Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs – Reproductive and Developmental Toxicity
Introduction- (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guideIntroduction
1. The Health Outcome Category “Reproductive and Developmental Toxicity” is considered in section 3.1.6 of the EFSA opinion. This paper is extensively based on the draft opinion and aims to provide a summary of the information covered in the chapter along with additional information where available. A number of the endpoints were taken forward for BMD analysis and it is noted in the main opinion that changes in ovarian follicle ratios was the second most sensitive endpoint and, had a TDI been set on this, the population would have exceeded it by 2-3 orders of magnitude.
Epidemiology - (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guideEpidemiology
2. A total of 47 epidemiology studies were considered. Details of their appraisal for internal validity is set out in Annex B to the EFSA opinion. The studies are listed alphabetically and may cover more than one endpoint. All epidemiology studies were considered to be tier 3, mainly because of low confidence in the exposure assessment.
3. Weight of evidence analysis was conducted on the following clusters and endpoints (see section 2.3.2 of the main opinion for further information on HOCs etc). The main information extracted from the studies is summarised in section C5 of Annex C to the opinion. The outcome of the weight of the evidence is described in the text of the opinion (and summarised below) and presented in a tabulated format in section D4 of Annex D to the opinion.
• C: Fetal and post-natal growth – Exp: Adulthood.
• C: Prematurity – Exp: Pregnancy.
• C: Pre-eclampsia – Exp: Adulthood.
• C: Male fertility – Exp: Adulthood.
• C: Female fertility – Exp: Adulthood.
Cluster Fetal and post-natal growth - Exposure during pregnancy
4. A total of 13 studies reported results on indices of fetal growth (Burstyn et al., 2013; Philippat et al., 2014; Smarr et al.2015; Veiga-Lopez et al., 2015; Birks et al., 2016; Casas et al., 2016; Ferguson et al., 2016; Huang YF et al., 2017a; Pinney et al., 2017; Woods et al., 2017; Lee YM et al., 2018; Lester et al., 2018; Mustieles et al., 2018b. Of these, two studies examined associations with measures of growth in utero based on ultrasound measures and two studies (Philippat et al., 2014; Lee YM et al., 2018) examined associations between maternal pregnancy BPA concentrations in urine with measures of post-natal growth up to 3 and 6 years of age. Overall, no consistent associations with birth weight, birth length, head circumference or other indices measured in utero or at birth were observed. In most cases the effect estimates were centred around the NULL with wide confidence intervals, but significant associations were observed in a few studies. As an example, the panel noted a study by Lee YM et al. (2018) which reported a significant increase in birth weight with higher maternal exposure while a significant decrease in femoral length in utero was observed. A second study by Mustieles et al. (2018) examined associations with birthweight among 346 subjects who were having their fertility status examined. In this study a significant inverse association between maternal pre-pregnancy urinary BPA concentration and birth weight was observed [~79 g decrease (95%CI: −153, −5) for ~3-fold increase in BPA exposure]. For maternal samples collected during pregnancy this association was in the same direction but non-significant [−38 g (95%CI: −101, 25)]. Although interesting, the panel considered that this inverse association for maternal pre-pregnancy BPA concentration would need to be replicated in another study before any robust conclusions can be drawn.
5. In terms of possible high exposures, Birks et al. (2016) used individual data from 13 European birth cohorts to identify pregnant women who had been occupationally exposed (based on self-report) to BPA during pregnancy. Of 133,957 individuals a total of 59 women with occupational exposure were identified. Mean birth weight among these exposed women was not significantly different from unexposed women. The few studies that examined more clinically relevant birth outcomes such as small for gestational age and low birth weight (Burstyn et al., 2013; Lester et al., 2018) did not find any association with maternal BPA exposure. However, only the case–control study by Burstyn et al. (2013) had sufficient statistical power to evaluate this outcome with some accuracy. Concerning post-natal growth, the study by Lee YM et al. (2018) also reported some significant associations between maternal concentrations of BPA in pregnancy and weight and weight for length from age 3 to 6 years of age. In contrast, no association with weight or length up to 3 years of age was observed in the study by Philippat et al. (2014).
Overall conclusions
6. On the basis of the above studies, the CEP Panel concluded that an association between maternal BPA exposure and impaired pre-natal and post-natal growth is Not Likely.
Cluster Prematurity - Exposure during pregnancy
7. A total of seven studies examined the association between maternal BPA concentrations and length of gestation or preterm delivery (Weinberger et al., 2014; Cantonwine et al., 2015; Smarr et al., 2015; Veiga-Lopez et al., 2015; Birks et al., 2016; Casas et al., 2016; Pinney et al., 2017. In a study of a cohort of 72 pregnant women, Weinberger et al. (2014) reported a significant inverse association between total BPA concentration in urine and length of gestation (approximately 1-day shorter gestation for each interquartile increase in exposure). In contrast, a relatively large case–control study sampling 130 preterm cases and 352 random controls taken from a larger birth cohort (n = 2246) did not find any association between maternal urinary BPA exposure (samples collected minimum three times during pregnancy) and preterm delivery (Cantonwine et al., 2015. In other studies looking at length of gestation, no association was consistently observed. In studies examining associations with length of gestation no associations were observed (Smarr et al., 2015 ; Veiga-Lopez et al., 2015; Casas et al., 2016; Pinney et al., 2017).
8. In terms of possible high exposures Birks et al. (2016) reported a slightly longer gestation period (approximately 4 days) among 59 women that were likely to have been occupationally exposed to BPA (based on self-report) compared with unexposed women (n = 116,358).
Overall conclusions
9. On the basis of these studies, the CEP Panel concluded an association between BPA exposure and shorter duration of gestation or increased risk of preterm delivery is Not Likely.
Cluster Pre-eclampsia - Exposure during adulthood
10. Two case–control studies, Ye et al. (2017) n = 173 (73 cases, 99 controls) and Cantonwine et al. (2016), n = 481 (50 cases, 431 controls) for a total number of cases of 123, assessed the association between BPA exposure measured in spot urine (n = 1) or serum (n = 1) samples in pregnancy and endpoints related to pre-eclampsia (onset and/or severity).
11. The detailed description and risk of bias assessment related to these studies are provided in Annexes C and D to the opinion respectively. The study by Cantonwine et al., (2016) was conducted in the US and did not report an association with pre-eclampsia: HR (95% CI), 10 weeks 1.53 (1.04–2.25), 18 weeks 1.12 (0.61–2.07), 26 weeks 0.68 (0.43–1.07), average 1.14 (0.73–1.79). The study by Ye et al., (2017) was conducted in China with similar endpoint definitions. In this study, BPA exposure (continuous and per tertile) was statistically significantly associated with pre-eclampsia Odds ratio (OR) (95% CI), continuous BPA, 1.39 (1.19–1.63); OR (95% CI), categorical BPA, medium 2.15 (0.98–4.75), high 16.46 (5.42–49.95), pre-eclampsia onset OR (95% CI), continuous BPA, mild severity 1.42 (1.21–1.67), severe 1.35 (1.14–1.60) and pre-eclampsia severity (OR (95% CI), continuous BPA, early onset 1.33 (1.07–1.66), late onset 1.41 (1.20–1.66) 20–1.66).
Overall conclusions
12. On the basis of the above, the CEP Panel concluded that the evidence for a positive association between BPA exposure and pre-eclampsia is ALAN.
Cluster Male fertility - Exposure during adulthood
13. A total of five studies (Buck Louis et al., 2014; Bae et al., 2015; Dodge et al., 2015; Goldstone et al., 2015; Buck Louis et al., 2018) in three different cohorts examined the relationship between urinary BPA concentrations and fertility in both males and females.
14. Among couples (n = 218) seeking fertility treatment, Dodge et al. (2015) found no association between urinary BPA concentrations in men and fertilisation or live birth following in vitro fertilisation or insemination. Similarly, Buck Louis et al. (2014) examined associations between urinary concentrations in both male and female couples (n = 501) with fecundability. No association was observed for urinary BPA concentrations in males and females.
15. In a later study of 339 males from the same cohort (Buck Louis et al., 2018) no association was observed between BPA concentrations in seminal plasma and fecundity.
16. These findings are in line with a study by Goldstone et al. (2015) where no association was observed between urinary BPA concentrations and semen quality (total count, concentration or morphology) in 418 males.
17. Finally, Bae et al. (2015) reported an association between paternal BPA exposure and fewer male births (lower male to female sex ratio). (Relative risk of male birth. per standard deviation change in the log- transformed maternal urinary BPA concentration. RR 1.16 (95% CI 1.03–1.31). The model was adjusted for paternal concentration, urinary creatinine, research site, maternal age, difference in couples' ages, annual income and maternal parity). The panel noted that as no other studies have reported on this outcome and in the light of the fact that none of the other studies found consistent associations with live birth rate, fecundability or other fertility outcomes, a chance finding seems plausible.
Overall conclusions
18. On the basis of the above, the CEP Panel concluded that an association between exposure to BPA measured in spot urine and reduced fertility is considered Not Likely.
Cluster female fertility- Exposure during adulthood
19. A total of 11 studies examined the association between exposure to BPA during adult life with fertility in females (Souter et al., 2013; Buck Louis et al., 2014; Lathi et al., 2014; Bae et al., 2015; Minguez-Alarcon et al., 2015; Chavarro et al., 2016; Jukic et al., 2016; Minguez-Alarcon et al., 2016; Chin et al., 2018; Pollack et al., 2018; Wang B et al., 2018). These included associations between BPA exposures during pregnancy with fecundability and miscarriage and offspring sex ratio in the more general population (Buck Louis et al., 2014; Lathi et al., 2014; Bae et al., 2015; Jukic et al., 2016; Chin et al., 2018; Wang B et al., 2018) or associations with fertility outcomes in a more selective group of women seeking fertility treatment (Souter et al., 2013; Minguez-Alarcon et al., 2015; Minguez-Alarcon et al., 2016.
20. For the studies among the general population, associations with fecundability were inconsistent, with three studies reporting no association (Buck Louis et al., 2014; Jukic et al., 2016; Chin et al., 2018. One study (Wang B et al., 2018) reported associations between maternal BPA exposure and decreased fecundability (OR 95% CI: 0.87 (0.78, 0.98) for ~3-fold increase in exposure]. OR < 1 reflects longer time to pregnancy. A study by Lathi et al. (2014) also reported a significant positive association between BPA and aneuploid pregnancy and risk of miscarriage (Association between BPA and aneuploid pregnancy risk ratio and respective 95% CI for every quartiles of BPA exposure (1st quartile as reference) 1Q–2Q: RR = 1.18 (95% CI: 0.57–2.45) 2Q to 3Q: RR = 1.63 (95% CI: 0.86–3.09) >3Q: RR = 1.97 (95% CI: 1.08–3.59) p < 0.05 Association between BPA and miscarriages. Risk ratio and respective CI for every quartiles of BPA exposure (3Q: RR = 1.83 (95% CI: 1.14–2.96) p <0.05).
21. In a group of selected women seeking fertility treatment, Chavarro et al. (2016) reported a significant decrease in live births among strata of women who did not consume soy (n = 100), while no such association were observed among majority of study participants that did consume soy (n = 247). As women who seek treatment may change their dietary habits before treatment it is possible that women who did not consume soy in a group of selected women may have underlying different fertility compared with those who reported to consume soy. The context of the study was that animal studies had shown that soy could ameliorate the effects of BPA, so the aim was to establish if this was the case in humans. Similarly, in a group of selected women Minguez-Alarcon et al., (2016) reported decreased probability of implantation and clinical pregnancies with higher BPA exposures.
Overall conclusions
22. Overall, the results from these studies conducted in both selected and more unselected group of women are inconsistent and an association between BPA exposure in adult life and reduced fertility was judged as ALAN.
Cluster Pubertal/endocrine - Exposure during pregnancy
23. Watkins et al. (2017a) examined the association between exposure to BPA during pregnancy and pubertal development in 109 male offspring aged 8 to 14 years. In a separate publication based on the same cohort, associations with pubertal development were also examined in 120–129 female offspring aged 8 to 13 years (Watkins et al., 2014 ; Watkins et al., 2017b). No associations with markers of pubertal development were observed in either of the studies.
24. Similarly, no association between exposure to BPA in pregnancy and markers of puberty was observed in 112 boys in a study by Ferguson KK et al. (2014). However, a study by Berger et al. (2018) examining the relation between maternal BPA exposure and pubertal development in 179 female and 159 male offspring at age 9 to 13 years found associations with later and earlier pubertal development in female and male offspring, respectively. Association between BPA exposure and thelarche onset in females (mean change in months with 95% CI). All girls: 3.0 (95% CI, 0.9–5.1); normal weight: 4.7 (95% CI, 2.5–7.0); overweight: 1.6 (95% CI, −1.6–4.8), inverse association between maternal BPA concentrations and pubarche onset in boys. Mean shift in months −3.1 (95% CI: −5.1 to −1.0) Inverse association between BPA exposure and gonadarche onset in boys. Mean shift in months −4.1 (95% CI: −6.6 to −1.6).
25. Finally, three studies reported associations with anogenital distance (Barrett et al., 2017; Arbuckle et al., 2018; Sun et al., 2018, an outcome that is often used as a predictor for later reproductive disorders. Overall, despite a few significant findings being observed the findings from these three studies were inconsistent.
26. On the basis of these studies, the CEP Panel concluded that an association between BPA exposure during pregnancy and pubertal development is considered as ALAN.
Cluster Pubertal/endocrine - Exposure during childhood
27. A total of five prospective studies examined associations between exposure to BPA measured in urine during childhood and pubertal development (Lee et al., 2013; Wolff et al., 2015; Kasper-Sonnenberg et al., 2017; Wolff et al., 2017; Binder et al., 2018. However, two of these studies were conducted in the same study population but with different lengths of follow-up (Wolff et al., 2015; Wolff et al., 2017). Overall, no association between childhood exposures to BPA and pubertal development were observed in these studies.
28. On the basis of these studies, the CEP Panel concluded that an association between BPA exposure during childhood and pubertal development is considered Not Likely.
Overall conclusions
29. On the basis of these studies, the CEP Panel concluded that an association between BPA exposure and pubertal development is ALAN.
Cross sectional studies
30. A number of cross-sectional studies were considered for each endpoint as described above (p181-182 of the opinion). In general, these did not add to the assessment and have not been considered further in this document. Some findings of interest are:
- Leclerc et al., 2014 found no statistical significance between maternal exposure and pre-eclampsia, analysing levels in maternal and fetal serum and placenta. In placental tissue, concentrations of BPA were higher in preeclamptic women compared with normotensive pregnant women (p-value = 0.04).
- 15 cross-sectional studies considered BPA and male fertility, assessing a number of different parameters. It was noted that although, the associations were not entirely consistent in terms of directionality, findings from some individual studies could be interpreted as being adverse for male reproductive function. However this was not supported by other lines of evidence – prospective studies and animal studies.
- Similarly, a number of cross-sectional studies reported associations between BPA concentrations and adverse effects in female fertility. It was noted that the measured BPA concentrations reflected exposure during the previous few hours and as such, in the absence of further evidence, are unlikely to reflect past exposure that may have led to development of the underlying disease condition. Differences in lifestyle factors that may affect exposure to BPA or rate of uptake or excretion among cases may equally explain the observed findings, particularly in the absence of similar findings from prospective studies.
Animal studies -(BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guideAnimal studies
31. As part of the HOC a Reproductive and Developmental Toxicity, a total of 153 animal studies were reviewed by the CEP panel. Endpoints for which statistically significant changes were reported were extracted from the available literature and grouped into three clusters:
- Developmental toxicity.
- Female reproductive toxicity.
- Male reproductive toxicity.
These clusters include endpoints that were identified as relevant in the 2015 EFSA opinion and were considered in the uncertainty analysis. These endpoints were endometrial hyperplasia, ovarian cysts and anogenital distance (AGD); the first two of these were also identified as relevant in the newly compiled studies. As it was previously identified as relevant, AGD was also included and considered relevant in the new assessment.
32. The information extracted from these studies is summarised and tabulated (Table G5) in Annex G to the opinion. The weight of evidence is discussed in the main opinion and presented in a tabulated format in Annex H (sheet H5). The clusters considered not likely or ALAN are outlined briefly below, with the clusters considered likely are described in more detail.
Cluster - Developmental toxicity
33. Within the cluster developmental toxicity, there were nine studies in mice; seven studies had exposure during development until weaning, two had exposure during development until adulthood and one had exposure during the growth phase. There were 13 studies on rats: 10 studies had exposure during development until weaning, four had exposure during development until adulthood and in one the exposure was to adults. Some studies assessed multiple exposure periods.
34. The specific endpoints that were included in this cluster were blastocyte outgrowth incidence, embryo development, age/day of first oestrus, AGD, mammary gland histology, mammary gland weight, bone development and body weight of F1/F2/F3 generation, as well as body weight of F0 dams. (Note- the assessment of body weight for each exposure period is described in detail in the Metabolic effects category (Chapter 3.1.4.2).
Developmental exposure (pre-natal and/or post-natal until weaning)
AGD
35. One Tier 2 study (Spörndly-Nees et al., 2018) in rats was considered. There was a not statistically significant increase in AGD at 12 months but not at PND 35. In the absence of a dose -response and without other studies available this endpoint was considered Not Likely.
Age at first oestrus
36. No effect was seen in a single Tier 1 study in mice (Tucker et al., 2018) using doses of 500, 5000 and 50000 µg/kg bw per day, twice daily, from GD10.5–17.5. The endpoint was judged Not Likely.
Bone development
37. Two Tier 1 studies in rats (Lejonklou et al., 2016; Lind et al., 2017 and one Tier 1 study in mice were available (van Esterik, 2014). The studies in rats showed inconsistent and no effects were observed in mice. The endpoint was judged ALAN.
Mammary gland weight
38. One Tier 1 study in rats was identified (Montévil et al., 2020, with mammary gland weight in female rats was determined at PND90. It was noted that the study authors described a Non Monotonic Dose Response (NMDR). However, when using more conventional statistical methods, e.g. modelling the data in PROAST (Hill and Exponential models) or using spline and polynomial fit (without overfitting the data) no dose response could be identified by the CEP Panel. The CEP Panel considered that alternative interpretations of the data were plausible, and the findings were likely explained by random fluctuations and variability in the data. The endpoint was judged Not Likely.
