of Herbal Medicinal Products Derived from Echinacea Species | Drug Safety
Husain, I., Dale, O.R., Martin, K., Gurley, B.J., Adams, S.J., Avula, B., Chittiboyina, A.G., Khan, I.A., Khan, S.I. (2023). Screening of medicinal plants for possible herb-drug interactions through modulating nuclear receptors, drug-metabolizing enzymes and transporters. J. Ethnopharmacol. 301, 115822. https://doi.org/10.1016/j.jep.2022.115822
Jacobsson, I., Jönsson, A.K., Gerdén, B., Hägg, S. (2009). Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol. Drug Saf. 18, 1039–1047. https://doi.org/10.1002/pds.1818
Jawad, M., Schoop, R., Suter, A., Klein, P., Eccles, R. (2012). Safety and Efficacy Profile of Echinacea purpurea to Prevent Common Cold Episodes: A Randomized, Double-Blind, Placebo-Controlled Trial. Evid. Based Complement. Alternat. Med. 2012, 1–7. https://doi.org/10.1155/2012/841315
Jeong, J.-S., Kim, J.-W., Kim, J.-H., Chung, E.-H., Lee, D.-R., Choi, B.-K., Ko, J.-W., Kim, T.-W. (2024). Oral toxicity and genotoxicity assessment of standardized Echinacea purpurea (L.) extract and the pharmacokinetic profile of its active ingredient chicoric acid. Toxicol. Res. 40, 457–472. Oral toxicity and genotoxicity assessment of standardized Echinacea purpurea (L.) extract and the pharmacokinetic profile of its active ingredient chicoric acid | Toxicological Research
Kemp, D.E., Franco, K.N. (2002). Possible leukopenia associated with long-term use of Echinacea. J. Am. Board Fam. Pract. 15, 417.
Khaksary Mahabady, M., Ranjbar, R., Arzi, A., Papahn, A.A., Najafzadeh, H. (2006). A comparison study of effects of Echinacea extract and levamisole on phenytoin-induced cleft palate in mice. Regul. Toxicol. Pharmacol. 46, 163–166. https://doi.org/10.1016/j.yrtph.2006.06.005
Kocaman, O., Hulagu, S., Senturk, O. (2008). Echinacea-induced severe acute hepatitis with features of cholestatic autoimmune hepatitis. Eur. J. Intern. Med. 19, 148. Echinacea-induced severe acute hepatitis with features of cholestatic autoimmune hepatitis - European Journal of Internal Medicine
Kovitvadhi, A., Gai, F., Dabbou, S., Ferrocino, I., Rotolo, L., Falzone, M., Vignolini, C., Gennero, M.S., Bergagna, S., Dezzutto, D., Barbero, R., Nebbia, P., Rosati, S., Cocolin, L., Zoccarato, I., Gasco, L. (2016). Rabbit dietary supplementation with pale purple coneflower. 2. Effects on the performances, bacterial community, blood parameters and immunity of growing rabbits. Animal 10, 1110–1117. https://doi.org/10.1017/S1751731115002980
Lee, A.N., Werth, V.P. (2004). Activation of Autoimmunity Following Use of Immunostimulatory Herbal Supplements. Arch. Dermatol. 140. Activation of Autoimmunity Following Use of Immunostimulatory Herbal Supplements | Complementary and Alternative Medicine | JAMA Dermatology | JAMA Network
Lee Soon, S., Crawford, R.I. (2001). Recurrent erythema nodosum associated with Echinacea herbal therapy. J. Am. Acad. Dermatol. 44, 298–299. Recurrent erythema nodosum associated with echinacea herbal therapy - Journal of the American Academy of Dermatology
Lenk, W. (1989). Akute toxizitat von verschiedenen polysacchariden Echinacea purpurea an der maus.[Acute toxicity of various polysaccharides from Echinacea purpurea in the mouse.]. Zeitschrift fur Phytotherapie, 10, pp.49-51.
