Introduction to physiologically based pharmacokinetic (PBPK) modelling - Handbook 2021 Workshop
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.Chemical risk assessments do not routinely evaluate internal dose. However, internal dose relates more directly (temporally and spatially) to a toxicological response than external dose, and thus provides a more accurate basis for the evaluation of health risk. Furthermore, there is a need for some means to translate biologically active concentrations in vitro to the equivalent doses in vivo. Computational approaches such as the use of PBPK modelling can be used for these purposes.
PBPK models are mathematical representations of physiological processes affecting a chemical’s in vivo toxicokinetics; absorption, distribution, metabolism and excretion (ADME) or, simply put, describe the relationships between external exposure and the concentration-time profile of a chemical within the body.
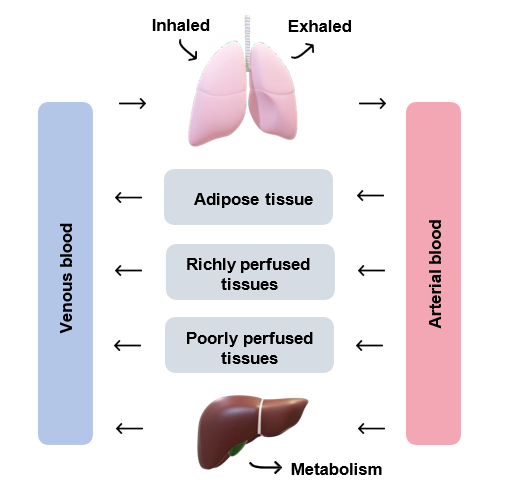
ADME processes are represented by a series of mathematical differential equations which describe the rate of change in the amount of a chemical in target tissues and blood (Figure 1).
Figure 1: a general schematic of a PBPK model (adapted from Tan et al., 2020).
The concept of PBPK modeling was first described by Thorsten Teorell in 1937 (Teorell, 1937). Each parameter in a PBPK model describes a physiological (e.g. ventilation rate), physicochemical (e.g. lipid solubility) or biochemical (e.g. metabolic Vmax) process.
The development and application of PBPK models has been described to have six steps (Rietjens et al., 2011). These are as follows:
i). Definition of the conceptual model;
ii). Translation into a mathematical model;
iii). Defining parameter values;
iv). Solving the equations;
v). Evaluation of model predictions and;
vi). Making predictions.
PBPK models are based on several general assumptions regarding ADME including: the mixing of the chemical in the effluent blood from the tissues is instantaneous and complete, blood flow is unidirectional, constant and non-pulsatile, and the presence of chemicals in the blood cells does not alter the blood flow rate. Any deviations from which should be justified and documented as recommended by the World Health Organisation (WHO) (WHO, 2010).
The WHO published the key principles and best practices for characterising and applying PBPK models in risk assessment (WHO, 2010). This was conducted within the International Programme on Chemical Safety (IPCS) project on the Harmonisation to Approaches to the Assessment of Risk from Exposure to Chemicals.
The WHO 2010 publication addressed model validation, which is required before a PBPK model can be used confidently as a tool in risk assessment. Validation is the “process by which the reliability and relevance of a particular model are established for a defined purpose” (IPCS, 2005). Validation should be conducted by considering the following factors (WHO, 2010):
i). The biological basis of the model structure and parameters;
ii). Closeness of the model simulations to actual pharmacokinetic data;
iii). Reliability of simulated dose metrics (as they relate to a specific purpose or application in risk assessment) and;
iv). Supplementary analyses of sensitivity, uncertainty and variability (might be important, depending upon the end use and extent of comparison with real life data).
The “dose metric” has been defined as the dose measure that is causally related to the toxic outcome (Andersen et al., 1987). It is more closely related with tissue response than external dose. When the direct measurement of a dose metric is unethical or not technically feasible, PBPK models can be used to simulate it.
The dose metric relates to the form of chemical (e.g. parent chemical or metabolite), its level (free or total concentration or amount), duration (instantaneous, daily, lifetime or a specific developmental period), intensity (maximum, average or integral), and the biological matrix (e.g. blood, target tissue) (US EPA, 2006).