Mammary gland histology
39. In males: Out of six rat and one mouse studies, three rat studies (all Tier 1) (Kass et al., 2015; Mandrup et al., 2016; NTP Clarity Report, 2018/Camacho et al., 2019 assessed the mammary gland histology of male pups. There were no neoplastic changes but changes in mammary gland growth were observed in Kass et al. (2015) and Mandrup et al. (2016). The study by Mandrup et al., (2016) showed a decrease in male and increase in female-like mammary gland structures in male rat pups assessed at PND100 when doses of 5,000 and 50000 µg/kg bw per day were given from GD7 until birth. Assessment of the same dose groups at PND22 did not show any changes. However, lower doses in this study (25 and 250 µg/kg bw per day), which were also assessed at PND22, showed an increase in mammary gland growth at 25 µg/kg bw per day. No effects were reported on mammary glands in males in the NTP Clarity Report (2018)/Camacho et al. (2019).
40. The CEP Panel judged the male mammary gland effects as ALAN.
41. In females: Non-neoplastic findings in female rat mammary gland were observed in six Tier 1 studies (Kass et al., 2015; Grassi et al., 2016; NTP Clarity Report, 2018/Camacho et al., 2019; Montévil et al., 2020; Tucker et al., 2018; Mandrup et al., 2016 studies and one Tier 3 study (Leung et al., 2017). The findings included delayed ductal growth, increased branching score and number of terminal ducts (TDs) and terminal end buds (TEB+TDs) a decreased incidence of ductal dilatation in F1 females at 1 year. Montévil et al 2020, who assessed samples from the Clarity study, reported several effects at single doses in the mammary glands of female F1 pups, these included increased gland density, a number of structural difference and an increased average branch length at BPA doses of 250 µg/kg bw per day. All effects with indications of a dose–response relationship for which individual data were available (gland density, Dimension 3D and angles of branches between beginning and end, thickness of epithelium, variation of ductal thickness, aspect ratio) were statistically re-analysed by the CEP Panel. This revealed that a formal dose–response could not be identified by fitting flexible biologically based functions or polynomials that are commonly used to describe biological systems except for ductal thickness and aspect ratio, for which a potential non- monotonic dose–response was identified statistically.
42. Another Tier 1 study (Tucker et al., 2018) reported increased branching density at low doses, increased length of TEBs, mammary gland development score and number of TEBs at mid dose. All effects occurred only at PND20 and not at later time points. These non-neoplastic were not consistent among studies with different study designs. Pre-neoplastic findings in adult females were seen in one Tier 1 study, Mandrup et al. (2016), showing an increase in intraductal hyperplasia. One Tier1 study (NTP Clarity Report, 2018/Camacho et al., 2019 revealed a neoplastic effect, an increase in adenoma and adenocarcinoma in the lowest dose, assessed at terminal sacrifice after 2 years. However, four other Tier 1 studies with shorter timepoints showed no pre-neoplastic effects. One neoplastic lesion was reported, i.e. stromal polyps, in the NTP Clarity Report, 2018/Camacho et al., 2019. For this endpoint, a decrease (no adverse effect) was seen in this Tier 1 study at the highest dose after 2 years.
43. The CEP Panel concluded that, based on the inconsistent findings of non-neoplastic effects as well as the unconfirmed neoplastic or pre-neoplastic effects, that effects on the female mammary gland are ALAN.
44. Overall, the CEP Panel assigned a likelihood level of ALAN to the developmental toxicity effects of BPA in the developmental exposure period based on bone development, mammary gland histology and body weight. Therefore, none of the endpoints were taken forward for BMD analysis. However, the ALAN endpoints were considered in the uncertainty analysis (which is set out in Appendix D to the main opinion).
Developmental and adult (pre-natal and post-natal in pups until adulthood)
AGD
45. Only one single-dose study (Tier 3) by Patel et al., 2013 was available. It was therefore judged that there is Inadequate evidence for this endpoint.
Embryo development
46. A single Tier 3 study was available (Dobrzynska et al., 2018). It was therefore judged that there is Inadequate evidence for this endpoint.
Bone development:
47. Only one single-dose study (Tier 2) by Auger et al., 2013 is available. It was therefore judged that there is Inadequate evidence for this endpoint.
Mammary gland histology
48. Two Tier 1 studies (Montévil et al., 2020; NTP Clarity Report 2018/Camacho et al., 2019) assessed the mammary glands of female rats. Non-neoplastic effects were only observed at single doses without any dose–response, apart for mammary gland scores, where the data were in line with the definition by the CEP Panel of indications for a NMDR. For Montévil et al. (2020), the CEP Panel re-evaluated the dose–response for gland density by fitting flexible biologically based functions or polynomials that are commonly used to describe biological systems and concluded that there was no dose–response relationship.
49. Among the non-neoplastic effects identified in this exposure group several changes were only reported in one dose group in the female rats, this was an increase in lobular alveolar budding (Montévil et al., 2020), changes in ductal dilatation which was increased at 1 year but decreased at 2 years (the adversity was noted to be unclear) and a decrease in lobular hyperplasia, (NTP Clarity Report 2018/Camacho et al.2019) was also observed. The BPA-induced decreases were different from the results following oestradiol treatment which resulted in clear increases in duct dilatation and lobular hyperplasia as well in adenocarcinomas. In the same study, an increase in alveolar dilatation was only reported in males at the lowest dose after 2 years while the effect was not significant in females in both studies. Therefore, no dose–response could be established for non-neoplastic effects. For neoplastic effects, the NTP Clarity Report (2018)/Camacho et al. (2019) reported an increase in atypical foci and adenocarcinomas at a dose of 2.5 µg/kg bw per day. In addition, there were non-significant increases in atypical foci in the 25 and 250 μg/kg bw per day dose groups at 1 year. One neoplastic lesion, stromal polyps, was also reported. For this endpoint, however, a significant dose trend towards increased incidence at the higher doses at 1 year was observed, while a negative trend (no adverse effect) was observed at 2 years in this Tier 1 study. The CEP Panel therefore considered this result biologically implausible.
50. Based on the above results, histological effects on mammary gland induced by BPA were judged as ALAN.
51. Overall, the CEP Panel assigned a likelihood level of ALAN to the developmental toxicity effects of BPA in the developmental and adult exposure period based on effects on body weight and mammary gland histology. Therefore, none of the endpoints was taken forward for BMD analysis. However, the ALAN endpoints were considered in the uncertainty analysis (see Appendix D to the opinion).
Growth phase/young age
Age at first oestrus
52. In the Tier 1 study by Li et al., (2016) in mice, a decreased age at first oestrus was observed at a BPA dose of 60 μg/kg bw per day, and an increased age at first oestrus at a dose of 600 μg/kg bw per day following dosing three times per day after 5 weeks of exposure starting at PND22.
53. The likelihood of changes in age at first oestrus was judged ALAN by the CEP Panel.
54. The CEP Panel assigned a likelihood of ALAN to the cluster of developmental toxicity of BPA in the growth phase/young age exposure period. Therefore, none of the endpoints was taken forward for BMD analysis. However, both body weight and age at first oestrus were considered in the uncertainty analysis (see Appendix D).
Adult exposure (after puberty)
Blastocyst outgrowth and F1 embryo development:
55. It was judged that there was Inadequate Evidence for these endpoints as they were both only assessed in a single dose Tier 1 Study (Martinez et al., 2015).
56. The CEP Panel assigned a likelihood level of Not Likely to the developmental toxicity effects of BPA in the adult exposure period based on body weight. Therefore, none of the endpoints were taken forward for BMD analysis.
Indirect (germline) exposure
Bone development
57. As only one Tier 2 single-dose study (Auger et al., 2013 was available for assessment, it was judged that there was inadequate Evidence for this endpoint.
58. The CEP Panel assigned a likelihood level of Not Likely to the developmental toxicity effects of BPA in the indirect germline exposure period based on body weight. Therefore, this endpoint was not taken forward for BMD analysis.
Overall cluster selection for endpoints/studies for BMD for developmental toxicity
59. Overall, the CEP Panel assigned a likelihood level of ALAN, to the developmental toxicity effects of BPA in the exposure periods developmental, developmental and adult and growth phase/young age, and of Not Likely in the adult and indirect (germline) exposure. The overall likelihood across all exposure periods, i.e. the highest likelihood given in the cluster developmental toxicity was ALAN.
60. The CEP Panel considered that the evidence from the studies available showed ALAN effects of BPA for the endpoints bone development, mammary gland histology, body weight (developmental exposure), body weight and mammary gland histology (developmental and adult exposure) as well as body weight and age at first oestrus (growth phase/young age). These endpoints were therefore not brought forward for BMD analysis.
Female reproductive toxicity - Animal Studies - (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guideFemale reproductive toxicity
61. In the cluster female reproductive toxicity, 17 studies were available in mice, of these seven studies had exposure during development until weaning, one had exposure during development until adulthood, one had exposure during the growth phase, in seven studies the mice were exposed as adults and four had indirect germline exposure: some of the studies tested multiple exposure periods. Of the 16 studies on rats, 11 had exposure during development until weaning, three had exposure during development until adulthood, and in four the rats were exposed as adults. There was one study in hamsters which were exposed during development until weaning. In addition, three studies were on sheep, two had exposure during the period development until weaning and one as adults.
62. The specific endpoints that were included for effects of BPA on female reproductive toxicity cluster were plasma/serum thyroid hormones, testosterone, oestrus cyclicity, age at first oestrus, fertilisation rate and implantation incidences, ovary weight and histology, uterus weight and histology.
Developmental exposure (pre-natal and/or post-natal until weaning)
Plasma/serum thyroid hormones
63. For this exposure period the following studies were identified.
- Triiodothyronine (T3): one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019
- Thyroxine (T4): two Tier 1 rat studies (Bansal and Zoeller, 2019; NTP Clarity Report, 2018/Camacho et al., 2019, one Tier 1 sheep study (Guignard et al., 2017 and one Tier 3 mouse study (Bodin et al., 2014.
- T3/ totalT4: one Tier 1 sheep study (Guignard et al., 2017).
64. No effect was seen on T3 levels in the Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019. In this study, the NCTR Sprague Dawley rats were dosed with 2.5, 25, 250, 2500 or 25000 μg/kg bw per day BPA by gavage; F0 dams from GD6 to PND0 and F1 pups from PND1 to PND21 at interim sacrifice (1 year).
65. No effect was seen on T4 levels in the Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019. Similarly, on PND15, no effect was observed in the Tier 1 rat study by Bansal and Zoeller, 2019 in which female NCTR Sprague Dawley rats were dosed from GD6–PND15 with 2.5, 25, 250, 2500 or 25000 μg/kg bw BPA per day by gavage (this study is a sub project of the Clarity study). No change in T4 was observed in fetuses (GD28–132/134) in the Tier 1 study in Lacaune sheep (Guignard et al., 2017) after sub cutaneous dosing with 5, 50 or 5000 µg/kg bw BPA per day (using dose conversion factors of 125 and 37 respectively, the doses were equivalent to oral doses of 625, 6250 or 625000 µg/kg bw per day and 185, 1850 or 185000 µg/kg bw per day).
66. No change was observed on the serum ratio T3/ total T4 (TT4) in the study in sheep by Guignard et al., 2017.
67. During this exposure period the likelihood of changes in thyroid hormones (T3, T4, rT3/TT4) were judged as Not Likely by the CEP Panel as no effects were observed in Tier 1 or Tier 2 studies in rats or in a Tier 1 study in sheep.
Plasma/serum testosterone:
68. Only three Tier 3 studies in rats (Castro et al., 2018; Leung et al., 2017; Johnson et al., 2016) along with two Tier 3 studies in mice (Mahalingam et al., 2017; Tucker et al., 2018) were identified for this endpoint. The CEP Panel noted that as only Tier 3 studies were available, the likelihood for this endpoint could not be determined because of Inadequate evidence.
Oestrus cyclicity
69. For this exposure period, four Tier 1 studies (Hass et al., 2016; Ferguson SA et al., 2014; NTP Clarity Report, 2018/Camacho et al., 2019; Franssen et al., 2016) and one Tier 2 study (Santamaria et al., 2016) in rats, one Tier 1 study (Tucker et al., 2018) and one Tier 2 study (Acevedo et al., 2018) in mice and one Tier 2 study (Veiga-Lopez et al., 2014) in sheep were identified. A Tier 3 rat study (Leung et al., 2017 and a Tier 3 mouse study (Wang W et al., 2014) were also identified.
70. No change in oestrus cyclicity was observed in the Tier 1 rat studies which used a range of doses and administration methods. Similarly, no change was observed in the Tier 1 mouse study. However, in a Tier 2 mouse study (Acevedo et al., 2018) only, a decrease in oestrus cyclicity by 6 months at the lowest dose was observed in F1 females.
71. In the Tier 2 study in sheep (Veiga-Lopez et al., 2014) a change in oestrus cyclicity (decrease in follicular count trajectories) in all dose groups was observed. Pregnant sheep were exposed to BPA from GD30–GD90 by subcutaneous injection of 50, 500 or 5000 µg/kg bw per day. The time of examination for oestrus cyclicity was not described but considered at least after 8 weeks (weaning) and when the F1 females weighed more than 40 kg.
72. Apart from the decreased oestrus cyclicity in only the lowest dose and one timepoint in the Tier 2 mouse study (Acevedo et al., 2018 and a decrease in oestrus cyclicity (decrease in follicular count trajectories) in the sheep study (Veiga-Lopez et al., 2014, no change in oestrus cycle was observed in the other five Tier 1 studies in rats and mice. Therefore, the CEP Panel considered that the likelihood of a change in oestrus cyclicity is Not Likely.
Ovary weight
73. One Tier 1 study (NTP Clarity Report, 2018/Camacho et al., 2019) and one Tier 2 study (Santamaria et al., 2016) in rats and one Tier 3 mouse study (Patel et al., 2013 were identified for this exposure period.
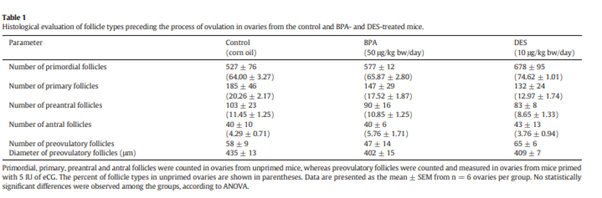
74. In the Clarity study, a decrease in ovary weight was observed in the high-dose group with a trend apparent in the other dose groups. Ovary weight was decreased in the F1 females at 7 weeks of age. The NTP report states that “mean absolute ovary weights, as well as ovary weights adjusted for brain and body weights, were decreased relative to the vehicle control mean by 18%, 16%, and 15%, respectively”.
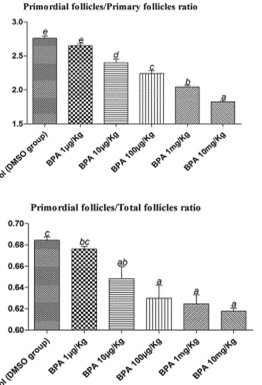
75. In the Tier 2 rat study by Santamaria et al., (2016) 10-12 F0 dams/group of a Wistar derived strain and their female offspring were given 0.5 μg or 50 μg/kg bw BPA per day (via drinking water) from GD9–PND21. An approximately equal decrease was observed in ovary weight in the F1 animals of both BPA-treated groups at PND90. The numbers are not given in the paper but from the figure, mean ovary weight appears to be around 50 mg in the controls and 40 mg in the treated groups, with no clear trend but a greater spread of data in the higher dose.
76. The CEP Panel considered the likelihood of the decrease of ovary weight as Likely as there was a trend in the Tier 1 study (supported by one Tier 2 study with effects at lower doses without dose–response (Santamaria et al., 2016).
Ovary histology (follicle count, cellular hypertrophy, follicular cysts):
77. For this exposure period one Tier 1 study (NTP Clarity Report, 2018/Camacho et al., 2019, one Tier 2 study (Santamaria et al., 2016 and one Tier 3 study (Patel et al., 2017 in rats and three Tier 3 studies in mice (Mahalingam et al., 2017; Wang W et al., 2014; Berger et al., 2016) were identified.
78. In the Tier 1 Clarity study, no change was observed in cell hypertrophy. The incidence in follicular cysts was increased in the highest dose group and in all dose groups there was an increased trend in the incidence of follicular cysts. In the Tier 2 (Santamaria et al., 2016) in rats, a decrease in the number of growing follicles was observed in the BPA-treated groups on PND90; the decrease was comparable in both groups.
79. The CEP Panel considered that for the exposure period developmental exposure (pre-natal and/or post-natal until weaning) the likelihood of histological changes in the ovary as Likely.
Uterus weight:
80. For this exposure period one Tier 1 study (NTP Clarity Report, 2018/Camacho et al., 2019) in rats, one Tier 1 study in hamsters (Radko et al., 2015) and one Tier 3 study in mice (Patel et al., 2013) were identified.
81. In the Tier 1 rat Clarity study, no change in uterus weight was observed. In the Tier 1 hamster study uterus weight (wet and dry) on PND21 was statistically significantly increased at the top dose of 160000 µg/kg bw BPA per day only.
82. The effect on uterus weight was considered as ALAN as no effect was observed in the Tier 1 study in rats and an increase in weaning hamsters (uterotropic assay) was only seen at the highest dose (160,000 µg/kg bw per day).
Uterus histology
83. This endpoint included observations of cystic endometrial hyperplasia, uterine dilation, squamous metaplasia, apoptosis in the luminal epithelial cells of the endometrium, endometrial hyperplasia, luminal epithelial anomalies and glands with cellular anomalies): Two Tier 1 studies in rats (NTP Clarity Report, 2018/Camacho et al., 2019; Vigezzi et al., 2015) were identified for this exposure period.
84. In the Tier 1 Clarity study, a statistically significant increase was observed in cystic endometrial hyperplasia at the interim sacrifice (1 year) in the highest dose group and at terminal sacrifice (2 years) in the two highest dose groups (2500 and 25,000 μg/kg bw BPA per day group). At the interim sacrifice, uterine dilation and squamous metaplasia were increased in the 250 μg/kg bw per day and in the 25,000 μg/kg bw per day dose groups, respectively. There was a non-significant increase observed in the incidence of apoptosis in the luminal epithelial cells in the endometrium in the high-dose group. No change was observed in endometrial hyperplasia in any of the dose groups in this study.