Li, Y., Wang, Y., Wu, Y., Wang, B., Chen, X., Xu, Xin, Chen, H., Li, W., Xu, Xiaogang, (2017). Echinacea pupurea extracts promote murine dendritic cell maturation by activation of JNK, p38 MAPK and NF-κB pathways. Dev. Comp. Immunol. 73, 21–26. https://doi.org/10.1016/j.dci.2017.03.002
Maass, N., Bauer, J., Paulicks, B.R., Böhmer, B.M., Roth‐Maier, D.A. (2005). Efficiency of Echinacea purpurea on performance and immune status in pigs. J. Anim. Physiol. Anim. Nutr. 89, 244–252. https://doi.org/10.1111/j.1439-0396.2005.00501.x
Mahajan, D., Sharma, N. R., Kancharla, S., Kolli, P., Tripathy, A., Sharma, A. K., Singh, S., Kumar, S., Mohanty, A. K., & Jena, M. K. (2022). Role of Natural Killer Cells during Pregnancy and Related Complications. Biomolecules, 12(1), 68. https://doi.org/10.3390/biom12010068
Maskatia, Z.K., Baker, K. (2010). Hypereosinophilia Associated with Echinacea Use: South. Med. J. 103, 1173–1174. https://doi.org/10.1097/smj.0b013e3181f1ed8b
Matthias, A., Merika, H., Addison, R., Bone, K., Lehmann, R. (2008). Bioavailability of Echinacea alkylamides in human breast milk. Planta Med. 74, s-0028-1083939. https://doi.org/10.1055/s-0028-1083939
Melchart D, Linde K, Worku F, Sarkady L, Holzmann M, Jurcic K, Wagner H (1995). Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. J Altern Complement Med. Summer;1(2):145-60. doi: 10.1089/acm.1995.1.145. PMID: 9395611. PMID: 9395611.
Melchart, D. (1998). Echinacea Root Extracts for the Prevention of Upper Respiratory Tract Infections: A Double-blind, Placebo-Controlled Randomized Trial. Arch. Fam. Med. 7, 541–545. https://doi.org/10.1001/archfami.7.6.541
Mengs, U., Clare, C.B., Poiley, J.A. (1991). Toxicity of Echinacea purpurea. Acute, subacute and genotoxicity studies. Arzneimittelforschung. 41, 1076–1081.
Merali, S., Binns, S., Paulin-Levasseur, M., Ficker, C., Smith, M., Baum, B., Brovelli, E., Arnason, J.T. (2003). Antifungal and Anti-inflammatory Activity of the Genus Echinacea. Pharm. Biol. 41, 412–420. https://doi.org/10.1076/phbi.41.6.412.17828
Modarai, M., Gertsch, J., Suter, A., Heinrich, M., Kortenkamp, A. (2010). Cytochrome P450 inhibitory action of Echinacea preparations differs widely and co-varies with alkylamide content. J. Pharm. Pharmacol. 59, 567–573. https://doi.org/10.1211/jpp.59.4.0012
Mrozikiewicz, P.M., Bogacz, A., Karasiewicz, M., Mikolajczak, P.L., Ozarowski, M., Seremak-Mrozikiewicz, A., Czerny, B., Bobkiewicz-Kozlowska, T., Grzeskowiak, E.(2010). The effect of standardized Echinacea purpurea extract on rat cytochrome P450 expression level. Phytomedicine 17, 830–833. https://doi.org/10.1016/j.phymed.2010.02.007
Mullins, R.J. (1998). Echinacea‐associated anaphylaxis. Med. J. Aust. 168, 170–171. https://doi.org/10.5694/j.1326-5377.1998.tb126773.x
Mullins, R.J., Heddle, R. (2002). Adverse reactions associated with Echinacea: the Australian experience. Ann. Allergy. Asthma. Immunol. 88, 42–51. https://doi.org/10.1016/S1081-1206(10)63591-0
Nordeng, H., Bayne, K., Havnen, G.C., Paulsen, B.S. (2011). Use of herbal drugs during pregnancy among 600 Norwegian women in relation to concurrent use of conventional drugs and pregnancy outcome. Complement. Ther. Clin. Pract. 17, 147–151. https://doi.org/10.1016/j.ctcp.2010.09.002
Ondrizek, R.R., Chan, P.J., Patton, W.C., King, A. (1999). Inhibition of Human Sperm Motility by Specific Herbs Used in Alternative Medicine. J. Assist. Reprod. Genet. 16, 87–91. https://doi.org/10.1023/A:1022568823262
Penzak, S.R., Robertson, S.M., Hunt, J.D., Chairez, C., Malati, C.Y., Alfaro, R.M., Stevenson, J.M., Kovacs, J.A. (2010). Echinacea purpurea Significantly Induces Cytochrome P450 3A Activity but Does Not Alter Lopinavir‐Ritonavir Exposure in Healthy Subjects. Pharmacother. J. Hum. Pharmacol. Drug Ther. 30, 797–805. https://doi.org/10.1592/phco.30.8.797
Perri D, Dugoua JJ, Mills E, Koren G. (2006): Safety and efficacy of Echinacea (Echinacea angustafolia, E. purpurea and E. pallida) during pregnancy and lactation. Can J Clin Pharmacol, 13(3): e262-267.