For application to risk assessment, the model should be able to simulate the dose metric of relevance to the chemical’s mode of action (MoA). The dose metric that is estimated may correspond to an external reference dose such as the no-observed-adverse-effect- level (NOAEL) or benchmark dose level (BMDL) or another exposure scenario of interest to risk assessors. When there are several dose metrics, the appropriate one for use in risk assessment should be chosen on the basis of plausibility. The plausibility of a particular dose metric is determined by its consistency with available information on the chemical’s MoA as well as the dose-response information for the toxicological endpoint of concern (WHO, 2010).
A review by Sager et al., (2015) found that published PBPK models for pharmaceutical agents in humans (n=366 articles) were most commonly used for drug-drug interaction predictions (28%), followed by interindividual variability and general clinical pharmacokinetic predictions (23%), formulation or absorption modelling (12%), and predicting age-related changes in pharmacokinetics and disposition (10%).
PBPK models are generally complex, data and resource intensive, however, they can be highly informative, provide platforms for: integration and scaling of in vitro data, extrapolation across a wide variety of conditions (e.g. different exposure scenarios, disease states, changes with age and co-exposure to other chemicals) and enable prediction of pharmacokinetic endpoints.
PBPK modelling: Considerations of the COT
In 2003, the COT hosted a workshop on PBPK modelling. The presentations considered the use of PBPK models in risk assessment, and the requirements to allow for their incorporation in risk assessment. The presentations were followed by a general discussion which focused on the strengths and weaknesses of PBPK modelling, whether PBPK models could be integrated into risk assessments conducted by the COT, and how this might be achieved: (TOX/2003/40 Annex B).
A COT statement on PBPK modelling was published in 2003, where the COT considered PBPK modelling to be an established technique capable of predicting the in vivo behaviour of chemicals. PBPK modelling is widely used in the development and risk assessment of pharmaceutical products, where there is often sufficient human data available with which to validate the models. However, for many chemicals evaluated by COT, it was noted there are limited or no human pharmacokinetic data available that can be used for model validation. Members expressed their reservations in assessing a PBPK model that had not been validated in this way.
Furthermore, the COT considered that animal data can provide partial validation if it can be assumed, or there is evidence, that the chemical behaves similarly in animals and humans. Additionally, validation could be enhanced by mechanistic studies in experimental animals that show human relevance. However, there would be less confidence in the predictions of such models, and this would need to be expressed as a source of greater uncertainty in the risk assessment.
The Committee concluded that it would not be feasible to undertake PBPK modelling routinely for COT risk assessments because the generation and validation of a PBPK model is resource and time intensive. However, the COT agreed that relevant published PBPK models should be incorporated into risk assessments when possible, for example when submitted to support a risk assessment by industry.
In 2007, the COT held an open workshop on “Evolving Approaches to Chemical Risk Assessment”. A statement was published that summarises the presentations and Committee’s discussions. PBPK models were briefly discussed as part of the presentation on exploring uncertainty using sensitivity analysis: [ARCHIVED CONTENT] COT: COT statement on the COT workshop on evolving approaches to chemical risk assessment (nationalarchives.gov.uk).
The COT’s overall conclusions were as follows: the need to more explicitly assess and describe the uncertainty in the available data, the use of more transparent and
reproducible methods (e.g. framework approaches and systematic rather than narrative reviews).
Additionally, new technologies should be carefully adopted, and only implemented if they offer a clear benefit in terms of improving the risk assessments by the Committee. Although, where appropriate, new approaches should be initially performed in parallel with existing methods, allowing for further investigation of divergent outcomes.
In 2009, the COT held a workshop on 21st century toxicology. The workshop addressed the US National Academy of Sciences report entitled Toxicity Testing in the 21st Century: A Vision and a Strategy | The National Academies Press. A statement was published: COT statement on the COT Workshop on 21st Century Toxicology where the COT welcomed the systematic approach of the strategy for the use of in vitro and in silico approaches to better understand toxicity.
More recently, in 2019, the COT reviewed PBPK modelling used for human health risk assessment (TOX/2019/34). The discussion of the Committee focused on ways to assess the reliability of human PBPK models in the absence of human pharmacokinetic data. Approaches that were considered to assess model reliability in this context included the use of the read-across approach (Read-across is a technique for predicting endpoint information for one substance (target substance), by using data from the same endpoint from (an)other substance(s); the source substance(s) and conducting interspecies extrapolations to animal species other than humans. The Committee agreed that it would be useful to have further information in the form of case studies, where in vitro data had been successfully extrapolated to in vivo, or cases where risk assessments considered in retrospect may have benefitted from PBPK modelling.