85. In the other Tier 1 study in Wistar rats (Vigezzi et al., 2015) the dams were given 0.5 or 50 µg/kg bw BPA per day in drinking water from GD9–PND21. The F1 females were necropsied on PND90 and 360. No changes in squamous metaplasia and luminal epithelial anomalies were observed. At necropsy on PND360, an increase in glands with cellular anomalies was observed in the 50 µg/kg bw per day; on PND90 but no effects were seen in the 50 µg/kg bw per day group or at both times of necropsy in the lower dose group.
86. The CEP Panel considered the endpoint uterus histology based on effects seen at histological examination of the uterus in two rat Tier 1 studies as Likely.
87. During developmental exposure (pre-natal and/or post-natal until weaning), the CEP Panel assigned a likelihood level of Likely to the cluster female reproductive toxicity of BPA. Since the likelihood level is Likely for the endpoint ovary weight in Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019, for the endpoint ovary histology (follicle count and follicle cysts) in one rat study (NTP Clarity Report, 2018/Camacho et al., 2019 (Tier1)) and the endpoint uterus histology in two Tier 1 rat studies (NTP Clarity Report, 2018/Camacho et al., 2019; Vigezzi et al., 2015), these were taken forward for BMD analysis and uncertainty analysis.
Developmental and adult exposure (pre-natal and post-natal in pups until adulthood)
Plasma/serum thyroid hormones
88. For this exposure period only one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019) was identified. No effect was seen on T3 or T4 in this study. Based on this study, the CEP Panel considered an effect on thyroid hormones (T3, T4) as Not Likely.
Oestrus cyclicity
89. For this exposure period only one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019 was identified. No effect was seen on oestrus cyclicity. Based on this study, the CEP Panel judged an effect on oestrus cyclicity as Not Likely.
Ovary weight
90. For this exposure period, the one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019 and one Tier 3 mouse study (Patel et al., 2013 ) were identified. No effect was seen on ovary weight and based on this Tier 1 rat study, the CEP Panel judged an effect on ovary weight as Not Likely.
Ovary histology (interstitial cell hypertrophy and follicle cysts):
91. For this exposure period one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019 was identified. In this, a statistically significant increase was observed in interstitial cell hypertrophy in the 2,500 and 25,000 μg/kg bw per day dose groups at the interim (1 year) sacrifice. In the same study, no change in follicular cysts was observed. The CEP Panel judged the effect on interstitial cell hypertrophy as Likely.
Uterus weight
92. For this exposure period two Tier 1 rat studies (NTP Clarity Report, 2018/Camacho et al., 2019; Leung et al., 2020 and one Tier 3 mouse study (Patel et al., 2013) were identified. No effect was seen on uterus weight in either of the Tier 1 studies. Based on this, the CEP Panel judged an effect on uterus weight as Not Likely.
Uterus histology
93. This endpoint included observations of squamous metaplasia, apoptosis, uterine dilation, endometrial hyperplasia and squamous metaplasia).
94. For this exposure period two Tier 1 rat studies (Leung et al., 2020; NTP Clarity Report, 2018/Camacho et al., 2019 were identified. In the Tier 1 rat study (Leung et al., 2020 – note this study is one of the grantee studies related to Clarity) no effects were observed on squamous metaplasia and apoptosis; the protocol was the same as for the Clarity study. However, in the Clarity study the following statistically significant effects were observed at interim sacrifice (1 year): increased uterine dilation (250 µg/kg bw per day), increased endometrial hyperplasia (2.5 or 250 µg/kg bw per day), apoptosis and squamous metaplasia (25,000 µg/kg bw per day) and a decreased cystic endometrial hyperplasia (2.5 µg/kg bw per day). No other statistically significant effects were observed at interim or terminal sacrifice in this study.
95. During the exposure period developmental until adult, no effect was seen on squamous metaplasia and apoptosis in one of the Tier 1 rat studies (Leung et al., 2020, but in the other in which the exposure time and dose range (2.5, 25, 250, 2500 and 25000 µg/kg bw per day) was the same, an effect was only observed at the highest dose tested. The other histological effects were only seen at low concentrations (uterine dilation at 250 µg/kg bw per day and cystic endometrial hyperplasia (2.5 µg/kg bw per day)). Therefore, the CEP Panel considered the likelihood for this endpoint to be ALAN.
Number of implantation sites
96. In the Tier 1 rat study (Boudalia et al., 2014) the dams were exposed from GD1 to last day of lactation (LD21) by micropipette with 5 μg/kg bw BPA. The examination of the dams at LD/PND21 revealed no change in the number of implantation sites. As this study is a single-dose study, the CEP Panel considered the available study data to be Inadequate evidence for any further conclusions.
97. During developmental and adult exposure (pre-natal and/or post-natal in pups until adulthood), the CEP Panel assigned a likelihood level of Likely to the cluster female reproductive toxicity of BPA. As the likelihood level is Likely for the endpoint ovary histology (interstitial cell hypertrophy in the Tier 1 study (NTP Clarity Report, 2018/Camacho et al., 2019; this study was taken forward for BMD analysis; the Likely and ALAN endpoints were considered for uncertainty analysis.
Growth phase/young age exposure
Oestrus cyclicity
98. For this exposure period one Tier 1 mouse study in which oestrus cyclicity was studied, was identified (Li et al., 2016). Mice were dosed from PND22 for 5 weeks (3 times per day) orally (via micropipette) with 0, 60 and 600 μg/kg bw per day BPA. In the high-dose group, the oestrous cyclicity was decreased (the females spent less time in pro-estrus and oestrus and more time in met and diestrus).
99. The CEP Panel judged this endpoint as ALAN.
Implantation rate
100. The implantation incidence was decreased in a dose-dependent manner in a Tier 1 mouse study (Li et al., 2016), in which mice were fed split doses of BPA from PND22 for 5 weeks (0, 60 and 600 μg/kg bw per day).
101. The CEP Panel judged this endpoint as Likely.
102. During the growth phase/young age, the CEP Panel assigned a likelihood level of Likely to the cluster of female reproductive toxicity based on one Tier 1 mouse study (Li et al., 2016) in which the implantation incidence was decreased in a dose-dependent manner. This study was taken forward for BMD analysis and the Likely and ALAN endpoints were considered for uncertainty analysis.
Adult exposure (after puberty)
Plasma/serum thyroid hormones
103. For this exposure period one Tier 1 sheep study (Guignard et al., 2017) and one Tier 2 rat study (Zhang J et al., 2017) were identified. The female sheep in the Tier 1 study were injected s.c. with 5, 50 or 5000 µg/kg bw per day (equivalent to oral doses of 185/625; 1850/6250; 185000/625000 µg/kg bw per day depending on the conversion factor of 37 or 125 used). In this study, no effect was observed on total thyroxine (T4). A decrease was observed in total T3 at 50 µg/kg bw per day and in free T4 at 50 or 5000 µg/kg bw per day. An increase in reverse T3/T4 was observed at a s.c. dose of at 50 µg/kg bw per day (equivalent to oral dose of 1850/6250 µg/kg bw per day).
104. In the Tier 2 rat study (Zhang J et al., 2017) the female rats were dosed with 250 and 1000 µg/kg bw per day from 6 weeks of age for 64 weeks. In this study, a statistically significant increase was seen on free T4 at 1000 µg/kg bw per day and no changes in free thyroid triiodothyronine (T3).
105. The effects on T3 were judged as Not Likely as, at approximately the same dose, no effects were measured in rats (Zhang J et al., 2017), but an effect without dose relationship was seen in sheep; This was considered to be a variation.
106. The effects on T4 were judged as Not Likely as no dose–response was seen in sheep (Guignard et al., 2017) for FT4 and an effect in opposite direction (increase) was observed in rats (Zhang J et al., 2017) at a similar dose was observed.
107. In the sheep study (Guignard et al., 2017), the increase on reverse T3/T4 was only seen at 50 µg/kg bw per day, the mid dose. Therefore, this effect was judged as Not Likely.
108. During this exposure period the likelihood of changes in thyroid hormones (T3, T4, FT4 rT3/TT4) was considered as Not Likely by the CEP Panel as no consistent effects were observed in a Tier 2 study in rats and in a Tier 1 study in sheep.
Plasma/serum testosterone:
109. For this exposure period one Tier 3 rat study (Rashid et al., 2018) and two Tier 3 mouse studies (Hu et al., 2018; Xu XH et al., 2015) were identified. Therefore, the data were deemed Inadequate to judge the likelihood of an effect of BPA on testosterone levels. (Note; Hu et al., 2018 is listed as being Tier 2 in Annex F to the opinion which lists the animal studies used and their tiers).
Fertilisation rate:
110. One Tier 1 mouse study (Moore-Ambriz et al., 2015) in which the fertilisation rate was determined was identified for this exposure period. In adult female mice exposed from the day of the first oestrus until the completion of three oestrous cycles orally (via pipette) at a dose of 50 μg/kg bw per day, a decreased fertilisation rate was observed. However, as only one single-dose Tier 1 mouse study was available data were Inadequate to judge the likelihood of an effect on fertilisation rate.
Implantation rate:
111. Only one single-dose Tier 1 study (Boudalia et al., 2014) in rats and two Tier 3 studies in mice (Yuan et al., 2018; Dobrzynska et al., 2018) are available for this endpoint, the panel concluded that there was Inadequate evidence to conclude on a likelihood of an effect.
Oestrus cyclicity:
112. For this exposure period one Tier 1 study (Moore-Ambriz et al., 2015 and one Tier 3 study (Cao et al., 2018) in mice and one Tier 2 rat study (Zaid et al., 2014) were identified.
113. No effect on oestrous cyclicity was observed in the Tier 1 study in adult female mice, exposed orally (via pipette) from the day of the first oestrus until the completion of three oestrous cycles at a dose of 50 μg/kg bw per day (Moore-Ambriz et al., 2015). In the Tier 2 rat study (Zaid et al., 2014) the number of females which were in persistent diestrous was increased after daily dosing of 10000 µg/kg bw per day (single-dose level) from PND28 for 6 weeks.
114. No effect was seen in the lower dose (50 μg/kg bw per day) Tier 1 mouse study, but an effect at higher dose level, 10000 µg/kg bw per day was observed in the Tier 2 rat study; both studies were single-dose studies. The effect on oestrous cyclicity was judged ALAN.
Ovary weight
115. One Tier 2 rat study (Zaid et al., 2014) was identified for this exposure period. No change in absolute ovary weight was observed after daily dosing of female rats from PND28 for 6 weeks with 10000 µg/kg bw per day. Therefore, data were Inadequate to judge the likelihood of an effect on ovary weight.
Ovary histology
116. For this endpoint, observations were follicle count, premature activation of primordial follicles, large antral-like and atretic cystic-like follicles): For this exposure period one Tier 1 study (Moore-Ambriz et al., 2015) and one Tier 2 study (Hu et al., 2018) in mice and one Tier 2 rat study (Zaid et al., 2014) were identified.
117. No effect on follicle count was observed in adult female mice (Tier 1 study Moore-Ambriz et al., 2015 exposed orally (via pipette) from the day of the first oestrus until the completion of three oestrous cycles at a dose of 50 μg/kg bw per day. In the Tier 2 mouse study (Hu et al., 2018) a dose-related decrease was observed in the number of primordial follicles and the premature activation of primordial follicles in all dose groups; in this study adult (6 week old) female CD-1 mice were dosed orally for 28 days with 1 µg, 10 µg, 100 µg, 1000 µg and 10000 µg/kg bw per day (oral -route not stated). In the Tier 2 rat study (Zaid et al., 2014) female rats were dosed orally from PND28 for 6 weeks with 10000 µg/kg bw per day (the only dose tested). In this study, the numbers of atretic follicles, atretic cystic-like and large antral-like follicles were increased in the BPA-treated group when compared with the controls.
118. The effects on ovary histology were judged as Likely. As the Tier 2 rat study (Zaid et al., 2014) is a single-dose study, only the Tier 2 mouse study (Hu et al., 2018 was taken forward for BMD analysis
Uterus histology
119. This endpoint included observations of gland nests density, gland nests.
120. For this exposure period a Tier 1 study in CD1 mice (Kendziorski and Belcher, 2015) and a Tier 3 study in C57Bl/6J mice (Kendziorski and Belcher, 2015 were identified. In the Tier 1 study, CD-1 mice were exposed for 12– 15 weeks via the diet to BPA doses equivalent to 4, 40, 400, 4000 and 40000 µg/kg bw per day. Gland nests density and the number of gland nests were increased in the high-dose group. The design of the Tier 3 study in C57Bl/6J mice was identical. In this study, no effect on the number of gland nests was observed but the gland nests density was increased in the 4, 4000 and 40000 µg/kg bw per day group. The reference related to strain differences and it is unclear why the study has a different tiering for each strain.
121. The likelihood of effects on uterus histology was considered as ALAN as gland nest number and density showed inconsistent effects; in the Tier 1 study in CD-1 mice an increase in gland nest number and density was observed only at the high dose, in the Tier 3 study with a low number of C57BI/6J mice, varying effects were seen.
122. The CEP Panel assigned a likelihood level of Likely to the female reproductive toxicity cluster in the exposure period adulthood. The likelihood for the endpoint ovary histology is Likely. In a Tier 2 mouse study (Hu et al., 2018), a dose-related decrease in the number of primordial follicles and the premature activation of primordial follicles was observed. This study was taken forward for BMD analysis. In addition, an increase in follicle abnormalities was reported in a single dose rat Tier 2 study (Zaid et al., 2014). As this study tested only one dose, it was not taken forward for BMD analysis. The Likely and ALAN endpoints were also considered for uncertainty analysis.
Indirect (germline) exposure
123. For this exposure period five studies were assessed: three Tier 3 mouse studies (Ziv-Gal et al., 2015; Berger et al., 2016; Mahalingam et al., 2017), in which the F2 and F3 generation were studied and two Tier 3 mouse studies (Dobrzynska et al., 2015; Mahalingam et al., 2017) in which the F2 generation were studied. In the study by Ziv- Gal et al. (2015) the age at first oestrus, in the study by Dobrzynska et al. (2015) the embryo implantation incidence and in the study by Berger et al. (2016) and Mahalingam et al. (2017) the follicle count (ovary histology) were reported.
124. The CEP Panel noted that since for indirect (germline) exposure only Tier 3 studies were available, the likelihood for this endpoint could not be determined due to Inadequate evidence.
Overall cluster selection for endpoints/studies for BMD for female reproductive toxicity
125. Overall, the CEP Panel assigned a likelihood level of Likely, to the female reproductive toxicity cluster in the exposure periods developmental (pre-natal and/or post-natal until weaning), developmental and adult (pre-natal and/or post-natal until adulthood) and growth phase/young age, and of Inadequate Evidence in the adult and indirect (germline) exposure periods.
126. The overall likelihood across all exposure periods, i.e. the highest likelihood given in the cluster female reproductive toxicity was Likely.
127. The CEP Panel considered that the evidence from the studies available showed a:
- Likely effect for ovary weight (NTP Clarity Report, 2018/Camacho et al., 2019, for uterus histology (NTP Clarity Report, 2018/Camacho et al., 2019 and Vigezzi et al., 2015)
- Likely effect for ovary histology (NTP Clarity Report, 2018/Camacho et al., 2019) during the developmental exposure period,
- Likely effect for ovary histology (NTP Clarity Report, 2018/Camacho et al., 2019) during developmental and adult exposure and
- Likely effect for ovary histology (Hu et al., 2018) during adult exposure
- Likely effect for decreased implantation incidence during the growth phase (Li et al., 2016).
Therefore, these endpoints were taken forward for BMD analysis.
Male reproductive toxicity - Animal Studies - (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guideMale reproductive toxicity
128. Within the cluster male reproductive toxicity, there were 15 studies in mice, of which six studies included exposure during the period development until weaning, two had exposure during development until adulthood, seven were exposed as adults and two had germline exposure; some studies tested multiple exposure periods). Of the 26 studies on rats, 14 included exposure during development until weaning, four had exposure during development until adulthood, five had exposure during the growth phase, five were exposed as adults. In addition, one study was on sheep, which had exposure during the development until weaning period and one study in monkeys which had exposure during adulthood.
129. The specific endpoints that were included for effects of BPA on the male reproductive toxicity cluster were plasma/serum thyroid hormones, testosterone, epididymis weight and histology, prostate histology, seminal vesicle weight, sperm count/morphology/motility/viability, testis weight and histology.
Developmental exposure (pre-natal and/or post-natal until weaning)
Plasma/serum thyroid hormones:
130. For this exposure period the following studies were identified.
- T3: one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019
- T4: two Tier 1 rat studies (NTP Clarity Report, 2018/Camacho et al., 2019; Bansal and Zoeller, 2019 and one Tier 1 sheep study (Guignard et al., 2017
- T3/TT4: one Tier 1 sheep study (Guignard et al., 2017).
131. No effect was seen on T3 in the Tier 1 rat study.
132. Apart from a decrease at the highest dose level in the Clarity study, no effects was seen on T4 in either Tier 1 rat study or in the sheep study (Guignard et al., 2017. In addition, in the latter study, no change was seen in the ratio T3/T4.
133. During this developmental exposure (pre-natal and/or post-natal until weaning) period changes in thyroid hormones (T3, T4, rT3/TT4) were judged as Not Likely by the CEP Panel.
Plasma/serum testosterone
134. for this endpoint four Tier 3 studies in rats (Quan et al., 2017; Wang C et al., 2014; Castro et al., 2018; Johnson et al., 2016 - the latter study is related to the Clarity study) and one Tier 3 mouse study (Shi et al., 2018 were identified. Therefore, data were considered Inadequate to judge the likelihood of an effect of BPA on testosterone levels.
Epididymis weight:
135. For this endpoint one Tier 1 (NTP Clarity Report, 2018/Camacho et al., 2019, one Tier 2 study (Spörndly-Nees et al., 2018, one Tier 3 rat study (Tarapore et al., 2017) and one Tier 2 mouse study (Meng Y et al., 2018) were identified.