Pilarska, G., Twarużek, M., Ałtyn, I. (2022). The Presence of Molds and Their Secondary Metabolites in Purple Coneflower-Based Dietary Supplements (Echinacea purpurea (L.) Moench). Toxins 14, 607. https://doi.org/10.3390/toxins14090607
Pleschka, S., Stein, M., Schoop, R., Hudson, J.B. (2009). Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian Influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol. J. 6, 197. https://doi.org/10.1186/1743-422X-6-197
Raman, P., Patino, L.C., Nair, M.G. (2004). Evaluation of Metal and Microbial Contamination in Botanical Supplements. J. Agric. Food Chem. 52, 7822–7827. https://doi.org/10.1021/jf049150
Raner, G.M., Cornelious, S., Moulick, K., Wang, Y., Mortenson, A., Cech, N.B. (2007). Effects of herbal products and their constituents on human cytochrome P4502E1 activity. Food Chem. Toxicol. 45, 2359–2365. https://doi.org/10.1016/j.fct.2007.06.012
Rininger, J.A., Kickner, S., Chigurupati, P., McLean, A., Franck, Z. (2002). Immunopharmacological activity of Echinacea preparations following simulated digestion on murine macrophages and human peripheral blood mononuclear cells. J. Leukoc. Biol. 68, 503–510. https://doi.org/10.1189/jlb.68.4.503
Rondanelli, M., Miccono, A., Lamburghini, S., Avanzato, I., Riva, A., Allegrini, P., Faliva, M.A., Peroni, G., Nichetti, M., Perna, S. (2018). Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds—Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds. Evid. Based Complement. Alternat. Med. 2018, 1–36. https://doi.org/10.1155/2018/5813095
Schapowal, A., Klein, P., Johnston, S.L. (2015). Echinacea Reduces the Risk of Recurrent Respiratory Tract Infections and Complications: A Meta-Analysis of Randomized Controlled Trials. Adv. Ther. 32, 187–200. https://doi.org/10.1007/s12325-015-0194-4
See, D.M., Broumand, N., Sahl, L., Tilles, J.G. (1997). In vitro effects of Echinacea and ginseng on natural killer and antibody-dependent cell cytotoxicity in healthy subjects and chronic fatigue syndrome or acquired immunodeficiency syndrome patients. Immunopharmacology 35, 229–235. https://doi.org/10.1016/S0162-3109(96)00125-7
Sharma, M., Anderson, S.A., Schoop, R., Hudson, J.B. (2009). Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antiviral Res. 83, 165–170. https://doi.org/10.1016/j.antiviral.2009.04.009
Signer, J., Jonsdottir, H.R., Albrich, W.C., Strasser, M., Züst, R., Ryter, S., Ackermann-Gäumann, R., Lenz, N., Siegrist, D., Suter, A., Schoop, R., Engler, O.B. (2020). In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol. J. 17, 136. https://doi.org/10.1186/s12985-020-01401-2
Svedlund, E., Larsson, M., Hägerkvist, R. (2017). Spontaneously Reported Adverse Reactions for Herbal Medicinal Products and Natural Remedies in Sweden 2007–15: Report from the Medical Products Agency. Drugs - Real World Outcomes 4, 119–125. https://doi.org/10.1007/s40801-017-0104-y
Tournas, V.H. (2009). Microbial contamination of select diatary supplements. J. Food Saf. 29, 430–442. https://doi.org/10.1111/j.1745-4565.2009.00167.x
Tsai, Y.-L., Chiou, S.-Y., Chan, K.-C., Sung, J.-M., Lin, S.-D. (2012a). Caffeic acid derivatives, total phenols, antioxidant and antimutagenic activities of Echinacea purpurea flower extracts. LWT - Food Sci. Technol. 46, 169–176. https://doi.org/10.1016/j.lwt.2011.09.026
Tsai, Y.-L., Chiu, C.-C., Yi-Fu Chen, J., Chan, K.-C., Lin, S.-D. (2012b). Cytotoxic effects of Echinacea purpurea flower extracts and chicoric acid on human colon cancer cells through induction of apoptosis. J. Ethnopharmacol. 143, 914–919. https://doi.org/10.1016/j.jep.2012.08.032
Upton, R. (Ed.) (2007). Echinacea purpurea aerial parts: Echinacea purpurea (L.) Moench ; standards of analysis, quality control, and therapeutics, American herbal pharmacopoeia and therapeutic compendium. American Herbal Pharmacopoeia, Scotts Valley, CA.