A follow-up paper (TOX/2019/73) was then presented in response to this request. The paper summarised a number of PBPK case studies that have been used in risk assessment (PFOS & PFOA, dioxins, bisphenol A, acrylamide & glycidamide, chloroform & carbon tetrachloride, vinyl acetate, methylene chloride, and vinyl chloride), in addition to cases where in vitro to in vivo extrapolation has been conducted (PFOS, triclosan, pyridaben & fluazinam, estragole, and trichloroethylene). Furthermore, examples involving 2-butoxyethanol, persistent organic pollutants, amphetamine analogues and electronic nicotine (and non-nicotine) delivery systems devices were described where the use of PBPK modelling may have facilitated their risk assessment.
The Committee noted that PBPK models have predominantly been developed and applied on a case-by-case basis, for example to assess exposures of chemicals with narrow margins of exposure or to fill in data gaps from more conventional approaches.
The Committee recognised that PBPK modelling is of current interest to regulators in the field of chemical risk assessment; however, it is still largely used more in research capacities to refine estimates of health risk. PBPK models are not routinely applied or assessed by regulatory bodies because they are generally complex and both labour and data intensive, for example in terms of the data required for model parameterisation.
However, despite the multitude of case-specific PBPK models, systems are being developed to enable generic PBPK models to be generated. Software platforms such as these may be used in conjunction with the read-across approach to assess human health risks without the need for animal testing.
In March 2020, the FSA COT held a workshop entitled “Exploring Dose Response” which was attended by scientists from regulatory agencies, government bodies, academia and industry. The workshop provided a platform from which to address and enable expert discussions on the latest in silico prediction models, new approach methodologies, PBPK modelling, future methodologies, integrated approaches to testing and assessment, as well as validation of methodology. Several case studies involving plastic particles, polymers, tropane alkaloids, and selective androgen receptor modulators were used to explore approaches fit for purpose in the context of future food safety assessment. Furthermore, possible future research initiatives were discussed, such as establishing points of departures using non-animal alternative models and improving use of exposure metrics in risk assessment.
The workshop report (TOX/2020/30) was discussed as reserved business in July 2020, as it is hoped to publish a peer reviewed paper. The key issues identified in respect to PBPK models would be further developed through the current workshop.
PBPK models
The below examples of PBPK models is not an exhaustive list but rather provides detail on some that are publicly available.
RVis
Rvis is a prototype, proof of concept application for the analysis of structure and performance of PBPK and other models, written in the free, open source syntax R or C++ developed by the Health Safety Laboratory with funding from the European Partnership for Alternative Approaches to Animal testing and the Health and Safety Executive. Further information is available on the Cefic website.
It is a general-purpose modelling platform, not just an in vitro and in vivo exposure predictor. The features of Rvis include the ability to load, run, visualise and plot graphical outputs from models. Model structures may be analysed using parameter elementary
effects screening and global sensitivity analysis for any species (e.g. human, rodent, farm animals), and parameter estimation using Markov Chain Monte Carlo simulation and Bayesian inference. The parameter estimation feature is used to perform “reverse dosimetry” to reconstruct human dose or exposure concentrations consistent with human biomonitoring data.
Rvis and a user-guide are available to download from the Rvis repository hosted on GitHub: GitHub - GMPtk/RVis: Open access PBPK modelling platform. Users can download the latest version and post issues that arise that should be addressed.
European Food Safety Authority (EFSA): A Web-based opensource tool for toxicokinetic (TK) and toxicodynamic (TD) modelling
An EFSA external report on a Web-based open source tool for TK and TD modelling has been recently published in November 2020 (Bossier et al., 2020). The software has been developed in R as a web-based tool that includes different components for the modelling of TK and TD processes in a structured workflow.
This workflow provides steps to perform TK-TD modelling for single chemicals involved in human health, animal health and ecological risk assessment. There are four main modules in the model, these are: chemical specific, physiological and life cycle trait, TK and TD modules.