136. No effect was seen on epididymis weight in any of the studies, and the likelihood for this endpoint was considered Not Likely.
Epididymis histology
137. This endpoint included non-neoplastic, inflammatory changes, inflammation). For this endpoint one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019 and one Tier 2 rat study (Spörndly-Nees et al., 2018 were identified.
138. No effect was seen on epididymis histology in the Tier 1 rat Clarity study (NTP Clarity Report, 2018/Camacho et al., 2019. An increase in inflammatory changes was seen at the top dose (40 µg/kg) bw per day in the other Tier 2 rat study (Spörndly-Nees et al., 2018): in this study animals were exposed via the drinking water to doses of 4 or 40 µg/kg per day from GD3.5–PND22 and necropsied at PND35 or 12 months.
139. As no effects were seen for the endpoint in one Tier 1 study (NTP Clarity Report, 2018/Camacho et al., 2019 and an effect only at the highest dose in a Tier 2 rat study, the likelihood assigned to this endpoint was Not Likely.
Prostate histology
140. For this endpoint four Tier 1 (NTP Clarity Report, 2018/Camacho et al., 2019; Bernardo et al., 2015; Prins et al., 2018; Brandt et al., 2014, one Tier 2 (Hass et al., 2016 and one Tier 3 (Prins et al., 2017) studies in rats were identified. In these studies, several histological effects were examined.
141. No effect was seen on prostate histology (non-neoplastic proliferative lesions, hyperplasia of the ventral prostate, epithelium hyperplasia and inflammatory changes, inflammation, dorsal/lateral prostate histology, suppurative inflammation) in the Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019.
142. At histological examination, an increase (same effect size) in the incidence of inflammatory changes, pre-neoplastic lesions (atypical hyperplasia), non-neoplastic proliferative lesions (reactive hyperplasia), in the prostate was seen at both doses tested in a Tier 1 rat study (Bernardo et al., 2015). In this study animals were given 25 or 250 μg/kg bw BPA per day from GD10–21 and were necropsied at PND180.
143. No effect was seen on prostate histology (non-neoplastic proliferative lesions, hyperplasia of the ventral prostate and inflammatory changes, inflammation, dorsal/lateral prostate histology, suppurative inflammation) in the Tier 1 rat study (Prins et al., 2018). This study is a component of the Clarity study. In a Tier 1 study by Brandt et al., (2014) rats were administered 25 and 250 μg/kg bw per day from GD10–21. F1 pups/adults were sacrificed on PND21/180. At histological examination on PND21 an increase in proliferation and hyperplasia/dysplasia of the prostate was observed in the lowest dose and of apoptosis in the highest dose. At both doses an increase (effect the same size) of multifocal inflammation in the ventral prostate on PND180 was observed.
144. In the Tier 2 rat study (Hass et al., 2016), no change in prostate histology (interstitial inflammation, proliferation or epithelial atypical hyperplasia) was observed when examined in F1 males at 3 or 8 months; F0 dams were treated at oral doses of 25, 250, 5000 or 50000 µg/kg bw per day GD7–PND22.
145. In the two Tier 1 rat studies (Bernardo et al., 2015; Brandt et al., 2014 from the same laboratory), inflammatory effects and reactive hyperplasia in the prostate were reported at doses of 25 and 250 μg/kg bw per day GD10–GD21. This effect was not confirmed in the two other Tier 1 rat studies or in the Tier 2 rat study (Hass et al., 2016). The likelihood for this endpoint was considered to be ALAN.
Seminal vesicle weight
146. For this endpoint one Tier 1 (NTP Clarity Report, 2018/Camacho et al., 2019 and one Tier 2 study (Spörndly-Nees et al., 2018 in rats and one Tier 3 mouse study (Patel et al., 2013) were identified.
147. No effect was seen in either of the Tier 1 or Tier 2 studies. Therefore, the likelihood assigned to this endpoint is Not Likely.
Sperm count
148. For this endpoint one Tier 1 (NTP Clarity Report, 2018/Camacho et al., 2019) and one Tier 3 study (Hass et al., 2016 in rats and two Tier 3 studies in mice (Rahman et al., 2017; Shi et al., 2018) were identified.
149. No effect was seen on epidydimal sperm count and count of testicular sperm heads in the Tier 1 rat studies. The likelihood assigned to this endpoint is Not Likely.
Sperm morphology
150. For this endpoint one Tier 1 study (NTP Clarity Report, 2018/Camacho et al., 2019 and one Tier 2 study (Spörndly-Nees et al., 2018 in rats and one Tier 3 mouse study (Kalb et al., 2016 were identified. No effects on sperm morphology were observed and the likelihood assigned to the endpoint was Not Likely.
Sperm motility
151. For this endpoint, one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019) and three Tier 3 studies in mice (Shi et al., 2018; Kalb et al., 2016; Rahman et al., 2017) were identified.
152. No effects were observed in the Tier 1 rat study so the likelihood assigned to this endpoint is Not Likely.
Sperm viability
153. For this endpoint one Tier 3 mouse study (Rahman et al., 2017) was identified. Therefore, the data were Inadequate to judge the likelihood of an effect of BPA on sperm viability.
Testis weight
154. For this endpoint two Tier 1 studies (NTP Clarity Report, 2018/Camacho et al., 2019; Cao et al., 2015), and one Tier 3 study (Tarapore et al., 2017 in rats and two Tier 2 studies (Meng Y et al., 2018; Shi et al., 2018) and one Tier 3 study (Patel et al., 2013) in mice were identified. No effect was reported on testis weight in the Tier 1 rat study Clarity study.
155. However, in a single-dose Tier 1 rat study (Cao et al., 2015), an increase in testis weight was observed on PND 120. In this study the F0 females were administered BPA via drinking water (2 mg BPA/L; equivalent to 100 μg/kg bw per day) and co-treated with soy in the diet from GD1–PND21. No change was observed in the BPA-treated group with a soy-free diet. As noted elsewhere, soy is thought to ameliorate the effects of BPA.
156. No effect was seen on testis weight in a Tier 2 mouse study (Meng Y et al., 2018). In this study animals were exposed via drinking water to doses equivalent to 18 or 180 µg/kg per day from GD6–PND21 and necropsied at PND50.
157. No effect was seen on testis weight in the other Tier 2 mouse study (Shi et al., 2018). In this study, animals were exposed via the drinking water to doses equivalent to 0.5, 20 or 50 µg/kg bw per day from GD11 to birth and necropsied at PND60.
158. As no effect was observed in either the Tier 1 rat study or the two Tier 2 mouse studies, the likelihood was considered to be Not Likely for this endpoint.
Testis histology
159. For this endpoint, one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019, two rat Tier 2 studies (Quan et al., 2017; Spörndly-Nees et al., 2018), two Tier 2 mouse studies (Shi et al., 2018; Xie et al., 2016) and one Tier 3 mouse study (Rahman et al., 2017) were identified.
160. At histological examination, an increased incidence of testis (and pancreas) polyarteritis was seen in the Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019) at a dose of 2500 μg/kg bw per day.
161. In the Tier 2 rat study (Quan et al., 2017) an increase was seen in seminiferous tubular changes in the testis, cell-specific and/or stage-specific: degeneration, germ cell, at all dose levels at PND50 in F1 Sprague Dawley rats; this was not dose related. In this study, the F0 animals were dosed with 1000; 10000 or 100000 µg/kg bw BPA by gavage from GD14–21.
162. At histological examination, following 12 months exposure, an increase was seen in inflammatory changes in the testis of the low-dose group (4 µg/kg per day from GD3.5–PND22) in a Tier 2 rat study (Spörndly-Nees et al., 2018). No such effects were seen at the other dose tested (40 µg/kg per day) or other histological effects in the testis (seminiferous tubular changes, non-specific seminiferous epithelial height and seminiferous tubule diameter) at either dose level.
163. At histological examination of the testis in a Tier 2 mouse study (Shi et al., 2018), a decrease in seminiferous tubular changes, cell and/or stage-specific, in stage VII seminiferous epithelial cells and an increase in stage VIII seminiferous epithelial cells was seen in the mid-dose group (20 μg/kg bw per day) at PND60. On PND12, an increase without dose–response was seen in testicular apoptosis in the mid and high-dose groups. In this study CD- 1 mice were dosed with 0.5, 20 or 50 μg/kg bw per day from GD11 to birth by micropipette.
164. In another Tier 2 mouse study (Xie et al., 2016) a dose-related increase was observed at histological examination of the testis (degeneration, germ cell). In this study male mice were s.c. injected with10, 100 or 5000 µg/kg bw per day BPA (equivalent to oral doses of 2222; 22220 or 1111000 µg/kg bw per day).
165. The Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019 reported an increased incidence of polyarteritis at 2500 µg/kg bw per day only (dose range from 2.5– 25000 µg/kg bw per day). The Tier 2 rat studies showed effects on inflammatory changes at only one dose (4 µg/kg bw per day) (Spörndly-Nees et al., 2018), or effects (apoptosis) without a dose response at doses of 1000–100000 µg/kg bw per day (Quan et al., 2017). In the Tier 2 mouse study (Shi et al., 2018) only the mid dose group (20 µg/kg bw per day) showed effects on the testis (decrease in stage VII and decrease in stage VIII seminiferous epithelial cells) and apoptosis in the mid and high dose, 20 or 50 µg/kg bw per day, with no dose–response. In another Tier 2 mouse study (Xie et al., 2016) with s.c. administration dose-related testicular findings (germ cell (apoptosis)) were observed at doses equivalent to oral doses of 2220; 22220 or 1111000 µg/kg bw per day.
166. The likelihood of the histological changes in the testis were considered to be ALAN: During developmental exposure (pre-natal and/or post-natal until weaning), the CEP Panel assigned a likelihood level of ALAN to the cluster male reproductive toxicity of BPA. Hence, none of these endpoints were taken forward for BMD analysis. However, the Likely and ALAN endpoints were considered in the uncertainty analysis.
Developmental and adult exposure (pre-natal and post-natal in pups until adulthood)
Plasma/serum thyroid hormones
167. For this exposure period only one Tier 1 rat study was identified (NTP Clarity Report, 2018/Camacho et al., 2019. In this study T3 and T4 were measured. No effect was seen on T3 but according to the authors, T4 levels in the serum showed a significant trend, however the panel noted that the nature of the trend was not evident from their inspection of the data. The likelihood of an effect on T4 was therefore considered as Not Likely.
168. During this exposure period the likelihood of changes in thyroid hormones (T3, T4) were considered as Not Likely by the CEP Panel.
Testosterone
169. For this endpoint one Tier 3 rat study (Gonzalez-Cadavid, 2018 – part of the Clarity study) was identified. Therefore, data were Inadequate to judge the likelihood of an effect of BPA on serum testosterone.
Epididymis weight
170. For this exposure period, two Tier 1 rat studies (Dere et al., 2018 NTP Clarity Report, 2018/Camacho et al., 2019) were identified.
171. No change in epididymis weight was seen in the Tier 1 rat study (NTP Clarity Report, 2018/Camacho 9251 et al., 2019). In the other Tier 1 rat study (Dere et al., 2018; this study is part of the Clarity consortium), rats were dosed with 2.5, 25, 250, 2500 or 25000 µg/kg bw per day; F0 dams from GD6 to PND0 and F1 pups from PND1 to PND90. In this study an extra satellite control and 250,000 μg/kg bw per day dose group was added. A decrease in epididymis weight was only seen in the 250000 μg/kg bw per day group when compared with the extra (satellite) control group. This satellite control group showed a higher epididymis weight than the other control group.
172. The likelihood of an effect on epididymis weight was considered as Not Likely.
Epididymis histology
173. For this exposure period the following Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019 was identified.
174. In this study (NTP Clarity Report, 2018/Camacho et al., 2019) an increased change in exfoliated germ cells and inflammation was seen at histological examination of the epididymis in the high-dose group (25,000 μg/kg bw per day) at interim sacrifice (1 year); these effects were not observed at terminal sacrifice (2 years).
175. The likelihood of the changes in epididymis histology (exfoliated germ cells and inflammation) was considered to be Likely, although as effects were only seen in the highest dose group at the interim and not at the terminal sacrifice, the effect was apparently transient.
Prostate histology
176. For this exposure period the following Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019) was identified; this study had two different times of sacrifice. In addition, another set of animals of the same study was examined (Prins et al., 2018).
177. In the Clarity study no change in hyperplasia of the epithelium (non-neoplastic, proliferative lesions) was observed at interim sacrifice and at terminal sacrifice an increase was only observed at the 250 μg/kg bw per day dose. At interim sacrifice, an increase without dose–response in inflammatory changes of the prostate was observed at 2.5, 250, 2500 or 25000 μg/kg bw per day; no change was seen at 25 μg/kg bw per day. At terminal sacrifice an increase in inflammatory changes of the prostate was only seen in the lowest dose group. There was no change in inflammation of the prostate in the Tier 1 rat study by Prins et al., (2018).
178. The likelihood of the changes in prostate histology were considered to be Not Likely as effects were not seen in different sets of animals and were only examined at interim sacrifice.
179. Seminal vesicle weight: For this exposure period one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019) and one Tier 3 mouse study (Patel et al., 2013) were identified. As no effects on seminal vesicle weight were observed in the Tier 1 study, the likelihood of an effect on seminal vesicle weight was considered as Not Likely.
Sperm count
180. For this endpoint one Tier 1 (NTP Clarity Report, 2018/Camacho et al., 2019 was identified. No effect was seen on epidydimal sperm count and count of testicular sperm heads. The likelihood of an effect on sperm count was considered as Not Likely.
Sperm morphology
181. For this endpoint one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019) and one Tier 3 mouse study (Dobrzynska et al., 2018) were identified. As no effect on sperm morphology was observed, the likelihood of an effect on sperm morphology was considered as Not Likely.
Sperm motility
182. For this endpoint, one Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019) and one Tier 3 mouse study (Dobrzynska et al., 2018) were identified. As no effect was seen on sperm motility in the Tier 1 rat study, the likelihood of an effect on sperm motility was considered as Not Likely.
Testis weight
183. For this exposure period two Tier 1 studies (NTP Clarity Report, 2018/Camacho et al., 2019; Dere et al., 2018) and one Tier 3 mouse study (Patel et al., 2013) were identified. No change in testis weight was seen in the Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019).
184. In the other Tier 1 rat study (Dere et al., 2018), The doses were the same as those used in the main clarity study, but extra satellite groups, 0 and 250000 μg/kg bw per day were added. A decrease in testis weight was only seen in the 250000 μg/kg bw per day group when compared with the extra control group. However, this extra control group showed a higher testis weight than the other control group.
185. The likelihood of an effect on testis weight was considered as Not Likely.
Testis histology
186. For this exposure period two Tier 1 studies (NTP Clarity Report, 2018/Camacho et al., 2019; Dere et al., 2018) were identified. At histological examination in the testis. No changes were observed in either study after histological examination.
187. The likelihood of an effect on testis histology was considered as Not Likely:
188. During developmental exposure (pre-natal and/or post-natal in pups until adulthood), the CEP Panel assigned a likelihood level of Likely to the cluster male reproductive toxicity of BPA.
189. Since the likelihood level is Likely for the endpoint epididymis histology (exfoliated germ cells and inflammation) in the Tier 1 study (NTP Clarity Report, 2018/Camacho et al., 2019, this study was taken forward for BMD analysis and uncertainty analysis.
Growth phase/young age
Testosterone
190. For this endpoint three Tier 3 rat studies (Ullah et al., 2018a; Ullah et al., 2018b; Gurmeet et al., 2014) were identified (note - the Gurmeet study has different tier ratings depending on the endpoint) As only Tier 3 studies were identified, the data were considered Inadequate to judge the likelihood of an effect on serum testosterone.
Epididymis weight:
191. For this endpoint, two Tier 1 rat studies (Ogo et al., 2018; Gurmeet et al., 2014) and one Tier 3 rat study (Ullah et al., 2018b) were identified. No effect on epididymis weight was seen when rats were dosed with 20 or 200 μg/kg bw BPA per day from PND36–66 (Ogo et al., 2018). In the Tier 1 rat study (Gurmeet et al., 2014) no effects were observed on epididymis weight when rats were dosed with 1000, 5000 or 100000 µg/kg bw per day from PND28–70.
192. The likelihood was considered as Not Likely as no effect was observed in either of two Tier 1 rat studies.
Seminal vesicle weight
193. For this endpoint one Tier 1 rat study (Gurmeet et al., 2014) and one Tier 3 rat study (Ullah et al., 2018b) were identified. No effect on seminal vesicle weight was observed when rats were administered BPA via the drinking water with doses equivalent to oral doses of 1000, 5000 and 100000 μg/kg bw per day from PND28– 70 weeks (Gurmeet et al., 2014).
194. The likelihood was considered as Not Likely as no effect was seen on seminal vesicle weight in one Tier 1 rat study.
Sperm count (testis/epididymis)
195. For this endpoint one Tier 1 rat study (Ogo et al., 2018) and one Tier 3 rat study (testicular count, Ullah et al., 2018b) were identified. No effect on epididymal sperm count was observed in the Tier 1 study when rats were dosed with 20 or 200 μg/kg bw per day from PND36–66 (Ogo et al., 2018).
196. The likelihood was considered as Not Likely as no effect was seen on epididymal sperm count in one Tier 1 rat study.
Sperm motility
197. For this endpoint, only one Tier 3 rat study (Ullah et al., 2018b) was identified. The data were therefore Inadequate to judge the likelihood of an effect of BPA on sperm motility.
Testis weight
198. For this endpoint two Tier 1 rat studies (Ullah et al., 2018a; Gurmeet et al., 2014), one Tier 2 rat study (Brouard et al., 2016) and one Tier 3 rat study (Ullah et al., 2018b) were identified.
199. In the Tier 1 rat study (Ullah et al., 2018a) no effect on testis weight was observed. In this study, rats were dosed for 4 weeks with 5000, 25000 or 50000 µg/kg bw per day from PND70–80. In another Tier 1 rat study (Gurmeet et al., 2014, no effects were observed on testis weight when rats were dosed with 1000, 5000 or 100000 µg/kg bw per day from PND28–70.
200. In a Tier 2 study (Brouard et al., 2016) rats were exposed from PND15–PND30 by s.c. injection with a single dose of 50 μg/kg bw per day (equivalent to an oral dose of 1800 µg/kg bw per day) and testis weight was increased.
