Handbook - COT FSA PBPK for Regulators Workshop 2021
Handbook Cover Page - 2021 Workshop
In this guide
In this guideHandbook table of contents - 2021 Workshop
In this guide
In this guide
| About the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) | 3 |
| About the UK Food Standards Agency (FSA) | 4 |
| Notes on data and presentations at the workshop | 4 |
| Preface and workshop objectives |
6 |
| PBPK for Regulators Workshop agenda | 7 |
| Introduction to physiologically based pharmacokinetic (PBPK) modelling | 8 |
| PBPK modelling: Considerations of the COT | 14 |
| PBPK models | 14 |
| RVis | 14 |
| European Food Safety Authority (EFSA): A Web-based open source tool for toxicokinetic (TK) and toxicodynamic (TD) modelling | 14 |
| In emerging approaches | 16 |
| Cumulative Risk Assessment Approach for Mixtures | 16 |
| Current regulatory landscape | 17 |
| Questions put forward for the discussion sessions | 20 |
| Limitations / Ensuring fit for purpose | 20 |
| Potential applications | 21 |
Validation for regulatory application |
21 |
| Duties as regulators | 21 |
| Speakers biosketches | 22 |
| Professor Alan Boobis OBE | 22 |
| Dr Melvin Ernest Andersen PhD, CIH, DABT, ATS | 22 |
| Professor Mark Cronin | 23 |
| Professor Amin Rostami-Hodjegan PhD, FCP, FAAPS, FJSSX | 23 |
| Dr Alexander J. Stevens BSc, MSc, PhD | 24 |
| Dr Sheila Annie Peters | 25 |
| Dr George Loizou PhD | 26 |
| Dr Judith Madden | 26 |
| Dr Harvey J. Clewell III PhD, DABT, FATS | 27 |
| COT Members | 28 |
| COT Secretariat | 28 |
| Abbreviations | 29 |
| References | 30 |
Introduction - Handbook 2021 Workshop
In this guide
In this guideAbout the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT)
The COT assesses chemicals for their potential to harm human health and provides advice on the risks to government departments and agencies.
Scientific evaluations are carried out at the request of the Food Standards Agency, Department of Health and Social Care, Public Health England, and other Government Departments and Regulatory Authorities. The Committee’s procedures for openness include the publication of these evaluations on the internet as statements or shorter position papers; meeting agendas, finalised minutes, agreed conclusions are also published on the committees website: COT website.
The COT further provides expert advice to other advisory committees, such as the Scientific Advisory Committee on Nutrition, as well as having collaborative links with the Science Council, Veterinary Products Committee and the Expert Committee on Pesticides (formerly known as the Advisory Committee on Pesticides).
COT Members are appointed as scientific and medical experts on the basis of their specialist knowledge and expertise. In addition, two non-specialist lay members of the Committee are appointed for their broader insight into consumer affairs.
Members are required to follow the Code of Practice for Scientific Advisory Committees: Scientific advisory committees: code of practice and map of connections - GOV.UK (www.gov.uk). As part of this, they must declare any potential conflicts of interest, and depending on the nature of such conflicts, they may (at the Chair’s discretion) be excluded from the discussion and formulation of the Committee’s conclusions and recommendations in relation to relevant agenda items.
The COT is supported in its work by a Secretariat, which is provided by the Food Standards Agency and Public Health England. The Secretariat has scientific expertise which enables them to provide Members with comprehensive background information and briefing papers that inform the decision-making process of the Committee.
About the UK Food Standards Agency (FSA)
The UK FSA is an independent Government department working across England, Wales and Northern Ireland to protect public dietary health and consumers’ wider interests in food.
Our job in the FSA is to use our expertise and influence, so that people can trust that the food they consume is safe and is what it says it is.
The Science, Evidence and Research Division (SERD) of the FSA provides strategic analysis, insight and evidence across the FSA’s remit to underpin the development of policies, guidance and advice on food safety. The FSA’s approach to science is summarised on the UK FSA website.
SERD is a multi-disciplinary team of approximately 100 staff that includes scientists, risk assessors, economists, statisticians, social scientists and operational researchers who provide high quality, timely and robust evidence. We strengthen our knowledge base using a range of external science capabilities, such as our independent Scientific Advisory Committees (independent groups of experts that advise the FSA on various aspects of food safety), by commissioning research and surveys, and engaging with academia and research councils through sponsoring PhDs and post-doctorate fellowships.
Notes on data and presentations at the workshop
Oral presentations are unedited and represent the views of individual speakers and not necessarily those of the FSA, COT nor the institutions and/or employers of the speakers. Delegates are asked to note that the presentation of any data at the workshop largely represents work in progress, much of which is preliminary and as yet unpublished and therefore not in the public domain.
Delegates are asked to respect the confidential nature of the unpublished data presented at the workshop. Although the nature of this COT workshop is open, participants are requested to observe the usual courtesy of not discussing or circulating the data, or copies of it, to other parties until these data are in the public domain. As the workshop is being held remotely due to the COVID-19 pandemic, additionally we kindly request that there is no electronic recording of any of the presentation or discussions.
COT Secretariat December 2020
Preface and workshop objectives - Handbook 2021 Workshop
In this guide
In this guideThe UK FSA and the COT would like to thank you for your attendance and contributions at this workshop.