Vimalanathan, S., Kang, L., Amiguet, V.T., Livesey, J., Arnason, J.T., Hudson, J. (2005). Echinacea purpurea. Aerial Parts Contain Multiple Antiviral Compounds. Pharm. Biol. 43, 740–745. https://doi.org/10.1080/13880200500406354
Vonau, B., Chard, S., Mandalia, S., Wilkinson, D., Barton, S.E. (2001). Does the extract of the plant Echinacea purpurea influence the clinical course of recurrent genital herpes? Int. J. STD AIDS 12, 154–158. https://doi.org/10.1258/0956462011916947
Yale, S.H., Glurich, I. (2005). Analysis of the Inhibitory Potential of Ginkgo biloba, Echinacea purpurea, and Serenoa repens on the Metabolic Activity of Cytochrome P450 3A4, 2D6, and 2C9. J. Altern. Complement. Med. 11, 433–439. https://doi.org/10.1089/acm.2005.11.433
WHO (1999). Radix echinaceae. In: WHO monographs on selected medicinal plants, Vol. 1, WHO Geneva 1999, Switzerland: 128-135. WHO monographs on selected medicinal plants
Zhai, Z., Liu, Y., Wu, L., Senchina, D.S., Wurtele, E.S., Murphy, P.A., Kohut, M.L., Cunnick, J.E. (2007). Enhancement of Innate and Adaptive Immune Functions by Multiple Echinacea Species. J. Med. Food 10, 423–434. https://doi.org/10.1089/jmf.2006.257
TOX/2024/43 Annex A
In this guide
In this guideSearch methodology
1. The following electronic databases were searched for relevant articles published between 2014 and 2024: LitFetch (which includes material from PubMed, Scopus, Ebsco (Food Science Source) and Springer), Google and Google Scholar. The searches were conducted on various dates between 13th May 2024 and 24th May 2024.
2. The search terms used included ‘echinacea’ AND: (‘pregnan*’ OR ‘maternal*’ OR ‘reproduction’ OR ‘gestation’ OR ‘lactation’ OR ‘preconception’ OR ‘development’ OR ‘tox*’ OR ‘safety’ OR ‘uses’ OR ‘consumption’ OR ‘indication’ OR ‘interaction’).
3. The references from extracted papers were searched for citations not captured in the literature search. Only articles published in English were included, due to the linguistic abilities of the reviewer.
4. The UKTIS was also asked for information on any enquiries relating to maternal echinacea use and any reports of adverse effects in pregnant women or their newborn infants received from 1983 to June 2024. This included information relating to the type, dosage, duration, and timing of echinacea taken and any pregnancy outcomes captured through follow-up.
TOX/2024/43 Annex B
In this guide
In this guideTable 8a: Combined acute consumption scenarios for 4-5 products of Echinacea (as dried root/herb) during pregnancy.
|
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
|
Tea |
Honey |
Lozenges |
Tablets/capsules |
Oral liquids |
Total consumed per day |
|
860 - 6,000 |
19 - 100 |
40 |
400 - 3,600 |
600 - 3,000 |
1,900 - 13,000 |
|
N/A |
19 - 100 |
40 |
400 - 3,600 |
600 - 3,000 |
1,100 - 6,700 |
|
860 - 6,000 |
N/A |
40 |
400 - 3,600 |
600 - 3,000 |
1,900 -13,000 |
|
860 - 6,000 |
19 - 100 |
N/A |
400 - 3,600 |
600 - 3,000 |
1,900 - 13,000 |
|
860 - 6,000 |
19 - 100 |
40 |
N/A |
600 - 3,000 |
1,500 - 9,100 |
|
860 - 6,000 |
19 - 100 |
40 |
400 - 3,600 |
N/A |
1,300 - 9,700 |
*Rounded to 2 significant figures.
Table 8b: Combined acute consumption scenarios for 3 products of Echinacea (as dried root/herb) during pregnancy.
|
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
|
Tea |
Honey |
Lozenges |
Tablets/capsules |
Oral liquids |
Total consumed per day |
|
860 - 6,000 |
19 - 100 |
40 |
N/A |
N/A |
920 - 6,100 |
|
860 - 6,000 |
19 - 100 |
N/A |
400 - 3,600 |
N/A |
1,300 - 9,700 |
|
860 - 6,000 |
19 - 100 |
N/A |
N/A |
600 - 3,000 |
1,500 - 9,100 |
|
860 - 6,000 |
N/A |
N/A |
400 - 3,600 |
600 - 3,000 |
1,900 - 13,000 |
|
860 - 6,000 |
N/A |
40 |
400 - 3,600 |
N/A |
1,300 - 9,600 |
|
860 - 6,000 |
N/A |
40 |
N/A |
600 - 3,000 |
1,500 - 9,000 |
|
N/A |
N/A |
40 |
400 - 3,600 |
600 - 3,000 |
1,000 - 6,700 |
|
N/A |
19 - 100 |
N/A |
400 - 3,600 |
600 - 3,000 |
1,000 - 6,700 |
|
N/A |
19 - 100 |
40 |
400 - 3,600 |
N/A |
460 - 3,700 |
|
N/A |
19 - 100 |
40 |
N/A |
600 - 3,000 |
660 - 3,100 |
*Rounded to 2 significant figures.