201. The likelihood was considered to be Not Likely as no effects on testis weight were seen in two Tier 1 oral (gavage) studies and an effect was only observed in the Tier 2 single-dose rat study via the s.c. route.
Testis histology
202. For this endpoint two Tier 2 rat studies (Gurmeet et al., 2014; Brouard et al., 2016) and two Tier 3 rat studies (Ullah et al., 2018a; Ullah et al., 2018b) were identified.
203. In the Tier 2 rat study by Gurmeet et al., 2014), a decrease of the seminiferous tubule diameter was observed in the high-dose group. In this study, the rats were dosed with 1000, 5000 or 100000 µg/kg bw per day from PND28–PND70.
204. In another Tier 2 study (Brouard et al., 2016), rats were exposed from PND15–PND30 by s.c. injection with a single dose of 50 μg/kg bw per day (equivalent to an oral dose of 1800 µg/kg bw per day), and incidences of seminiferous tubules with lumen, with acrosomal vesicles and acrosome reaction were increased in the BPA-treated group.
205. The likelihood of the effect was considered as Likely as in a Tier 2 rat study (Gurmeet et al., 2014) a decrease in seminiferous tubule diameter at dose level of 100000 µg/kg bw per day was observed. These effects were supported by testicular effects in another Tier 2 rat study (Brouard et al., 2016) at a s.c. dose equivalent to an oral dose of 1800 µg/kg bw per day. In this study, the incidence of seminiferous tubules with lumen, acrosomal vesicles and acrosome reaction was increased. This single-dose study was not taken forward for BMD analysis.
206. During the exposure during the growth phase, the CEP Panel assigned a likelihood level of Likely to the cluster male reproductive toxicity of BPA. Since the likelihood level is Likely for the endpoint testis histology (decrease in seminiferous tubule diameter) in the Tier 2 rat study (Gurmeet et al., 2014); this study was taken forward for BMD analysis and uncertainty analysis.
Adult exposure (after puberty)
Plasma/serum testosterone
207. For this endpoint, four Tier 3 rat studies (Srivastava and Gupta, 2018; Wu et al., 2016; Huang DY et al., 2018; Rashid et al., 2018), three Tier 3 mouse studies (Xu XH et al., 2015; Chouhan et al., 2015; Gao et al., 2018) and one Tier 3 monkey study (Vijaykumar et al., 2017) were identified.
208. As only Tier 3 studies were identified, data were considered Inadequate to judge the likelihood of an effect of BPA on testosterone.
Epididymis weight
209. For this exposure period only one Tier 3 mouse study (Dobrzynska et al., 2014 was identified, data were therefore considered Inadequate to judge the likelihood of an effect on epididymis weight.
Prostate histology
210. For this exposure period one Tier 2 (Olukole et al., 2018) and two Tier 3 studies (Huang DY et al., 2018; Wu et al., 2016) in rats were identified.
211. In the Tier 2 study (Olukole et al., 2018), rats were dosed with 10000 µg/kg bw per day for 14 days. The following observations were increased in the prostate histology of the BPA-treated group: degenerative changes, atrophy (atrophic tubules); non-neoplastic proliferative lesions: functional hyperplasia, proliferative lesions, reduced glandular diameter, hyperplasia reactive (accompanied by inflammation); inflammatory changes: inflammation and vascular digestion; atypical hyperplasia.
212. The likelihood of the effects on prostate histology was judged as Inadequate evidence as the effects on prostate histology were observed in a Tier 2 rat study using only a single dose level (Olukole et al., 2018).
Seminal vesicle weight
213. For this exposure period one Tier 3 mouse study (Chouhan et al., 2015) was identified. The data were therefore considered Inadequate to judge the likelihood of an effect on seminal vesicle weight.
Sperm count
214. For this exposure period one Tier 1 study (Wang HF et al., 2016), two Tier 2 studies (Park et al., 2018; Yin et al., 2017) and three Tier 3 studies (Dobrzynska et al., 2014; Gao et al., 2018; Chouhan et al., 2015) in mice and one Tier 3 rat study (Srivastava and Gupta, 2018) were identified.
215. In the Tier 1 study (Wang HF et al., 2016) mice were dosed for 8 weeks with 10, 50 or 250 μg/kg BPA per day; no effect on sperm count was noted. Similarly, in the Tier 2 mouse study (Yin et al., 2017) no effect was observed on sperm count after 5 weeks dosing with 3000, 30000 or 300000 µg/kg bw per day. In another Tier 2 mouse study (Park et al., 2018) a decrease in sperm count was seen at the only dose tested (10000 µg/kg bw per day) for 12 weeks.
216. The likelihood of the effect on sperm count was judged Not Likely as no effects were seen in either the Tier 1 or Tier 2 study in mice. While a decrease in sperm count was seen in the study by Park et al., (2018a) this used only a single dose level of 10000 µg/kg bw per day.
Sperm motility
217. For this exposure period one Tier 1 study (Wang HF et al., 2016), one Tier 2 study (Park et al., 2018) and one Tier 3 study (Dobrzynska et al., 2014) in mice were identified.
218. In the Tier 1 study (Wang HF et al., 2016) mice were dosed for 8 weeks with 10, 50 or 250 μg/kg bw per day; a dose-related decrease in sperm motility was observed. In the Tier 2 mouse study (Park et al., 2018) a decrease in sperm motility was observed at the only dose tested (10000 µg/kg bw per day) for 12 weeks.
219. The likelihood of the decrease in sperm motility was judged as Likely based on the dose-related decrease in the Tier 1 mouse study (Wang HF et al., 2016) at doses 10, 50 or 250 μg/kg bw per day. This effect was supported by decrease in sperm motility seen in the single dose (10000 µg/kg bw per day) in the Tier 2 study (Park et al., 2018). This single-dose level study was not brought forward for BMD analysis.
Sperm morphology:
220. For this exposure period one Tier 2 study (Park et al., 2018) and one Tier 3 study (Dobrzynska et al., 2014) in mice were identified.
221. In the Tier 2 mouse study (Park et al., 2018) an increase in abnormal sperm was observed at the only dose tested (10000 µg/kg bw per day) for 12 weeks.
222. The likelihood of the increase of abnormal sperm was judged as Inadequate as the increase was observed at a single dose level (10000 µg/kg bw per day) in a Tier 2 mouse study (Park et al., 2018).
Sperm viability
223. For this exposure period one Tier 1 mouse (Wang HF et al., 2016) study was identified. In this, groups of 8 C57BL/6 mice/dose group aged 15-17 weeks were dosed for 8 weeks with 10, 50 or 250 μg/kg bw BPA per day; a significant dose-related decrease in sperm motility (as assessed by Computer Assisted Sperm Analysis) CASA was observed at the highest dose level and a non statistically significant decrease seen in the mid-dose group. Body weights and testicular weights were unaffected.
224. The likelihood of the decrease in sperm viability was judged as Likely based on this study.
Sperm acrosome reaction
225. For this exposure period one Tier 1 mouse study (Wang HF et al., 2016 was identified (see above for details. A dose-related decrease in acrosome reaction was seen at the two highest dose levels. This was assessed by chlortetracycline staining.
226. The likelihood of the decrease in acrosome reaction was considered to be Likely.
Testis weight
227. For this exposure period one Tier 1 study (Wang HF et al., 2016) and three Tier 3 studies (Dobrzynska et al., 2014, Gao et al., 2018, Chouhan et al., 2015) in mice were identified.
228. In the Tier 1 study; no effect on testis weight was noted.
229. The likelihood for an effect on testis weight was judged as Not Likely.
230. Overall, the CEP Panel assigned a likelihood of Likely to the cluster male reproductive toxicity during adult exposure. As the likelihood level for male reproductive toxicity is Likely for the endpoint sperm motility, viability and acrosome reaction in the Tier 1 mouse study (Wang HF et al., 2016) these data were taken forward for BMD analysis and uncertainty analysis.
Indirect (germline) exposure
231. For this exposure period two Tier 3 mouse studies (Dobrzynska et al., 2015; Dobrzynska et al., 2018), in which epididymis weight, sperm count and sperm motility were measured, were identified. In addition, in the Tier 3 mouse study (Dobrzynska et al., 2015) sperm morphology was examined and in the other Tier 3 study (Dobrzynska et al., 2018) testis weight was measured.
232. However, the CEP Panel noted that as only Tier 3 studies were available, the evidence was considered Inadequate for this exposure period.
Overall cluster selection for endpoints/studies for BMD analysis for male reproductive toxicity
233. Overall, the CEP Panel assigned a likelihood level of:
- ALAN to the male reproductive toxicity cluster in the developmental exposure period.
- Likely in the developmental and adult, growth phase/young age and adult exposure periods.
- Inadequate evidence in the indirect (germline) exposure period.
234. The overall likelihood across all exposure periods, i.e. the highest likelihood given in the cluster male reproductive toxicity was Likely. The CEP Panel considered that the evidence from the studies available showed a Likely effect for epididymis histology (exfoliated germ cells and inflammation) in the Tier 1 rat study (NTP Clarity Report, 2018/Camacho et al., 2019) during the developmental exposure (pre-natal and/or post- natal until adult) period. In addition, with exposure during the growth phase a Likely effect was reported for testis histology (decrease in seminiferous tubule diameter) in a Tier 2 rat study (Gurmeet et al., 2014) and during adult exposure effects on sperm (motility; viability; acrosome reaction) were observed in a Tier 1 mouse study (Wang HF et al., 2016). Therefore, these endpoints were taken forward for BMD analysis.
Description of key studies - Animal Studies - (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guideDescription of key studies
Clarity study
235. The Clarity study was a 2 year study NTP based study in which NCTR Sprague-Dawley rats were given doses of BPA (0, 2.5, 25, 250, 2,500,and 25,000 μg/kg body weight (bw)/day) by gavage in a 0.3% carboxymethylcellulose vehicle (Camacho et al, 2019). The rats were dosed from gestation day (GD) 6 through to the start of parturition and then the pups were dosed directly from the day after birth until either postnatal day 21 (the stop-dose arm) or continuously until termination at one (interim sacrifice) or two years. The stop-dose arm was included to assess the potential for any BPA effects that were due to developmental exposure. BPA was not detected in bedding extracts, cage leachates or drinking water.
Vigezzi et al., 2015
236. In the rat study by Vigezzi et al (2015) the dams were given 0.5 or 50 µg/kg bw BPA per day in drinking water from GD9–PND21; a Wistar derived strain was used. The F1 females were necropsied on PND90 and 360. The aim of the study was to assess the effect of long term BPA exposure on the uterus and the uterine response to oestrogen replacement therapy (this phase of the experiment started 1 year after BPA treatment). The group size was between 10-16. No changes in squamous metaplasia and luminal epithelial anomalies were observed. At necropsy on PND360 an increase in glands with cellular anomalies was observed in the 50 µg/kg bw per day; on PND90 but no effects were seen in the 50 µg/kg bw per day group or at both times of necropsy in the lower dose group.
Santamaria et al., 2016
237. In the study by Santamaria et al., 2016, 10-12 F0 dams/group of a Wistar derived strain and their female offspring were given 0.5 μg and 50 μg/kg bw per day (via drinking water) from GD9–PND21. The numbers are not given in the paper but from the figure, mean ovary weight appears to be around 50 mg in the controls and 40 mg in the treated groups, with no clear trend but a greater spread of data in the higher dose.
Hu et al., 2018
238. The aim of the study by Hu et al., (2018) was to investigate primary ovarian insufficiency, this the premature exhaustion of primordial follicles in the follicle pool, which is caused by the excessive premature activation of primordial follicles after birth. The authors state that BPA exposure promotes the transition of primordial follicles to primary follicles, thus the number of primordial follicles in the primordial follicle pool decreases significantly. However, the molecular mechanisms underlying abnormal follicle activation are poorly understood. The study aimed to investigate the role of the Phosphatase and tensin homologue (PTEN) signal system which is a negative regulator of follicle activation. This study was judged to be Tier 2.
239. Groups of 13 6 week old female mice were treated with oral doses of 0, 1, 10, 100, 1000 or 10,000 µg BPA/kg bw/day in corn oil with 0.1% DMSO for 28 days. The mice were killed in the oestrous cycle as determined by vaginal lavage and the ovaries collected. Ovaries from one side were used for serial section and hematoxylin and eosin (H&E) staining to evaluate the follicles, while ovaries from the other side were used for fluorescence immunocytochemistry, reverse transcription-polymerase chain reaction (RT-PCR), and Western blotting to observe the expression of PTEN. There were no significant differences in body weight between the two groups.
240. The hu of primordial follicles to primary follicles and the ratios of primordial follicles to total follicles in animals administered were significantly lower than in the control group, with a significant dose–response relationship being apparent. This is shown in the figure below, taken from the original paper. It was suggested that this indicated BPA promoted the premature activation of primordial follicles. Numerical results are not provided.
241. The results are expressed as mean + Standard Error of the Mean (SEM). Different lower-case letters above the columns, such as a, b, c, d, and e, indicate P < 0.05, and if 2 columns have the same lower case letter, it indicates no statistical significance. It is not stated in the paper but this suggests that the lowest dose is not significantly different from the controls when analysed by ANOVA.
Moore-Ambriz et al., 2015
242. The aim of the study by Moore-Ambriz et al., 2015, was to investigate how BPA impaired ovulation, therefore the study evaluated whether BPA altered ovulation by affecting folliculogenesis, the number of corpora lutea or eggs shed to the oviduct, ovarian gonadotropin responsiveness, hormone levels, and oestrous cyclicity. Young adult (39 days old) female C57BL/6J mice were treated with corn oil (vehicle) or 50 μg/kg bw/day BPA (via pipette) for a period encompassing the first three reproductive cycles (12–15 days). The dose of 50 µg was selected as it was the US EPA’s safe exposure limit. Diethylstilbestrol (DES) was given as a positive control. The result on follicles are presented in Table 1, taken from the paper.
243. It was concluded that BPA exposure did not alter any parameters related to ovulation. This study was considered to be Tier 1.
Integration of likelihoods from human and animal studies - (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guide244. Table 13 of the EFSA opinion (and reproduced below) presents the overall likelihood per cluster for the human and animal stream separately, as well as the integration of the likelihoods from the human and animal studies for reproductive toxicity. The integration is described by the panel as below:
245. AGD, bone development and breast development are endpoints assessed in two different clusters: in pubertal/endocrine in the human stream and in the developmental toxicity cluster in the animal stream. The CEP panel concluded that overall, the animal and human clusters were not comparable. It was considered of relevance to analyse the endpoints on which the final conclusion for the likelihood is based, both for animal and human clusters. In case of any similar outcomes the evidence of the respective likelihood would increase.
246. In the human stream, the fetal and post-natal growth cluster contains results for body weight and femoral length which can be compared to body weight effects and effects on bone development in the developmental cluster for animals. While human studies show Not Likely effects, animal studies result in ALAN effects on body weight and bone development.
247. The further likelihood of effects observed in developmental animal studies is based on ALAN effects on mammary gland development and age of first estrus. Neither of these effects have been described as changed nor as references of a similar likelihood in human studies. The pubertal/endocrine cluster of the human stream bases its ALAN evaluation on changes to the AGD. The assessment of the AGD in animals revealed, however, no effects. Sex hormones are included in the human pubertal/endocrine and in the animal Male and Female reproductive toxicity clusters, both without any influence on the overall conclusion on the respective likelihoods. Thus, finally there is no substantiation of the ALAN likelihood for the animal cluster developmental toxicity nor of the ALAN likelihood for the human cluster pubertal/endocrine.
248. In the animal Male and Female reproductive toxicity clusters, there were Likely effects on sperm, follicles and implantation, but not on fertility. There was no clear overlap between the endpoints assessed in human and animal studies that focused on reproductive toxicity in females. As for effects on mammary gland development, ovary and uterus weight and histology, there are no studies available in human to be compared and integrated with the animal evidence.
Integration of likelihoods from the human and animal studies- reproduced from Table 13 of the EFSA draft opinion.
|
Human Stream |
Animal Stream |
Integrated likelihood |
|
Cluster: Developmental toxicity |
Cluster: Developmental toxicity |
Integrated likelihood |
|
Not applicable |
Developmental exposure (pre-natal and/or post-natal until weaning) – ALAN.
Developmental and adult exposure (pre-natal and/or post-natal in pups until adulthood) – ALAN.
Growth phase/young age exposure – ALAN.
Adult exposure (after puberty) - Not Likely.
Indirect (germline) exposure - Not Likely. Overall likelihood ALAN. |
ALAN |
|
Cluster: Fetal and Post-natal Growth |
Cluster: Fetal and Post-natal Growth |
Integrated likelihood |
|
Exposure during pregnancy |
Not applicable. |
Overall Likelihood: Not Likely |
|
Cluster: Pubertal Endocrine |
Cluster: Pubertal Endocrine |
Integrated likelihood |
|
Exposure during pregnancy – ALAN. |
Not applicable. |
Overall Likelihood: ALAN – Likely. |
|
Exposure during adulthood – Not likelihood. |
Not Applicable. |
Overall Likelihood: ALAN – Likely. |
|
Cluster: Female fertility |
Cluster: Female reproductive toxicity |
Integrated likelihood |
|
Exposure during adulthood – ALAN. |
Developmental exposure (pre-natal and/or post-natal until weaning) – Likely. Developmental and adult exposure (pre-natal and/or post-natal in pups until adulthood) – Likely. Growth phase/young age exposure – Likely. Adult exposure (after puberty) - Likely. Indirect (germline) exposure - Inadequate evidence. |
Overall likelihood – ALAN – Likely. |
|
Cluster: Male fertility |
Cluster: Male reproductive toxicity |
Integrated likelihood |
|
Exposure during adulthood – Not Likely. Overall likelihood – Not Likely. |
Developmental exposure (pre-natal and/or post-natal until weaning) – ALAN. Developmental and adult exposure (pre-natal and/or post-natal in pups until adulthood) - Likely. Growth phase/young age exposure – Likely. Adult exposure (after puberty) - Likely. Indirect (germline) exposure - Inadequate evidence. Overall likelihood - Likely |
Overall likelihood –Likely. |
|
Cluster: Prematurity |
Cluster: Prematurity |
Integrated likelihood |
|
Exposure during pregnancy Not Likely |
Not Applicable. |
Not Applicable. |
|
Overall likelihood – Not Likely |
Not Applicable. |
Not Likely. |
|
Cluster: Pre-eclampsia |
Cluster: Pre-eclampsia |
Integrated likelihood |
|
Exposure during adulthood. |
Not applicable. |
Not Applicable. |
|
Overall likelihood - ALAN |
Not applicable. |
ALAN |
249. The Mode of Action studies for clusters and endpoints judged to be ALAN or Likely are discussed in section 3.1.6.4 of the opinion. These have not been considered in detail here. However, mechanisms including epigenetic changes, enhanced sensitivity to carcinogenic changes, altered proliferation and pathological processes, changes to gene expression and oxidative stress, effects on hormonal signalling pathways are considered in this cluster.
