Current regulatory landscape - Handbook 2021 Workshop
In this guide
In this guideThe use of PBPK models has increased over the last several decades throughout various working sectors, including academia and industry. Particularly in conjunction with other emerging alternative methods to in vivo animal testing (e.g. in vitro studies and data- driven in silico quantitative-structure-activity-relationship (QSAR) predictions), where the generated data allows for increased confidence in models for chemicals without in vivo data for model calibration.
Despite this growing use and advantages offered by these applications, there remains some hesitation from public health and other regulatory agencies for the integration of these models for use in the risk assessment process due to lack of harmonised guidance, human data, or expertise in computational modelling (Paini et al., 2017).
In Europe, the European Union Reference Laboratory for Alternatives to Animal Testing (EURL-ECVAM) have recently published a status report on the Development, Validation and Regulatory Acceptance of Alternative Methods and Approaches in March 2020 (JRC news and updates - European Commission (europa.eu) in which the Rvis platform was mentioned. International cooperation with the Health and Environmental Sciences Institute (HESI) PBPK Models Committee (HESI website.) was also summarised. It was mentioned that there are two main efforts ongoing from this Committee. Firstly, the establishment of a harmonised template to report information, and provide recommendations to model reviewers to facilitate the uptake of PBK models in regulatory risk assessment and secondly, the development of a framework and decision tree on PBPK applications based on different degrees of data availability.
The proposed PBPK model reporting template has now been published (Tan et al., 2020). In brief, the authors expanded the existing guidance designed for pharmaceutical applications (WHO, 2010; US FDA, 2018; EMA, 2019) by recommending additional elements that are relevant to environmental chemicals. There are 8 main sections which includes 3 to 8 sub-sections:
i). Executive summary;
ii). Background/Introduction – chemical’s physicochemical, PK and PD properties; known exposure, toxicity and efficacy; PBPK-related regulatory history; cross- referencing other PBPK efforts; relevant data used for model calibration; relevant data used for model evaluation;
iii). Model purpose;
iv). Materials and methods – modeling strategy; summary of data for model development and evaluation; model development and structure; model equations; model parameters; model simulations; software;
v). Results – model evaluation; sensitivity, uncertainty, and variability analyses; model applicability;
vi). Discussions and conclusions;
vii). Electronic files and Supporting Documents and;
viii). Appendices.
The authors note that the template can be adapted and customised, as well as serving as a general guidance for submitting PBPK-related studies for publication in journals or other modeling sharing purposes. The authors hoped that the use of the template will help in standardising PBPK model reporting and communication and thereby enhance their application and regulatory acceptance.
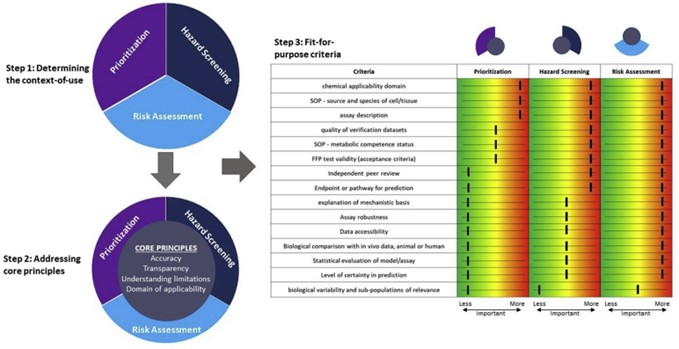
Considering the bigger picture, PBPK modelling is part of new approach methodologies (NAMs) for human health and safety assessment. An evaluation framework guideline for evaluating NAMs has been published by Parish et al., (2020), which is comprised of three steps. These are: determining the context of use (i.e. will the NAM be used in prioritization, hazard screening or risk assessment), addressing the core principles (which must be addressed, irrespective of the context-of-use) and the fit for purpose criteria. Figure 2 provides a schematic representation of the framework.
Figure 2: a schematic representation of the three steps of the evaluation framework for new approach methodologies (NAMs) as recommended by Parish et al., (2020) (reproduced from Parish et al., 2020).
The authors note that, their recommendations do not constitute regulatory guidance and are not meant to supersede or supplant any existing regulatory policy or address how NAMs could be implemented. The framework contextualizes the importance of the derived criteria from regulatory guidance (e.g. the Organisation for Economic Cooperation and Development (OECD) Guidance Document No.34 (OECD, 2005)), in such a way that NAM evaluations can be performed based on their level of importance (i.e. high or low), which is driven by the context-of-use. There is emphasis on ensuring that a NAM is fit for its intended purpose, as determined by problem formulation.
In this context, the application of PBPK models are considered under the ‘explanation of mechanistic basis’ criteria, where there are current efforts on supporting the integration of toxicokinetics into in vitro evaluations of toxicodynamics. Methods that enable in vitro to in vivo extrapolation (IVIVE) are necessary to accurately estimate relevant human exposures that correspond to observed in vitro bioactivity. The use of IVIVE approaches with PBPK modelling was suggested by the authors to quantitatively bridge in vitro and in vivo data and to explore the key mechanisms dictating the pharmacokinetics. Combining in vitro methods with appropriate exposure data will improve applicability in a risk assessment framework and thus allow specific consideration with regard to route of exposure, target-specificity, and the potential for human extrapolation.