Table 8c: Combined acute consumption scenarios for 2 products of Echinacea (as dried root/herb) during pregnancy.
|
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
|
Tea |
Honey |
Lozenges |
Tablets/capsules |
Oral liquids |
Total consumed per day |
|
860 - 6,000 |
19 - 100 |
N/A |
N/A |
N/A |
900 - 6,100 |
|
860 - 6,000 |
N/A |
40 |
N/A |
N/A |
900 - 6,000 |
|
860 - 6,000 |
N/A |
N/A |
400 - 3,600 |
N/A |
1,300 - 9,600 |
|
860 - 6,000 |
N/A |
N/A |
N/A |
600 - 3,000 |
1,500 - 9,600 |
|
N/A |
19 - 100 |
40 |
N/A |
N/A |
60 - 140 |
|
N/A |
19 - 100 |
N/A |
400 – 3,600 |
N/A |
420 - 3,700 |
|
N/A |
19 - 100 |
N/A |
N/A |
600 - 3,000 |
620 - 3,100 |
|
N/A |
N/A |
40 |
400 - 3,600 |
N/A |
440 - 3,600 |
|
N/A |
N/A |
40 |
N/A |
600 - 3,000 |
640 - 3,000 |
|
N/A |
N/A |
N/A |
400 - 3,600 |
600 - 3,000 |
1,000 - 6,600 |
*Rounded to 2 significant figures.
Table 9a: Combined chronic consumption scenarios for 4-5 products of Echinacea (as dried root/herb) during pregnancy.
|
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
|
Tea |
Honey |
Lozenges |
Tablets/capsules |
Oral liquids |
Total consumed per day |
|
580 – 4,000 |
10-53 |
40 |
400 - 3,600 |
600 - 3,000 |
1,600 - 11,000 |
|
N/A |
10-53 |
40 |
400 - 3,600 |
600 - 3,000 |
1,100 – 6,700 |
|
580 – 4,000 |
N/A |
40 |
400 - 3,600 |
600 - 3,000 |
1,600 – 11,000 |
|
580 – 4,000 |
10-53 |
N/A |
400 - 3,600 |
600 - 3,000 |
1,600 – 11,000 |
|
580 – 4,000 |
10-53 |
40 |
N/A |
600 - 3,000 |
1,200 – 7,100 |
|
580 – 4,000 |
10-53 |
40 |
400 - 3,600 |
N/A |
1,000 – 7,700 |
*Rounded to 2 significant figures.
Table 9b: Combined chronic consumption scenarios for 3 products of Echinacea (as dried root/herb) during pregnancy.
|
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
|
Tea |
Honey |
Lozenges |
Tablets/capsules |
Oral liquids |
Total consumed per day |
|
580 – 4,000 |
10-53 |
40 |
N/A |
N/A |
600 - 4,100 |
|
580 – 4,000 |
10-53 |
N/A |
400 - 3,600 |
N/A |
1,000 – 7,700 |
|
580 – 4,000 |
10-53 |
N/A |
N/A |
600 - 3,000 |
1,200 – 7,100 |
|
580 – 4,000 |
N/A |
N/A |
400 - 3,600 |
600 - 3,000 |
1,600 –11,000 |
|
580 – 4,000 |
N/A |
40 |
400 - 3,600 |
N/A |
1,000 – 7,600 |
|
580 – 4,000 |
N/A |
40 |
N/A |
600 - 3,000 |
1,200 – 7,000 |
|
N/A |
N/A |
40 |
400 - 3,600 |
600 - 3,000 |
1,000 – 6,600 |
|
N/A |
10 - 53 |
N/A |
400 - 3,600 |
600 - 3,000 |
1,000 - 6,700 |
|
N/A |
10 - 53 |
40 |
400 - 3,600 |
N/A |
450-3,700 |
|
N/A |
10 - 53 |
40 |
N/A |
600 - 3,000 |
650-3,100 |
*Rounded to 2 significant figures.