250. For the changes in follicles, the mechanisms discussed are considered below as the finding of Hu et al., (2018) were taken forward for BMD modelling.
251. In animals, Thilagavathi et al. (2017) reported that the number of antral follicles in rats was decreased after treatment with BPA at 10 mg/kg bw per day for 12 weeks, but increased at doses of 50 and 100 mg/kg bw per day. They proposed that BPA-induced oxidative stress leads to antioxidant depletion in the ovary, which leads to elevated level of Thiobarbituric acid reactive substances (TBARS – these are byproducts of lipid oxidation and indicate oxidative damage), which leads to overexpression of endothelial nitric oxide (eNOS), which prevented steroidogenic acute regulatory protein (StAR) transport from the outer to the inner mitochondria membrane, which inhibited CYP11A1, leading to the downregulation of aromatase.
252. Berger et al. (2016) reported that in mice dosed during gestation with 0.5–50 µg/kg bw BPA per day, germ cell nest breakdown was directly inhibited, probably by altering gene expression for apoptosis, oxidative stress and autophagy. Preantral follicle numbers were decreased, potentially by causing death of these follicles at time points earlier than 3 months. They concluded that in utero BPA exposure has different effects on the gene expression of steroidogenic enzymes and these effects depend on the dose of BPA, age and the generation of the mice.
253. Santamaria et al. (2016) reported reduced primordial to primary follicle transition and altered steroidogenesis in adult rats dosed during gestation and lactation with 0.5 or 50 mg/kg bw BPA per day. The androgen receptor (AR) was increased in primordial follicles at 5 mg/kg bw per day and decreased in primary follicles at 50 mg/kg bw per day. They concluded that BPA could affect ovulation through different mechanisms depending on dose but did not speculate on what was causing the altered steroidogenesis.
254. Santamaria et al. (2017) reported reduced ovulatory response to exogenous gonadotrophins in pre-pubertal female rats dosed during gestation and lactation at 50 mg/kg bw per day. They suggested that different mechanisms might be leading the follicles to atresia and that loss of AR in the later stages of follicular development might be part of a paracrine mechanism that affords protection against premature luteinisation and atresia.
255. Cao et al. (2018) reported that BPA decreased the ovarian reserve in adult mice dosed with 5–500 mg/kg bw per day for 28 days. They hypothesised that BPA may reduce ovarian granulosa cell activity and accelerate its apoptosis, leading to the decreased synthesis of AMH. They did not speculate on upstream mechanisms.
256. Soleimani Mehranjani and Mansoori (2016) reported that adult rats dosed with BPA at 60 mg/kg bw per day for 20 days had reduced antral follicles and increased atretic follicles. Concomitant vitamin C ameliorated these adverse effects. They did not propose a MoA, but antagonism of BPA- induced oxidative stress is a plausible possibility.
257. Ganesan and Keating (2016) reported that in vitro incubation of perinatal rat ovary with very high dose of BPA (440 mM) reduced primary and secondary follicle numbers after 2 days, followed by a reduction in primordial follicle numbers after four days and induced ovarian DNA damage. They concluded that BPA, via biotransformation, may be converted to a DNA alkylating agent. It is noted that the concentration tested was likely many orders of magnitude higher than achievable in vivo levels.
258. Overall, at lower doses, oxidative stress leading to steroid alterations is a plausible mechanism for reduced follicle development. DNA damage is only reported at a much higher dose/concentration.
Cluster overview for Reproductive and developmental toxicity - (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guide259. The CEP panel noted that the effects BPA are reported over a huge range of effective concentrations/doses (low nM and mg/kg bw per day to high µM and >1,000 µg/kg). This range needed consideration since there could be qualitative as well as quantitative differences in mechanisms, depending on dose.
260. It was considered clear that many of the reported effects of BPA on male reproductive endpoints are measuring downstream intermediate pleiotropic effects (e.g. altered expression of many genes and proteins, DNA methylation and histone changes, activation of MAPK, PI3K, PKA, Akt/mTOR pathways, INSL3, StAR, etc.). It was stated that details of upstream mechanisms, which are more likely to be BPA specific, are unclear in many cases. For example, mechanisms for BPA-induced sperm mitochondrial membrane potential changes could include altered ion channels, receptors (and receptor cross-talk) and ROS.
261. Receptor interactions are an obvious candidate mechanism for early BPA effects (AR, ERα, ERβ, GPER, ERRg, PPARg, etc.). Some studies have reported receptor-dependency of BPA effects, but the results are difficult to generalise, because steroid receptor properties and interactions are dynamic; substance effects can vary by e.g. tissue, life-stage and dose.
262. A plausible upstream mechanism for BPA effects is oxidative stress generation, at both low and high doses.
263. In summary, it seems plausible that BPA-induced oxidative stress (with modulation of androgen and oestrogen pathways, and downstream inflammation) is an early key event in the adverse effects of BPA on adult male and female reproductive organs.
Conclusion on hazard identification for Reproductive and developmental toxicity of BPA
In this guide
In this guide264. The conclusions of the CEP panel are reproduced below:
- In the previous opinion on BPA assessment (EFSA CEF Panel, 2015), the CEF Panel concluded that the evidence is not sufficient to infer a causal link between BPA exposure and reproductive and developmental effects in humans but re-confirmed that BPA is a reproductive toxicant in experimental animal studies at high doses (above a Human Equivalent Dose (HED) of 3.6 mg/kg bw per day, corresponding to the No Observed Adverse Effect Level (NOAEL) HED for General toxicity). The CEF Panel assigned a likelihood level of ALAN to reproductive and developmental effects of BPA in animals at low doses (below HED 3.6 mg/kg bw per day).
- In adult animals exposed at doses lower than 3.6 mg/kg bw per day, reproductive effects were considered to be ALAN; the data suggested that low-dose BPA may have adverse effects on testis function, especially various measures of spermatogenesis, although these effects were modest, and in several multigeneration studies no effects were observed at dose levels from as low as 3 µg/kg bw per day up to at least 50 mg/kg bw per day. There was less evidence that BPA will significantly impair testis morphology or reproductive endocrinology, especially in the longer term.
- In animals exposed in utero at doses lower than 3.6 mg/kg bw per day, reported effects on reproductive function were contradictory and highly variable between studies. A likelihood level of Likely was assigned to BPA-induced proliferative changes in the mammary gland. The CEF Panel established a tolerable daily intake which was designated as temporary (t-TDI), pending the outcome of a long-term study in rats involving pre-natal as well as post-natal exposure to BPA then being undertaken by NTP/FDA.
- Based on the human data, none of the reproduction clusters was considered Likely. Female fertility and pre-eclampsia after adult exposure, pubertal development after exposure during pregnancy were considered ALAN. Male fertility after exposure during adulthood, prematurity, fetal and post-natal growth after exposure during pregnancy and pubertal development after exposure during childhood were considered Not Likely.
- In the animal studies, the likelihood of reproductive effects was assessed by WoE in three clusters (developmental toxicity, male reproductive toxicity, female reproductive toxicity), each subdivided according to exposure periods (developmental, developmental and adult, growth phase/young age, adult and indirect (germline) exposure).
- Based on the animal data, several endpoints in both female and male reproductive toxicity clusters were judged as Likely.
- In the female reproductive toxicity cluster, there were Likely effects on ovary weight and histology after developmental exposure, on implantation rate after growth phase/young age exposure and on follicle counts after adult exposure. Therefore, these endpoints were taken forward for BMD analysis.
- In the male reproductive toxicity cluster, there were Likely effects on epididymis (exfoliated germ cells and inflammation) after developmental and adult exposure, on testis histology (increased seminiferous tubules with lumen and acrosomal vesicles) after growth phase/young age exposure and effects on sperm motility, morphology, viability and acrosome reaction after adult exposure. Therefore, these endpoints were taken forward for BMD analysis.
- In all three clusters studied in animal studies (developmental toxicity, male reproductive toxicity, female reproductive toxicity), several endpoints were rated ALAN.
- In the Developmental Toxicity cluster, mammary gland and bone development (both sexes) and body weight (described in Metabolic hazard identification section) after developmental exposure, and also for age at first oestrus and body weight effects after exposure during growth phase, were judged as ALAN.
- In the Female reproductive toxicity cluster, effects on uterus histology (increase in apoptosis and squamous metaplasia) after developmental and adult exposure, and on oestrus cyclicity after adult exposure, were judged as ALAN.
- In the Male reproductive toxicity cluster, effects on prostate (inflammation, reactive hyperplasia and apoptosis) and testis (polyarteritis, inflammation, reduced stage VIII seminiferous epithelial cells and increased germ cell degeneration) after developmental exposure were judged as ALAN.
- After the integration of the human and animal evidence, the overall likelihood of BPA effects was considered Likely for the clusters Female reproductive toxicity and Male reproductive toxicity, and ALAN for Developmental toxicity.
- Based mainly on the animal data and in reasonable agreement with the human data, a female reproduction hazard is identified in terms of likely effects on ovary weight and histology after developmental exposure, on implantation rate after growth phase/young age exposure and on follicle counts after adult exposure.
- Male reproductive toxicity effects identified from the animal data as Likely were epididymis (exfoliated germ cells and inflammation) after developmental and adult exposure, testis histology (increased seminiferous tubules with lumen and acrosomal vesicles) after growth phase/young age exposure and sperm (motility, morphology, viability and acrosome reaction) after adult exposure. This is broadly in agreement with the previous EFSA conclusion (EFSA CEF Panel, 2015) that doses of BPA below 3.6 mg/kg bw per day may have modest adverse effects on testis function, especially on various measures of spermatogenesis.
- Mechanisms of action for the identified BPA reproductive toxicity endpoints have been non-systematically explored in the literature. They include oestrogen and AR interactions and associated downstream and cross-stream effects, including epigenetic changes. Other possible mechanisms, including notably BPA-induced generation of oxidative stress, have been less explored.
Discussion and conclusions - (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guide265. The CEP panel reviewed the available human and animal data on reproductive and developmental toxicity. Findings from the human studies were judged to be Not Likely or ALAN. A number of endpoints were taken forward for BMD analysis. Most notably, this included the changes on follicle ratios observed in the study by Hu et al., 2018 as it was noted that this would have provided the second most sensitive endpoint.
Questions for the Committee
266. Do Members have any comments on:
a) The human data
b) The animal data
c) The study by Hu et al., (2018) or changes in follicles more widely.
d) The WoE and integration.
e) The overall conclusions reached.
Secretariat
February 2022
Abbreviations - (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guide|
AGD |
Ano genital distance |
|
ALAN |
As Likely as Not |
|
ANOVA |
Analysis of Variance |
|
AR |
Androgen Receptor |
|
BMD |
Benchmark Dose |
|
BPA |
Bisphenol A |
|
CEP |
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids |
|
DES |
Diethylstilbestrol |
|
EFSA |
European Food Safety Authority |
|
GD |
Gestational Day |
|
HBGV |
Health Based Guide Value |
|
HOC |
Health Outcome Category |
|
HED |
Human Equivalent Dose |
|
HR |
Hazard Ratio |
|
Kg |
kilogram |
|
LD |
Lactation Day |
|
µg |
microgram |
|
mg |
miiligram |
|
MoA |
mode of action |
|
NOAEL |
No Observed Adverse Effect Level |
|
NMDR |
Non Monotonic Dose Response |
|
Ng |
nanogram |
|
NTP |
US National Toxicology Program |
|
OR |
Odds Ratio |
|
PND |
postnatal day |
|
ROS |
Reactive Oxygen Species |
|
RR |
Relative Risk |
|
TBARS |
Thiobarbiturc Acid Reactive Substances |
|
TDI |
Tolerable Daily Intake |
|
TDs |
Terminal Ducts |
|
TEB |
Terminal End Buds |
References - (BPA) in foodstuffs – Reproductive and Developmental Toxicity
In this guide
In this guideAcevedo N, Rubin BS, Schaeberle CM and Soto AM, 2018. Perinatal BPA exposure and reproductive axis function in CD-1 mice. Reproductive Toxicology, 79, 39–46. https://doi.org/10.1016/j.reprotox.2018.05.002.
Arbuckle TE, Agarwal A, MacPherson SH, Fraser WD, Sathyanarayana S, Ramsay T, Dodds L, Muckle G, Fisher M, Foster W, Walker M and Monnier P, 2018. Prenatal exposure to phthalates and phenols and infant endocrine-sensitive outcomes: The MIREC study. Environment International, 120, 572–583. https://doi.org/10.1016/j.envint.2018.08.034.
Auger J, Le Denmat D, Berges R, Doridot L, Salmon B, Canivenc-Lavier MC and Eustache F, 2013. Environmental levels of oestrogenic and antiandrogenic compounds feminize digit ratios in male rats and their unexposed male progeny. Proceedings of the Royal Society of London. Series B, 280(1768), 8.
Bae J, Kim S, Kannan K and Buck Louis GM, 2015. Couples’ urinary bisphenol A and phthalate metabolite concentrations and the secondary sex ratio. Environmental Research, 137, 450–457. https://doi.org/10.1016%2Fj.envres.2014.11.011.
Bansal R and Zoeller RT, 2019. CLARITY-BPA: Bisphenol A or propylthiouracil on thyroid function and effects in the developing male and female rat brain. Endocrinology, 160(8), 1771–1785. https://doi.org/10.1210/en.2019-00121.
Barrett ES, Sathyanarayana S, Mbowe O, Thurston SW, Redmon JB, Nguyen RHN and Swan SH, 2017. First-trimester urinary bisphenol A concentration in relation to anogenital distance, an androgen- sensitive measure of reproductive development, in infant girls. Environmental Health Perspectives, 125(7), 077008.
Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou CQ and Flaws JA, 2016. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reproductive Toxicology, 60, 39–52. https://doi.org/10.1016/j.reprotox.2015.12.004.
Berger K, Eskenazi B, Kogut K, Parra K, Lustig RH, Greenspan LC, Holland N, Calafat AM, Ye X and Harley KG, 2018. Association of Prenatal Urinary Concentrations of Phthalates and Bisphenol A and pubertal timing in boys and girls. Environmental Health Perspectives, 126(9), 97004. https://doi.org/10.1289/EHP3424.
Bernardo BD, Brandt JZ, Grassi TF, Silveira LTR, Scarano WR and Barbisan LF, 2015. Genistein reduces the noxious effects of in utero bisphenol A exposure on the rat prostate gland at weaning and in adulthood. Food and Chemical Toxicology, 84, 64–73. https://doi.org/10.1016/j.fct.2015.07.011.
Binder AM, Corvalan C, Pereira A, Calafat AM, Ye X, Shepherd J and Michels KB, 2018. Pre-pubertal and pubertal endocrine disrupting chemicals exposure and breast density among Chilean adolescents. Cancer Epidemiology, Biomarkers and Prevention, 1491–1499. https://doi.org/10.1158/1055-9965.epi-17-0813.
Birks L, Casas M, Garcia AM, Alexander J, Barros H, Bergström A, Bonde JP, Burdorf A, Costet N, Danileviciute A, Eggesbø M, Fernández MF, González-Galarzo MC, Regina Gražulevičienė, Hanke W, Jaddoe V, Kogevinas M, Kull I, Lertxundi A, Melaki V, Andersen AN, Olea N, Polanska K, Rusconi F, Santa-Marina L, Santos AC, Vrijkotte T, Zugna D, Nieuwenhuijsen M, Cordier S and Vrijheid M, 2016. Occupational exposure to endocrine-disrupting chemicals and BW and length of gestation: A European meta-analysis. Environmental Health Perspectives, 124(11), 1785–1793.
Bodin J, Bølling AK, Becher R, Kuper F, Løvik M and Nygaard UC, 2014. Transmaternal bisphenol A exposure accelerates diabetes Type 1 development in NOD mice. Toxicological Sciences, 137(2), 311–323. https://doi.org/10.1093/toxsci/kft242.
Boudalia S, Berges R, Chabanet C, Folia M, Decocq L, Pasquis B, Abdennebi-Najar L and Canivenc-Lavier MC, 2014. A multi-generational study on low-dose BPA exposure in Wistar rats: Effects on maternal behavior, flavor intake and development. Neurotoxicology and Teratology, 41, 16–26. https://doi.org/10.1016/j.ntt.2013.11.002.
Brandt JZ, Silveira LTR, Grassi TF, Anselmo-Franci JA, Fávaro WJ, Felisbino SL, Barbisan LF and Scarano WR, 2014. Indole-3-carbinol attenuates the deleterious gestational effects of bisphenol A exposure on the prostate gland of male F1 rats. Reproductive Toxicology, 43, 56–66. https://doi.org/10.1016/j.reprotox.2013.11.001.
Brouard V, Guénon I, Bouraima-Lelong H and Delalande C, 2016. Differential effects of bisphenol A and estradiol on rat spermatogenesis’ establishment. Reproductive Toxicology, 63, 49–61. https://doi.org/10.1016/j.reprotox.2016.05.003.
Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J and Kannan K, 2014. Urinary bisphenol A, phthalates, and couple fecundity: The Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertility and Sterility, 101(5), 1359–1366. https://doi.org/10.1016/j.fertnstert.2014.01.022.
Buck Louis GM, Smarr MM, Sun LP, Chen Z, Honda M, Wang W, Karthikraj R, Weck J and Kannan K, 2018. Endocrine disrupting chemicals in seminal plasma and couple fecundity. Environmental Research, 163, 64–70. https://doi.org/10.1016/j.envres.2018.01.028.
