Description of key studies - Animal Studies - (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guideDescription of key studies
Clarity study
235. The Clarity study was a 2 year study NTP based study in which NCTR Sprague-Dawley rats were given doses of BPA (0, 2.5, 25, 250, 2,500,and 25,000 μg/kg body weight (bw)/day) by gavage in a 0.3% carboxymethylcellulose vehicle (Camacho et al, 2019). The rats were dosed from gestation day (GD) 6 through to the start of parturition and then the pups were dosed directly from the day after birth until either postnatal day 21 (the stop-dose arm) or continuously until termination at one (interim sacrifice) or two years. The stop-dose arm was included to assess the potential for any BPA effects that were due to developmental exposure. BPA was not detected in bedding extracts, cage leachates or drinking water.
Vigezzi et al., 2015
236. In the rat study by Vigezzi et al (2015) the dams were given 0.5 or 50 µg/kg bw BPA per day in drinking water from GD9–PND21; a Wistar derived strain was used. The F1 females were necropsied on PND90 and 360. The aim of the study was to assess the effect of long term BPA exposure on the uterus and the uterine response to oestrogen replacement therapy (this phase of the experiment started 1 year after BPA treatment). The group size was between 10-16. No changes in squamous metaplasia and luminal epithelial anomalies were observed. At necropsy on PND360 an increase in glands with cellular anomalies was observed in the 50 µg/kg bw per day; on PND90 but no effects were seen in the 50 µg/kg bw per day group or at both times of necropsy in the lower dose group.
Santamaria et al., 2016
237. In the study by Santamaria et al., 2016, 10-12 F0 dams/group of a Wistar derived strain and their female offspring were given 0.5 μg and 50 μg/kg bw per day (via drinking water) from GD9–PND21. The numbers are not given in the paper but from the figure, mean ovary weight appears to be around 50 mg in the controls and 40 mg in the treated groups, with no clear trend but a greater spread of data in the higher dose.
Hu et al., 2018
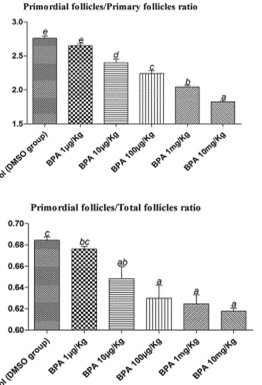
238. The aim of the study by Hu et al., (2018) was to investigate primary ovarian insufficiency, this the premature exhaustion of primordial follicles in the follicle pool, which is caused by the excessive premature activation of primordial follicles after birth. The authors state that BPA exposure promotes the transition of primordial follicles to primary follicles, thus the number of primordial follicles in the primordial follicle pool decreases significantly. However, the molecular mechanisms underlying abnormal follicle activation are poorly understood. The study aimed to investigate the role of the Phosphatase and tensin homologue (PTEN) signal system which is a negative regulator of follicle activation. This study was judged to be Tier 2.
239. Groups of 13 6 week old female mice were treated with oral doses of 0, 1, 10, 100, 1000 or 10,000 µg BPA/kg bw/day in corn oil with 0.1% DMSO for 28 days. The mice were killed in the oestrous cycle as determined by vaginal lavage and the ovaries collected. Ovaries from one side were used for serial section and hematoxylin and eosin (H&E) staining to evaluate the follicles, while ovaries from the other side were used for fluorescence immunocytochemistry, reverse transcription-polymerase chain reaction (RT-PCR), and Western blotting to observe the expression of PTEN. There were no significant differences in body weight between the two groups.
240. The hu of primordial follicles to primary follicles and the ratios of primordial follicles to total follicles in animals administered were significantly lower than in the control group, with a significant dose–response relationship being apparent. This is shown in the figure below, taken from the original paper. It was suggested that this indicated BPA promoted the premature activation of primordial follicles. Numerical results are not provided.
241. The results are expressed as mean + Standard Error of the Mean (SEM). Different lower-case letters above the columns, such as a, b, c, d, and e, indicate P < 0.05, and if 2 columns have the same lower case letter, it indicates no statistical significance. It is not stated in the paper but this suggests that the lowest dose is not significantly different from the controls when analysed by ANOVA.
Moore-Ambriz et al., 2015
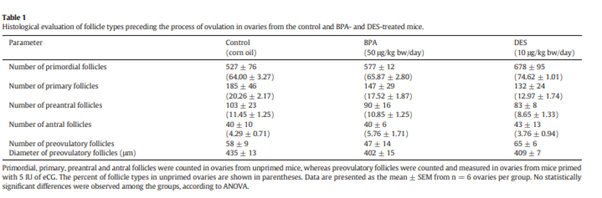
242. The aim of the study by Moore-Ambriz et al., 2015, was to investigate how BPA impaired ovulation, therefore the study evaluated whether BPA altered ovulation by affecting folliculogenesis, the number of corpora lutea or eggs shed to the oviduct, ovarian gonadotropin responsiveness, hormone levels, and oestrous cyclicity. Young adult (39 days old) female C57BL/6J mice were treated with corn oil (vehicle) or 50 μg/kg bw/day BPA (via pipette) for a period encompassing the first three reproductive cycles (12–15 days). The dose of 50 µg was selected as it was the US EPA’s safe exposure limit. Diethylstilbestrol (DES) was given as a positive control. The result on follicles are presented in Table 1, taken from the paper.
243. It was concluded that BPA exposure did not alter any parameters related to ovulation. This study was considered to be Tier 1.