Table 9c: Combined chronic consumption scenarios for 2 products of Echinacea (as dried root/herb) during pregnancy.
|
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
Estimated consumption of Echinacea (mg/day) |
|
Tea |
Honey |
Lozenges |
Tablets/capsules |
Oral liquids |
Total consumed per day |
|
580 – 4,000 |
10-53 |
N/A |
N/A |
N/A |
600 - 4,100 |
|
580 – 4,000 |
N/A |
40 |
N/A |
N/A |
620 - 4,000 |
|
580 – 4,000 |
N/A |
N/A |
400 - 3,600 |
N/A |
1,000 - 7,600 |
|
580 – 4,000 |
N/A |
N/A |
N/A |
600 - 3,000 |
1,200 - 7,000 |
|
N/A |
10-53 |
40 |
N/A |
N/A |
50 - 93 |
|
N/A |
10-53 |
N/A |
400 – 3,600 |
N/A |
410 - 3,700 |
|
N/A |
10-53 |
N/A |
N/A |
600 - 3,000 |
610 - 3,100 |
|
N/A |
N/A |
40 |
400 - 3,600 |
N/A |
440 - 3,600 |
|
N/A |
N/A |
40 |
N/A |
600 - 3,000 |
640 - 3,000 |
|
N/A |
N/A |
N/A |
400 - 3,600 |
600 - 3,000 |
1,000 - 6,600 |
*Rounded to 2 significant figures.
Table 10: Echinacea products (oral dosage forms) with THR in the UK.
|
Product name |
Dosage form |
Echinacea species and plant part |
Composition |
Directions for use |
Daily dose Echinacea (mg) |
|
Echinaflu Soft Capsules. |
Capsules. |
Echinacea purpurea (L.) Moench herb. |
176 mg of dried pressed juice from fresh flowering herb equivalent to 3.5-4.9 g of fresh herb (DER 20-28:1). |
1-2 capsules daily for no longer than 10 days. |
176 - 352 mg dried pressed juice equivalent to 3.5 - 9.8 g fresh herb. |
|
Echinacea Cold and Flu Capsules. |
Capsules. |
Echinacea purpurea (L.) Moench) root. |
140 mg dry extract from root equivalent to 838 - 1117 mg root (DER 6-8:1). |
1 capsule twice a day. |
280 mg dry root extract equivalent to 1,676- 2,234 mg root. |
|
Ekinalife. |
Capsules. |
Echinacea pallida (Nutt.) Nutt. root and Echinacea purpurea (L.) Moench root. |
200mg of Echinacea pallida. Nutt. and 200mg of Echinacea purpurea (L.) Moench powedered root. |
1 capsule twice a day for no longer than 10 days. |
400 mg E. pallida powdered root and 400 mg E. purpurea powdered root. |
|
Solgar Echinacea Cold and Flu Capsules. |
Capsules. |
Echinacea purpurea (L.) Moench root. |
140 mg dry extract from root equivalent to 838 - 1117 mg root (DER 6-8:1). |
1 capsule twice a day for no longer than 10 days. |
280 mg dry root extract equivalent to 1,676- 2,234 mg root. |
|
Phytocold. |
Capsules. |
Echinacea purpurea (L.) Moench root. |
250 mg powdered root. |
1-2 capsules three times a day fo no longer than 10 days. |
750 - 1,500 mg powdered root. |
|
Echinaflu Effervescent Tablets. |
Effervescent Tablets. |
Echinacea purpurea (L.) Moench herb. |
176 mg of dried pressed juice from fresh flowering herb equivalent to 3.5-4.9 g of fresh herb (DER 20-28:1). |
1-2 tablets daily for no longer than 10 days. |
176 - 352 mg dried pressed juice equivalent to 3.5 - 9.8 g fresh herb. |
|
Echineeze. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
70 mg dry extract from root equivalent to 460 – 530 mg root (DER 6.5-7.5:1). |
1 tablet 3 times a day for no longer than 10 days. |
210 mg dry root extract equivalent to 1,380 - 1,590 mg root. |
|
Echinaforce Forte Cold & Flu Tablets. |
Tablets. |
Echinacea purpurea (L.) Moench herb and root. |
1,140 mg dry extract from fresh herb (DER 1:12) and 60 mg (DER 1:11) dry extract from fresh root. |
1 tablet two to three times a day for no longer than 10 days. |
2,280 - 3,420 mg dry herb extract and 120-180 mg dry root extract. |
|
Herbal Cold And Flu Relief Tablets. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
71.5 mg dry extract from root equivalent to 429 - 500 mg root (DER 6-7:1). |
1-2 tablets twice daily for no longer than 10 days. |
143-286 mg dry root extract equivalent to 858 - 2,000 mg root. |
|
High Strength Herbal Cold And Flu Relief Tablets. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
143 mg dry extract from root equivalent to 858 - 1000 mg root (DER 6-7:1). |
1 tablet three times a day for no longer than 10 days. |
429 mg dry root extract equivalent to 2,574 - 3,000 mg root. |
|
Echinacea Skin Care Tablets. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