Burstyn I, Martin JW, Beesoon S, Bamforth F, Li QZ, Yasui Y and Cherry NM, 2013. Maternal exposure to bisphenol-A and fetal growth restriction: A case-referent study. International Journal of Environmental 12706 Research and Public Health, 10(12), 7001–7014. https://doi.org/10.3390/ijerph10127001.
Camacho L, Lewis SM, Vanlandingham MM, Olson GR, Davis KJ, Patton R, Twaddle NC, Doerge DR, Churchwell MI, Bryant MS, Mclellen FM, Woodling K, Felton RP, Maisha MP, Juliar BE, Gamboa da Costa G and Delclos KB, 2019. NTP CLARITY-BPA report (2018). A two-year toxicology study of bisphenol A (BPA) in Sprague-Dawley rats: CLARITY-BPA core study results. Food and Chemical Toxicology, 132. https://doi.org/10.1016/j.fct.2019.110728.
Cantonwine DE, Ferguson KK, Mukherjee B, McElrath TF and Meeker JD, 2015. Urinary bisphenol A levels during pregnancy and risk of preterm birth. Environmental Health Perspectives, 123(9), 895–901. https://doi.org/10.1289/ehp.1408126.
Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R and McElrath TF, 2016. Urinary concentrations of bisphenol A and phthalate metabolites measured during pregnancy and risk of preeclampsia. Environmental Health Perspectives, 124(10), 1651–1655. https://doi.org/10.1289/EHP188.
Cao YM, Qu XL, Ming Z, Yao YR and Zhang YZ, 2018. The correlation between exposure to BPA and the decrease of the ovarian reserve. International Journal of Clinical and Experimental Pathology, 11(7), 12753 3375–3382.
Casas M, Valvi D, Ballesteros-Gomez A, Gascon M, Fernández MF, Garcia-Esteban R, Iñiguez C, Martínez D, Murcia M, Monfort N, Luque N, Rubio S, Ventura R, Sunyer J and Vrijheid M, 2016. Exposure to bisphenol A and phthalates during pregnancy and ultrasound measures of fetal growth in the INMA- Sabadell cohort. Environmental Health Perspectives, 124(4), 521–528. https://doi.org/10.1016/j.envres.2015.07.024.
Castro B, Sánchez P, Torres JM and Ortega E, 2018. Effects of perinatal exposure to bisphenol A on the intraprostatic levels of aromatase and 5α-reductase isozymes in juvenile rats. Food and Chemical Toxicology, 115, 20–25. https://doi.org/10.1016/j.fct.2018.02.060.
Chavarro JE, Mínguez-Alarcón L, Chiu YH, Gaskins AJ, Souter I, Williams PL, Calafat AM, Hauser R and EARTH Study Team, 2016. Soy intake modifies the relation between urinary bisphenol A concentrations and pregnancy outcomes among women undergoing assisted reproduction. Journal of Clinical Endocrinology and Metabolism, 101(3), 1082–1090. https://doi.org/10.1210/jc.2015-3473.
Chin HB, Jukic AM, Wilcox AJ, Weinberg CR, Ferguson KK, Calafat AM, McConnaughey DR and Baird DD, 2019. Association of urinary concentrations of phthalate metabolites and bisphenol A with early pregnancy endpoints. Environmental Research, 168, 254–260. https://doi.org/10.1016/j.envres.2018.09.037.
Chouhan S, Yadav SK, Prakash J, Westfall S, Ghosh A, Agarwal NK and Singh SP, 2015. Increase in the expression of inducible nitric oxide synthase on exposure to bisphenol A: A possible cause for decline in steroidogenesis in male mice. Environmental Toxicology and Pharmacology, 39(1), 405–416. https://doi.org/10.1016/j.etap.2014.09.014.
Dere E, Anderson LM, Huse SM, Spade DJ, McDonnell-Clark E, Madnick SJ, Hall SJ, Camacho L, Lewis SM, Vanlandingham MM and Boekelheide K, 2018. Effects of continuous bisphenol A exposure from early gestation on 90 day old rat testes function and sperm molecular profiles: A CLARITY-BPA consortium study. Toxicology and Applied Pharmacology, 347, 1–9. https://doi.org/10.1016/j.taap.2018.03.021.
Dobrzyńska MM, Gajowik A, Radzikowska J, Tyrkiel EJ and Jankowska-Steifer EA, 2015. Male-mediated F1 effects in mice exposed to bisphenol A, either alone or in combination with X-irradiation. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 789–790, 36–45. https://doi.org/10.1016/j.mrgentox.2015.06.015
Dobrzyńska MM, Gajowik A, Jankowska-Steifer EA, Radzikowska J and Tyrkiel EJ, 2018. Reproductive and developmental F1 toxicity following exposure of pubescent F0 male mice to bisphenol A alone and in a combination with X-rays irradiation. Toxicology, 142–151. https://doi.org/10.1016/j.tox.2018.10.007.
Dodge LE, Williams PL, Williams MA, Missmer SA, Toth TL, Calafat AM and Hauser R, 2015. Paternal urinary concentrations of parabens and other phenols in relation to reproductive outcomes among couples from a fertility clinic. Environmental Health Perspectives, 123(7), 665–671. https://doi.org/10.1289/ehp.1408605.
Ferguson KK, Meeker JD, Cantonwine DE, Chen YH, Mukherjee B and McElrath TF, 2016b. Urinary phthalate metabolite and bisphenol A associations with ultrasound and delivery indices of fetal growth. Environment International, 94, 531–537. https://doi.org/10.1016%2Fj.envint.2016.06.013.
Ferguson SA, Law CD and Kissling GE, 2014. Developmental treatment with ethinyl estradiol, but not bisphenol A, causes alterations in sexually dimorphic behaviors in male and female Sprague Dawley rats. Toxicological Sciences, 140(2), 374–392. https://doi.org/10.1093/toxsci/kfu077.
Franssen D, Gérard A, Hennuy B, Donneau AF, Bourguignon JP and Parent AS, 2016. Delayed neuroendocrine sexual maturation in female rats after a very low dose of bisphenol A through altered GABAergic neurotransmission and opposing effects of a high dose. Endocrinology, 157(5), 1740–1750. https://doi.org/10.1210/en.2015-1937.
Ganesan S and Keating AF, 2016. Bisphenol A-induced ovotoxicity involves DNA damage induction to which the ovary mounts a protective response indicated by increased expression of proteinS involved in DNA repair and xenobiotic biotransformation. Toxicological Sciences, 152(1), 169–180. https://doi.org/10.1093/toxsci/kfw076.
Gao GZ, Zhao Y, Li HX and Li W, 2018. Bisphenol A-elicited miR-146a-5p impairs murine testicular steroidogenesis through negative regulation of Mta3 signaling. Biochemical and Biophysical Research Communications, 501(2), 478–485. https://doi.org/10.1016/j.bbrc.2018.05.017.
Goldstone AE, Chen Z, Perry MJ, Kannan K and Louis GM, 2015. Urinary bisphenol A and semen quality, the LIFE Study. Reproductive Toxicology, 51, 7–13. https://doi.org/10.1016/j.reprotox.2014.11.003.
Gonzalez-Cadavid NF, 2019. Cellular-molecular signature and mechanism of BPA effects on penile erection – CLARITY-BPA grantee study results. https://doi.org/10.22427/NTP-DATA-018-00009-0001-000-9.
Grassi TF, da Silva GN, Bidinotto LT, Rossi BF, Quinalha MM, Kass L, Muñoz-de-Toro M and Barbisan LF, 2016. Global gene expression and morphological alterations in the mammary gland after gestational exposure to bisphenol A, genistein and indole-3-carbinol in female Sprague-Dawley offspring. Toxicology and Applied Pharmacology, 303, 101–109. https://doi.org/10.1016/j.taap.2016.05.004.
Guignard D, Gayrard V, Lacroix MZ, Puel S, Picard-Hagen N and Viguié C, 2017. Evidence for bisphenol A- induced disruption of maternal thyroid homeostasis in the pregnant ewe at low level representative of human exposure. Chemosphere, 182, 458–467. https://doi.org/10.1016/j.chemosphere.2017.05.028.
Gurmeet KSS, Rosnah I, Normadiah MK, Das S and Mustafa AM, 2014. Detrimental effects of bisphenol A on development and functions of the male reproductive system in experimental rats. Excli Journal, 13,151–160.
Hass U, Christiansen S, Boberg J, Rasmussen MG, Mandrup K and Axelstad M, 2016. Low-dose effect of developmental bisphenol A exposure on sperm count and behaviour in rats. Andrology, 4(4), 594–607. https://doi.org/10.1111/andr.12176.
Hu Y, Yuan DZ, Wu Y, Yu LL, Xu LZ, Yue LM, Liu L, Xu WM, Qiao XY, Zeng RJ, Yang ZL, Yin WY, Ma YX 13401 and Nie Y, 2018. Bisphenol A initiates excessive premature activation of primordial follicles in mouse ovaries via the PTEN signalling pathway. Reproductive Sciences, 25(4), 609–620. https://doi.org/10.1177/1933719117734700.
Huang DY, Zheng CC, Pan Q, Wu SS, Su X, Li L, Wu JH and Sun ZY, 2018. Oral exposure of low-dose bisphenol A promotes proliferation of dorsolateral prostate and induces epithelial-mesenchymal transition in aged rats. Scientific Reports, 8(1), 490. https://doi.org/10.1038/s41598-017-18869-8.
Huang YF, Pan WC, Tsai YA, Chang CH, Chen PJ, Shao YS, Tsai MS, Hou JW, Lu CA and Chen ML, 2017a. Concurrent exposures to nonylphenol, bisphenol A, phthalates, and organophosphate pesticides on birth outcomes: A cohort study in Taipei, Taiwan. Science of the Total Environment, 607–608, 1126–1135. https://doi.org/10.1016/j.scitotenv.2017.07.092.
Johnson SA, Javurek AB, Painter MS, Ellersieck MR, Welsh TH, Camacho L, Lewis SM, Vanlandingham MM, Ferguson SA and Rosenfeld CS, 2016. Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: A CLARITY-BPA study. Hormones and Behavior, 80, 139–148. https://doi.org/10.1016/j.yhbeh.2015.09.005.
Jukic AM, Calafat AM, McConnaughey DR, Longnecker MP, Hoppin JA, Weinberg CR, Wilcox AJ and Baird DD, 2016. Urinary concentrations of phthalate metabolites and bisphenol A and associations with follicular-phase length, luteal-phase length, fecundability, and early pregnancy loss. Environmental Health Perspectives, 124(3), 321–328. doi:10.1289/ehp.1408164. https://doi.org/10.1289/ehp.1408164.
Kalb AC, Kalb AL, Cardoso TF, Fernandes CG, Corcini CD, Varela Junior AS and Martínez PE, 2016. Maternal transfer of bisphenol A during nursing causes sperm impairment in male offspring. Archives of Environmental Contamination and Toxicology, 70(4), 793–801. doi.org/10.1007/s00244-015-0199-7. Maternal Transfer of Bisphenol A During Nursing Causes Sperm Impairment in Male Offspring | SpringerLink.
Kasper-Sonnenberg M, Wittsiepe J, Wald K, Koch HM and Wilhelm M, 2017. Pre-pubertal exposure with phthalates and bisphenol A and pubertal development. PLoS ONE, 12(11), e0187922. https://doi.org/10.1371/journal.pone.0187922.
Kass L, Durando M, Altamirano GA, Manfroni-Ghibaudo GE, Luque EH and Muñoz-de-Toro M, 2015. Prenatal bisphenol A exposure delays the development of the male rat mammary gland. Reproductive Toxicology, 54, 37–46. https://doi.org/10.1016/j.reprotox.2014.02.001.
Kendziorski JA and Belcher SM, 2015. Strain-specific induction of endometrial periglandular fibrosis in mice exposed during adulthood to the endocrine disrupting chemical bisphenol A. Reproductive Toxicology, 58, 119–130. https://doi.org/10.1016/j.reprotox.2015.08.00.
Lathi RB, Liebert CA, Brookfield KF, Taylor JA, vom Saal FS, Fujimoto VY and Baker VL, 2014. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertility and Sterility, 102(1), 123–128. https://doi.org/10.1016/j.fertnstert.2014.03.024.
Leclerc F, Dubois MF and Aris A, 2014. Maternal, placental and fetal exposure to bisphenol A in women with and without preeclampsia. Hypertension in Pregnancy, 33(3), 341–348. https://doi.org/10.3109/10641955.2014.892607.
Lee HA, Kim YJ, Lee H, Gwak HS, Park EA, Cho SJ, Kim HS, Ha EH and Park H, 2013. Effect of urinary bisphenol A on androgenic hormones and insulin resistance in preadolescent girls: A pilot study from the Ewha birth & growth cohort. International Journal of Environmental Research and Public Health, 10(11), 5737–5749. https://doi.org/10.3390/ijerph10115737.
Lee YM, Hong YC, Ha M, Kim Y, Park H, Kim HS and Ha EH, 2018. Prenatal bisphenol-A exposure affects fetal length growth by maternal glutathione transferase polymorphisms, and neonatal exposure affects child volume growth by sex: From multiregional prospective birth cohort MOCEH study. Science of the Total Environment, 612, 1433–1441. https://doi.org/10.1016/j.scitotenv.2017.08.317.
Lejonklou MH, Christiansen S, Örberg J, Shen L, Larsson S, Boberg J, Hass U and Lind PM, 2016. Low-dose developmental exposure to bisphenol A alters the femoral bone geometry in Wistar rats. Chemosphere 164, 339–346. https://doi.org/10.1016/j.chemosphere.2016.08.114.
Lester F, Arbuckle TE, Peng Y and McIsaac MA, 2018. Impact of exposure to phenols during early pregnancy on birth weight in two Canadian cohort studies subject to measurement errors. Environment International 120, 231–237. https://doi.org/10.1016/j.envint.2018.08.005.
Leung YK, Govindarajah V, Cheong A, Veevers J, Song D, Gear R, Zhu XG, Ying J, Kendler A, Medvedovic M, Belcher S and Ho SM, 2017. Gestational high-fat diet and bisphenol A exposure heightens mammary cancer risk. Endocrine-Related Cancer 24(7), 365–378. https://doi.org/10.1530/erc-17-0006.
Leung YK, Biesiada J, Govindarajah V, Ying J, Kendler A, Medvedovic M and Ho SM, 2020. Low-dose bisphenol A in a rat model of endometrial cancer: A CLARITY-BPA study. Environmental Health Perspectives 128(12) https://doi.org/10.1289/EHP6875.
Li QX, Davila J, Kannan A, Flaws JA, Bagchi MK and Bagchi IC, 2016. Chronic exposure to bisphenol A affects uterine function during early pregnancy in mice. Endocrinology 157(5), 1764–1774. https://doi.org/10.1210/en.2015-2031.
Lind T, Lejonklou MH, Dunder L, Rasmusson A, Larsson S, Melhus H and Lind PM, 2017. Low-dose developmental exposure to bisphenol A induces sex-specific effects in bone of Fischer 344 rat offspring. Environmental Research 159, 61–68. https://doi.org/10.1016/j.envres.2017.07.020.
Mahalingam S, Ther L, Gao LY, Wang W, Ziv-Gal A and Flaws JA, 2017. The effects of in utero bisphenol A exposure on ovarian follicle numbers and steroidogenesis in the F1 and F2 generations of mice. Reproductive Toxicology, 74, 150–157. https://doi.org/10.1016/j.reprotox.2016.09.013.
Mandrup K, Boberg J, Isling LK, Christiansen S and Hass U, 2016. Low-dose effects of bisphenol A on mammary gland development in rats. Andrology, 4(4), 673–683. https://doi.org/10.1111/andr.12193.
Martinez AM, Cheong A, Ying J, Xue JC, Kannan K, Leung YK, Thomas MA and Ho SM, 2015. Effects of high-butterfat diet on embryo implantation in female rats exposed to bisphenol A. Biology of Reproduction, 93(6), 147. https://doi.org/10.1095/biolreprod.115.131433.
Meng Y, Lin R, Wu F, Sun Q and Jia L, 2018. Decreased capacity for sperm production induced by perinatal bisphenol A exposure is associated with an increased inflammatory response in the offspring of C57BL/6 male mice. International Journal of Environmental Research and Public Health, 15(10). https://doi.org/10.3390/ijerph15102158.
Mínguez-Alarcón L, Gaskins AJ, Chiu YH, Williams PL, Ehrlich S, Chavarro JE, Petrozza JC, Ford JB, Calafat AM, Hauser R and EARTH Study Team, 2015. Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Human Reproduction, 30(9), 2120– 2128. https://doi.org/10.1093/humrep/dev183.
Mínguez-Alarcón L, Gaskins AJ, Chiu YH, Souter I, Williams PL, Calafat AM, Hauser R, Chavarro JE and EARTH Study team, 2016. Dietary folate intake and modification of the association of urinary bisphenol A concentrations with in vitro fertilization outcomes among women from a fertility clinic. Reproductive Toxicology, 65, 104–112. https://doi.org/10.1016/j.reprotox.2016.07.012.
Montévil M, Acevedo N, Schaeberle CM, Bharadwaj M, Fenton SE and Soto AM, 2020. A combined morphometric and statistical approach to assess nonmonotonicity in the developing mammary gland of rats in the CLARITY-BPA study. Environmental Health Perspectives, 128(5), 57001. https://doi.org/10.1289/ehp6301.
Moore-Ambriz TR, Acuña-Hernández DG, Ramos-Robles B, Sánchez-Gutiérrez M, Santacruz-Márquez R, Sierra-Santoyo A, Piña-Guzmán B, Shibayama M and Hernández-Ochoa I, 2015. Exposure to bisphenol A in young adult mice does not alter ovulation but does alter the fertilization ability of oocytes. Toxicology and Applied Pharmacology, 289(3), 507–514. https://doi.org/10.1016/j.taap.2015.10.010.
Mustieles V, Williams PL, Fernandez MF, Mínguez-Alarcón L, Ford JB, Calafat AM, Hauser R, Messerlian C and Environment and Reproductive Health (EARTH) Study Team, 2018. Maternal and paternal preconception exposure to bisphenols and size at birth. Human Reproduction, 1528–1537. https://doi.org/10.1093/humrep/dey234.