71.5 mg dry extract from root equivalent to 429 - 500 mg root (DER 6-7:1). |
1-2 tablets three times a day for no longer than 10 days. |
143-286 mg dry root extract equivalent to 858 - 2,000 mg root. |
|
Herbal Classics Echinacea Cold Relief Film-Coated Tablets. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
40 mg dry extract from root equivalent to 260 mg root (DER 6.5:1). |
2-3 tablets three times a day. |
240-360 mg dry root extract equivalent to 1,560 -2,340 mg root. |
|
HRI Cold And Flu Echinacea Tablets. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
56 mg dry extract from root equivalent to 338 - 450 mg root (DER 6-8:1). |
1-2 tablets twice daily. |
112-224 mg dry root extract equivalent to 676-1,800 mg root. |
|
Echinapret Coated Tablets. |
Tablets. |
Echinacea purpurea (L.) Moench herb. |
175 mg of dried pressed juice from fresh flowering herb equivalent to 6.7 - 9.8 g fresh herb (DER 38 - 56:1). |
1 tablet three times a day for no longer than 10 days. |
525 mg dried pressed juice equivalent to 18.4 - 29.4 mg fresh herb. |
|
Thompson and Capper Echinacea Cold-n-Flu-Eze. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
105 mg dry extract from root equivalent to 630 - 840 mg root (DER 6-8:1). |
1 tablet twice a day for no longer than 10 days. |
210 mg dry root extract equivalent to 1,260 -1,680 mg root. |
|
Fuerte Tablets. |
Tablets. |
Wild indigo root (Baptisia tinctoria (L.) R.Br.), Echinacea purpurea root (Echinacea purpurea (L.) Moench), Echinacea pallida root (Echinacea pallida (Nutt.) Nutt.), White cedar tips and leaves (Thuja occidentalis L.). |
3.2 mg dry root extract (DER 4-9:1) from Wild indigo root, E. purpurea root, E. pallida root and Wild cedar tips and leaves (4.92:1.85:1.85:1). |
5 tablets three times a day for no longer than 10 days. |
9.2 mg E. pallida and 9.2 mg E. purpurea dry root extract. Total 18.4 mg dry root extract equivalent to 73.6-165.6 mg root. |
|
Healthsense Echinashield Cold and Flu Tablets. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
70 mg dry extract from root equivalent to 420 - 560 mg root (DER 6-8:1). |
1 tablet three times a day for no longer than 10 days. |
210 mg dry root extract equivalent to 1,260 -1,680 mg root. |
|
Lamberts Echinacea Cold & Flu relief tablets Nature’s Best Echinacea Cold & Flu Relief tablets. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
105 mg dry extract from root equivalent to 630 - 840 mg root (DER 6-8:1). |
1 tablet twice a day for no longer than 10 days. |
210 mg dry root extract equivalent to 1,260 -1,680 mg root. |
|
Vitabiotics Echinacea Tablets. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
200 mg dry extract from root equivalent to 1200 - 1600 mg root (DER 6-8:1). |
1 tablet twice a day for no longer than 10 days. |
400 mg dry root extract equivalent to 2,400 -3,200 mg root. |
|
Potter’s Skin Clear Tablets. |
Tablets. |
Echinacea angustifolia (D.C) root. |
110 mg dry extract from root equivalent to 500 mg root (DER 4.5:1). |
2 tablets three times a day for no longer than 10 days. |
660 mg dry root extract equivalent to 2,970 mg root. |
|
EKINACLEAR. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
50 mg dry extract from root equivalent to 300 - 400 mg root (DER 6-8:1). |
1-2 tablets three times a day for no longer than 10 days. |
150-300 mg dry root extract equivalent to 900-2,400 mg root. |
|
Lifeplan Echinacea Cold and Flu Relief Tablets. |
Tablets. |
Echinacea purpurea (L.) Moench root. |
140 mg dry extract from root equivalent to 840 - 1120 mg root (DER 6-8:1). |
1 tablet twice a day for no longer than 10 days. |
150-300 mg dry root extract equivalent to 900-2,400 mg root. |
|
Echinaforce Chewable Cold & Flu Tablets. |
Chewable tablets. |
Echinacea purpurea (L.) Moench herb and root. |
380 mg dry extract from fresh herb (DER 1:12) and 20 mg dry extract (DER 1:11) from fresh root. |
2 tablets two to three times a day for no longer than 10 days. |
1,520 - 2,280 mg dry herb extract and 80-120 mg dry root extract. |
|
Herbal Cold And Flu Sachets. |
Sachets. |
Echinacea purpurea (L.) Moench root. |
71.5 mg dry extract from root equivalent to 429 - 500 mg root (DER 6-7:1). |
1 sachet three times a day for no longer than 10 days. |
214.5 mg dry root extract equivalent to 1,287-1,500 mg root. |
|
Cystorelief Cystitis Uva-ursi & Echinacea oral dropsa. |
Tincture. |
Echinacea purpurea (L.) Moench herb, Uva-ursi herb (Arctostaphylos uva-ursi (l.) Spreng, Herb). |
240 mg of tincture from fresh herb (DER 1:12) per 1 ml. |
15 drops in a little water 2-5 times daily. 1mL is equivalent to 30 drops. |
240-600 mg of tincture from fresh herb. |
|
Potter’s Elixir of Echinacea Plus/Napiers Elixir of Echinacea Complexb. |
Oral solution. |
Echinacea angustifolia (D.C) root, Wild Indigo root, Fumitory herb. |
0.64 mL liquid extract from root equivalent to 640 mg root per 5 mL (DER 1:1). |
5 mL three times a day for no longer than 10 days. |
1.92 mL liquid root extract equivalent to 1.92 g root. |
|
Echinacin Juice MADAUS. |
Oral solution. |
Echinacea purpurea (L.) Moench) herb. |
117 mg of dried pressed juice from fresh flowering herb equivalent to 3.7 - 6.3 g of fresh herb per 5 mL (DER 31.5-53.6:1). |
5 mL three times a day for no longer than 10 days. |
351 mg dried pressed juice equivalent to 11.1 - 18.9 g fresh herb. |
|
Echinacin Liquidum MADAUSc. |
Oral solution. |
Echinacea purpurea (L.) Moench) herb. |
1.99 g of pressed juice from fresh flowering herb equivalent to 3.4 - 5 g of fresh herb per 2.5 mL (DER 1.7-2.5:1). |
2.5 mL three times a day for no longer than 10 days. |
5,970 mg pressed juice equivalent to 10.2-15 g fresh herb. |
|
Echinaforce hot drink cold & flu echinacea concentrate for oral solution. |
Tincture. |
Echinacea purpurea (L.) Moench herb and root. |
1,140 mg extract (as tincture) from fresh herb (DER 1:12-13) and 60 mg extract (as tincture) from fresh root (DER 1:11-12) per 5 ml. |
Days 1-3: Take 5 ml diluted in hot water five times daily. |
3,420 - 5,700 mg herb extract (tincture) and 180-300 mg root extract (tincture). |
|
Echinaforce Sore Throat Sprayd. |
Oromucosal spray. |
Echinacea purpurea (L.) Moench herb and root, Sage leaves, (Salvia officinalis L. folium). |
863.3 mg tincture from fresh herb (DER 1:12) and 45.5 mg tincture from fresh root (DER 1:11) per 1 mL. |
1 spray (0.22 mL) six to ten times a day for no more than 7 days. |
1,147 - 1,910 mg herb tincture and 60 - 100 mg root tincture. |
|
Duchy Herbals Echina-Relief Tincturee. |
Tincture. |
Echinacea purpurea (L.) Moench root. |
1mL of tincture from dried root (1:3) (equivalent to 33 mg dried root) per 1 mL tincture. |
2.5 ml of tincture, in water, two or three times daily for no longer than 10 days. |
5-7.5 mL tincture equivalent to 165 - 248 mg dried root. |
a 1 mL contains 426 mg ethanol equivalent to 10.8 mL beer or 4.5 mL wine (43% v/v ethanol content).
b 5 mL contains 760 mg ethanol equivalent to 19 mL beer or 7.9 mL wine (19 % v/v ethanol content).
c 1 mL contains 179 mg ethanol equivalent to 4 mL beer or 1.6 mL wine (18% v/v ethanol content).
d 1 mL contains 370 mg ethanol equivalent to 8.4 mL beer or 3.4 mL wine (38-42% v/v ethanol content).
e 2.5 mL contains 900 mg ethanol equivalent to 23 mL beer or 10 mL wine (38-45% v/v ethanol content).
Table 11: Summary of doses and preparations of THR products in the UK and EMA monographs.
|
Echinacea species |
Daily dose THR products UK |
EMA monographs daily doses |
|
Echinacea purpurea. |
Pressed juice from herb (DER 1.7-2.5:1): Dried pressed juice from herb (DER 20-28:1): Dry root extract (DER 6-7:1): |
Pressed juice from herb (DER 1.5-2.5:1): 6 - 9 g (equivalent to 9 - 22.5 g fresh herb.). Dried pressed juice: Corresponding to the expressed juice above. (EMA monograph, 2014). Dry root extract (DER 5.5-7.5:1): 360 mg (equivalent to 1,980 - 2,700 mg root). (EMA monograph, 2017). |
|
Echinacea angustifolia. |
Dry root extract (DER 4.5:1): |
Powdered root: (EMA monograph 2018). |
|
Echinacea pallida. |
Dry root extract (DER 4-9:1): Powdered root: |
Dry root extract (DER 4-8:1): (EMA monograph 2012). |