NTP CLARITY-BPA report (2018). A two-year toxicology study of bisphenol A (BPA) in Sprague-Dawley rats: CLARITY-BPA core study results. Food and Chemical Toxicology, 132. 12728. https://doi.org/10.1016/j.fct.2019.110728.
Ogo FM, Lion Siervo GEM, Staurengo-Ferrari L, Oliveira Mendes L, Luchetta NR, Vieira HR, Fattori V and Verri WA, Jr, 2018. S and W. RF. Ogo FM, de Lion Siervo GEM, Staurengo-Ferrari L, de Oliveira Mendes L, Luchetta NR, Vieira HR, Fattori V, Verri WA, Scarano WR and Fernandes GSA, 2018. Bisphenol A exposure impairs epididymal development during the peripubertal period of rats: Inflammatory profile and tissue changes. Basic and Clinical Pharmacology and Toxicology, 122(2), 262–270. https://doi.org/10.1111/bcpt.12894.
Olukole SG, Ajani SO, Ola-Davies EO, Lanipekun DO, Aina OO, Oyeyemi MO and Oke BO, 2018. Melatonin ameliorates bisphenol A-induced perturbations of the prostate gland of adult Wistar rats. Biomedicine and Pharmacotherapy, 105, 73–82. https://doi.org/10.1016/j.biopha.2017.05.125.
Park B, Kwon JE, Cho SM, Kim CW, Lee DE, Koo YT, Lee SH, Lee HM and Kang SC, 2018. Protective effect of Lespedeza cuneata ethanol extract on bisphenol A-induced testicular dysfunction in vivo and in vitro. Biomedicine and Pharmacotherapy, 102, 76–85. https://doi.org/10.1016/j.biopha.2018.03.045.
Patel BB, Raad M, Sebag IA and Chalifour LE, 2013. Lifelong exposure to bisphenol A alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicological Sciences, 133(1), 174–185. https://doi.org/10.1093/toxsci/kft026.
Pinney SE, Mesaros CA, Snyder NW, Busch CM, Xiao R, Aijaz S, Ijaz N, Blair IA and Manson JM, 2017. Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reproductive Toxicology, 67, 1–9. https://doi.org/10.1016/j.reprotox.2016.11.007.
Philippat C, Botton J, Calafat AM, Ye XY, Charles MA, Slama R and EDEN Study Group, 2014. Prenatal exposure to phenols and growth in boys. Epidemiology, 25(5), 625–635. https://doi.org/10.1097/ede.0000000000000132.
Pollack AZ, Mumford SL, Krall JR, Carmichael AE, Sjaarda LA, Perkins NJ, Kannan K and Schisterman EF, 2018. Exposure to bisphenol A, chlorophenols, benzophenones, and parabens in relation to reproductive 1hormones in healthy women: A chemical mixture approach. Environment International, 120, 137–144. https://doi.org/10.1016/j.envint.2018.07.028.
Prins GS, Ye SH, Birch L, Zhang X, Cheong A, Lin H, Calderon-Gierszal E, Groen J, Hu WY, Ho SM and van Breemen RB, 2017. Prostate cancer risk and DNA methylation signatures in aging rats following developmental BPA exposure: a dose–response analysis. Environmental Health Perspectives, 125(7), 077007. https://doi.org/10.1289/EHP1050.
Prins GS, Hu WY, Xie L, Shi GB, Hu DP, Birch L and Bosland MC, 2018. Evaluation of bisphenol A (BPA) exposures on prostate stem cell homeostasis and prostate cancer Risk in the NCTR-Sprague-Dawley rat: An NIEHS/FDA CLARITY-BPA consortium study. Environmental Health Perspectives, 126(11), 117001. https://doi.org/10.1289/EHP3953.
Quan C, Wang C, Duan P, Huang W and Yang KD, 2017. Prenatal bisphenol A exposure leads to reproductive hazards on male offspring via the Akt/mTOR and mitochondrial apoptosis pathways. Environmental Toxicology, 32(3), 1007–1023. https://doi.org/10.1002/tox.22300.
Radko L, Minta M, Jasik A, Stypuła-Trębas S and Żmudzki J, 2015. Usefulness of immature golden hamster (Mesocricetus auratus) as a model for uterotrophic assay. Bulletin of the Veterinary Institute in Pulawy, 59(4), 533–540. https://doi.org/10.1515/bvip-2015-0080.
Rahman MS, Kwon WS, Karmakar PC, Yoon SJ, Ryu BY and Pang MG, 2017. Gestational exposure to bisphenol A affects the function and proteome profile of F1 spermatozoa in adult mice. Environmental Health Perspectives, 125(2), 238–245. https://doi.org/10.1289/EHP378.
Rashid H, Sharma S, Beigh S, Ahmad F and Raisuddin S, 2018. Bisphenol A-induced endocrine toxicity and male Reprotoxicopathy are modulated by the dietary iron deficiency. Endocrine, Metabolic and Immune Disorders Drug Targets, 18(6), 626–636. https://doi.org/10.2174/1871530318666180521095443.
Santamaría C, Durando M, Muñoz de Toro MM, Luque EH and Rodriguez HA, 2016. Ovarian dysfunctions in adult female rat offspring born to mothers perinatally exposed to low doses of bisphenol A. Journal of Steroid Biochemistry and Molecular Biology, 158, 220–230. https://doi.org/10.1016/j.jsbmb.2015.11.016.
Santamaría CG, Rodriguez HA, Abud JE, Rivera OE, Muñoz-de-Toro M and Luque EH, 2017. Impaired ovarian response to exogenous gonadotropins in female rat offspring born to mothers perinatally exposed to bisphenol A. Reproductive Toxicology, 73, 259–268. https://doi.org/10.1016/j.reprotox.2017.06.050.
Shi M, Sekulovski N, MacLean JA and Hayashi K, 2018. Prenatal exposure to bisphenol A analogues on male reproductive functions in mice. Toxicological Sciences, 163(2), 620–631. https://doi.org/10.1093/toxsci/kfy061.
Smarr MM, Grantz KL, Sundaram R, Maisog JM, Kannan K and Buck Louis GM, 2015. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environmental Health, 14, 11. doi:10.1186/s12940-015-0060-5. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes | Environmental Health | Full Text (biomedcentral.com).
Soleimani Mehranjani MS and Mansoori T, 2016. Stereological study on the effect of vitamin C in preventing the adverse effects of bisphenol A on rat ovary. International Journal of Reproductive Biomedicine, 14496 14(6), 403–410. doi:10.29252/ijrm.14.6.403. World Journal of Diabetes - Baishideng Publishing Group (wjgnet.com).
Souter I, Smith KW, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM and Hauser R, 2013. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reproductive Toxicology, 42, 224–231. https://doi.org/10.1016/j.reprotox.2013.09.008.
Spörndly-Nees E, Boberg J, Ekstedt E, Holm L, Fakhrzadeh A, Dunder L, Kushnir MM, Lejonklou MH and Lind PM, 2018. Low-dose exposure to bisphenol A during development has limited effects on male reproduction in midpubertal and aging Fischer 344 rats. Reproductive Toxicology, 81, 196–206. https://doi.org/10.1016/j.reprotox.2018.08.007.
Srivastava S and Gupta P, 2018. Alteration in apoptotic rate of testicular cells and sperms following administration of bisphenol A (BPA) in Wistar albino rats. Environmental Science and Pollution Research International, 25(22), 21635–21643 doi:10.1007/s11356-018-2229. Alteration in apoptotic rate of testicular cells and sperms following administration of Bisphenol A (BPA) in Wistar albino rats | SpringerLink
Sun X, Li D, Liang H, Miao M, Song X, Wang Z, Zhou Z and Yuan W, 2018. Maternal exposure to bisphenol A and anogenital distance throughout infancy: A longitudinal study from Shanghai, China. Environment International, 121(1), 269–275. https://doi.org/10.1016/j.envint.2018.08.055.
Tarapore P, Hennessy M, Song D, Ying J, Ouyang B, Govindarajah V, Leung YK and Ho SM, 2017. High butter-fat diet and bisphenol A additively impair male rat spermatogenesis. Reproductive Toxicology, 68, 191–199. https://doi.org/10.1016/j.reprotox.2016.09.008.
Thilagavathi S, Pugalendhi P, Rajakumar T and Vasudevan K, 2017. Monotonic dose effect of bisphenol-A, an estrogenic endocrine disruptor, on estrogen synthesis in female Sprague-Dawley rats. Indian Journal of Clinical Biochemistry, 1–10. Monotonic Dose Effect of Bisphenol-A, an Estrogenic Endocrine Disruptor, on Estrogen Synthesis in Female Sprague-Dawley Rats | SpringerLink
Tucker DK, Hayes Bouknight SH, Brar SS, Kissling GE and Fenton SE, 2018. Evaluation of prenatal exposure to bisphenol analogues on development and long-term health of the mammary gland in female mice. Environmental Health Perspectives, 126(8), 087003. https://doi.org/10.1289/EHP3189.
Ullah A, Pirzada M, Jahan S, Ullah H, Shaheen G, Rehman H, Siddiqui MF and Butt MA, 2018a. Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere, 209, 508–516. https://doi.org/10.1177/0748233719831528.
Ullah A, Pirzada M, Jahan S, Ullah H, Turi N, Ullah W, Siddiqui MF, Zakria M, Lodhi KZ and Khan MM, 2018b. Impact of low-dose chronic exposure to bisphenol A and its analogue bisphenol B, bisphenol F and bisphenol S on hypothalamo-pituitary-testicular activities in adult rats: A focus on the possible hormonal mode of action. Food and Chemical Toxicology, 121, 24–36. https://doi.org/10.1016/j.fct.2018.08.024.
van Esterik JCJ, Dollé MET, Lamoree MH, van Leeuwen SPJ, Hamers T, Legler J and van der Ven LTM, 2014. Programming of metabolic effects in C57BL/6JxFVB mice by exposure to bisphenol A during gestation and lactation. Toxicology, 321, 40–52. https://doi.org/10.1016/j.tox.2014.04.001.
Veiga-Lopez A, Kannan K, Liao CY, Ye W, Domino SE and Padmanabhan V, 2015. Gender-specific effects on gestational length and birth weight by early pregnancy BPA exposure. Journal of Clinical Endocrinology and Metabolism, 100(11), E1394–E1403. https://doi.org/10.1210/jc.2015-1724.
Vigezzi L, Bosquiazzo VL, Kass L, Ramos JG, Muñoz-de-Toro M and Luque EH, 2015. Developmental exposure to bisphenol A alters the differentiation and functional response of the adult rat uterus to estrogen treatment. Reproductive Toxicology, 52, 83–92. https://doi.org/10.1016/j.reprotox.2015.01.011.
Vijaykumar T, Singh D, Vanage GR, Dhumal RV and Dighe VD, 2017. Bisphenol A-induced ultrastructural changes in the testes of common marmoset. Indian Journal of Medical Research, 146(1), 126–137. https://doi.org/10.4103/ijmr.ijmr_927_15.
Wang B, Zhou W, Zhu WT, Chen L, Wang WY, Tian Y, Shen LS, Zhang J and Shanghai Birth Cohort Study, 2018. Associations of female exposure to bisphenol A with fecundability: Evidence from a preconception cohort study. Environment International, 117, 139–145. https://doi.org/10.1016/j.envint.2018.05.003.
Wang C, Li ZH, Han HJ, Luo GY, Zhou BR, Wang SL and Wang JD, 2016. Impairment of object recognition memory by maternal bisphenol A exposure is associated with inhibition of Akt and ERK/CREB/BDNF pathway in the male offspring hippocampus. Toxicology, 341–343, 56–64. https://doi.org/10.1016/j.tox.2016.01.010.
Wang HF, Liu M, Li N, Luo T, Zheng LP and Zeng XH, 2016. Bisphenol A impairs mature sperm functions by a CatSper-relevant mechanism. Toxicological Sciences, 152(1), 145–154. https://doi.org/10.1093/toxsci/kfw070.
Wang W, Hafner KS and Flaws JA, 2014. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicology and Applied Pharmacology, 276(2), 157–164. https://doi.org/10.1016/j.taap.2014.02.009.
Watkins DJ, Téllez-Rojo MM, Ferguson KK, Lee JM, Solano-Gonzalez M, Blank-Goldenberg C, Peterson KE and Meeker JD, 2014. In utero and peripubertal exposure to phthalates and BPA in relation to female sexual maturation. Environmental Research, 134, 233–241. https://doi.org/10.1016%2Fj.envres.2014.08.010.
Watkins DJ, Sánchez BN, Téllez-Rojo MM, Lee JM, Mercado-García A, Blank-Goldenberg C, Peterson KE and Meeker JD, 2017a. Impact of phthalate and BPA exposure during in utero windows of susceptibility on reproductive hormones and sexual maturation in peripubertal males. Environmental Health: A Global Access Science Source, 16(1), 69. https://doi.org/10.1186/s12940-017-0278-5.
Watkins DJ, Sánchez BN, Téllez-Rojo MM, Lee JM, Mercado-García A, Blank- Goldenberg C, Peterson KE and Meeker JD, 2017b. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environmental Research, 159, 143–151. https://doi.org/10.1016/j.envres.2017.07.051.
Weinberger B, Vetrano AM, Archer FE, Marcella SW, Buckley B, Wartenberg D, Robson MG, Klim J, Azhar S, Cavin S, Wang L and Rich DQ, 2014. Effects of maternal exposure to phthalates and bisphenol A during pregnancy on gestational age. Journal of Maternal-Fetal and Neonatal Medicine, 27(4), 323–327. https://doi.org/10.3109/14767058.2013.815718.
Wolff MS, Teitelbaum SL, McGovern K, Pinney SM, Windham GC, Galvez M, Pajak A, Rybak M, Calafat AM, Kushi LH, Biro FM and Breast Cancer and Environment Research Program, 2015. Environmental phenols and pubertal development in girls. Environment International, 84, 174–180. [PDF] Environmental phenols and pubertal development in girlsbyundefined · 10.1016/j.envint.2015.08.008 · Citationsy.
Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez M, Rybak M, Silva MJ, Ye X, Calafat AM, Kushi LH, Biro FM, Teitelbaum SL and Breast Cancer and Environment Research Program, 2017. Associations of urinary phthalate and phenol biomarkers with menarche in a multiethnic cohort of young girls. Reproductive Toxicology, 67, 56–64. https://doi.org/10.1016/j.reprotox.2016.11.009.
Woods MM, Lanphear BP, Braun JM and McCandless LC, 2017. Gestational exposure to endocrine disrupting chemicals in relation to infant birth weight: A Bayesian analysis of the HOME Study. Environmental Health: A Global Access Science Source, 16(1), 115. https://doi.org/10.1186/s12940-017-0332-3.
Wu JH, Huang DY, Su X, Yan H and Sun ZY, 2016. Oral administration of low-dose bisphenol A promotes proliferation of ventral prostate and upregulates prostaglandin D-2 synthase expression in adult rats. Toxicology and Industrial Health, 32(11), 1848–1858. https://doi.org/10.1177/0748233715590758.
Xie MN, Bu PL, Li FJ, Lan SJ, Wu HJ, Yuan L and Wang Y, 2016. Neonatal bisphenol A exposure induces meiotic arrest and apoptosis of spermatogenic cells. Oncotarget, 7(9), 10606–10615. doi:10.18632/oncotarget.7218. https://doi.org/10.18632/oncotarget.7218.
Xu XH, Dong FN, Yang YL, Wang Y, Wang R and Shen XY, 2015. Sex-specific effects of long-term exposure to bisphenol-A on anxiety- and depression-like behaviors in adult mice. Chemosphere, 120, 258–266. https://doi.org/10.1016/j.chemosphere.2014.07.021.
Ye YZ, Zhou QJ, Feng LP, Wu JN, Xiong Y and Li XT, 2017. Maternal serum bisphenol A levels and risk of pre-eclampsia: A nested case-control study. European Journal of Public Health, 27(6), 1102–1107. doi:10.1093/eurpub/ckx148. Maternal serum bisphenol A levels and risk of pre-eclampsia: a nested case-control study - PubMed (nih.gov).
Yin L, Dai YL, Cui ZH, Jiang X, Liu WB, Han F, Lin A, Cao J and Liu JY, 2017. The regulation of cellular apoptosis by the ROS-triggered PERK/EIF2a chop pathway plays a vital role in bisphenol A-induced male reproductive toxicity. Toxicology and Applied Pharmacology, 314, 98–108. doi:10.1016/j.taap.2016.11.013 The regulation of cellular apoptosis by the ROS-triggered PERK/EIF2α/chop pathway plays a vital role in bisphenol A-induced male reproductive toxicity (researchgate.net).
Yuan M, Hu M, Lou Y, Wang Q, Mao L, Zhan Q and Jin F, 2018. Environmentally relevant levels of bisphenol A affect uterine decidualization and embryo implantation through the estrogen receptor/serum and glucocorticoid-regulated kinase 1/epithelial sodium ion channel alpha-subunit pathway in a mouse model. Fertility and Sterility, 109(4), 735–744 https://doi.org/10.1016/j.fertnstert.2017.12.003.
Zaid SSM, Othman S and Kassim NM, 2014. Potential protective effect of Tualang honey on BPA-induced ovarian toxicity in prepubertal rat. BMC Complementary and Alternative Medicine, 14(1), 509 doi:10.1186/1472-6882-14-509 https://doi.org/10.1186/1472-6882-14-509.
Zhang J, Zhang XC, Li YN, Zhou ZZ, Wu CL, Liu ZY, Hao LX, Fan SS, Jiang F, Xie Y and Jiang L, 2017. Low dose of bisphenol A enhance the susceptibility of thyroid carcinoma stimulated by DHPN and iodine excess in F344 rats. Oncotarget, 8(41), 69874–69887 doi:10.18632/oncotarget.1943 (PDF) Low dose of Bisphenol A enhance the susceptibility of thyroid carcinoma stimulated by DHPN and iodine excess in F344 rats (researchgate.net).
Ziv-Gal A, Wang W, Zhou CQ and Flaws JA, 2015. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicology and Applied Pharmacology, 284(3), 354–362. https://doi.org/10.1016/j.taap.2015.03.003.