The future of food safety assessment in the UK depends on our adaptability, flexibility and revolutionary principles in order to respond to the accelerating developments in science and technology. The Tox21 approach4 is an example of one of the major recent scientific advancements in the development of alternative toxicity testing and computer modelling strategies for the evaluation of hazard and exposure. (Toxicology in the 21st Century (Tox21) is a US federal research collaboration testing thousands of environmental chemicals using non-animal methods for potential health effects. Further information is available on the Tox21 website. See also the US EPA’s website for adopting new approach methodologies). A key aspect of this strategy is linking active concentrations in vitro to likely concentrations in vivo, for which physiologically based pharmacokinetic (PBPK) modelling is essential.
The application of such alternative strategies to health risk assessment in a regulatory context requires effective collaborations between scientists including chemists, toxicologists, informaticians, computational biologists, risk assessors, and policy makers. As such, this workshop has invited speakers with varied backgrounds including; academia, industry and regulatory agencies whose collective experience is diverse and multi-disciplinary.
This workshop on PBPK modelling techniques thus provides a platform from which to address the following objectives;
- To gain a better understanding of what PBPK models are and their application to risk assessment in regulatory fields;
- Advantages and limitations of PBPK modelling;
- What must be achieved to overcome limitations for integration into current health risk assessment practices;
- An interactive session involving a model run-through and;
- Any lessons learnt from authoritative bodies or industry.
We plan to publish a summary of proceedings from this workshop (either as a COT statement output and/or in the literature).
PBPK for Regulators Workshop agenda 2nd December 2020 - Handbook 2021 Workshop
In this guide
In this guide|
Time |
Topic |
Speaker |
|
09:45-10:00 |
Welcome and Introductions |
Prof Alan Boobis |
|
10:00-10:30 |
Introduction to, and Research Needs of, PBPK modelling in Chemical Risk Assessment |
Prof Mark Cronin |
|
10:30-11:00 |
PBPK: What is all the fuss about? |
Prof Amin Rostami-Hodjegan |
|
11:00-11:15 |
Break |
Break |
|
11:15-11:45 |
Including Variability in Pharmacokinetic Modelling and Simulation Approaches to Reduce Uncertainty in Risk Assessments |
Dr Alexander J. Stevens |
|
11:45-12:15 |
PBPK applications in the pharmaceutical industry today |
Dr Sheila-Annie Peters |
|
12:15-12:45 |
Panel discussion |
Prof Alan Boobis |
|
12:45-13:25 |
Lunch |
Lunch |
|
13:25-13:30 |
Afternoon Introduction |
Prof Alan Boobis |
|
13:30-14:30 |
RVis: An Open Access PBPK Modelling Platform |
Dr George Loizou |
|
14:30-14:45 |
Break |
Break |
|
14:45-15:15 |
Review of guidance on application and reporting of PBK models in regulatory settings |
Dr Judith Madden |
|
15:15-15:45 |
Applications of PBPK modelling by regulatory agencies: Examples and lessons learned |
Dr Harvey J. Clewell III |
|
15:45-16:15 |
Panel discussion |
Dr Melvin Ernest Andersen |
|
16:15-16:45 |
Future Research Needs - Open discussion |
Speaker |
|
16:45-17:00 |
Round up and closing remarks |
Prof Alan Boobis |
Introduction to physiologically based pharmacokinetic (PBPK) modelling - Handbook 2021 Workshop
In this guide
In this guideChemical risk assessments do not routinely evaluate internal dose. However, internal dose relates more directly (temporally and spatially) to a toxicological response than external dose, and thus provides a more accurate basis for the evaluation of health risk. Furthermore, there is a need for some means to translate biologically active concentrations in vitro to the equivalent doses in vivo. Computational approaches such as the use of PBPK modelling can be used for these purposes.
PBPK models are mathematical representations of physiological processes affecting a chemical’s in vivo toxicokinetics; absorption, distribution, metabolism and excretion (ADME) or, simply put, describe the relationships between external exposure and the concentration-time profile of a chemical within the body.
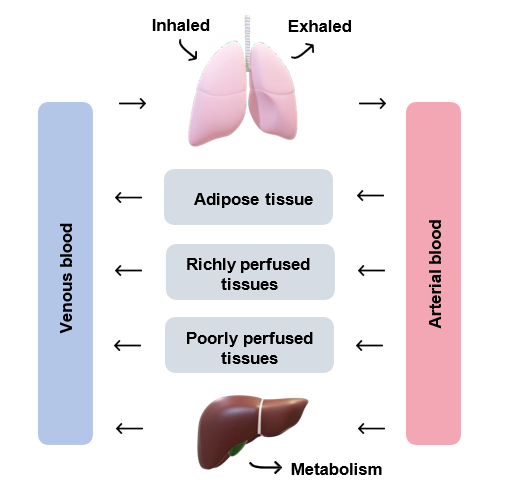
ADME processes are represented by a series of mathematical differential equations which describe the rate of change in the amount of a chemical in target tissues and blood (Figure 1).
Figure 1: a general schematic of a PBPK model (adapted from Tan et al., 2020).
The concept of PBPK modeling was first described by Thorsten Teorell in 1937 (Teorell, 1937). Each parameter in a PBPK model describes a physiological (e.g. ventilation rate), physicochemical (e.g. lipid solubility) or biochemical (e.g. metabolic Vmax) process.
The development and application of PBPK models has been described to have six steps (Rietjens et al., 2011). These are as follows:
i). Definition of the conceptual model;
ii). Translation into a mathematical model;
iii). Defining parameter values;
iv). Solving the equations;
v). Evaluation of model predictions and;
vi). Making predictions.
PBPK models are based on several general assumptions regarding ADME including: the mixing of the chemical in the effluent blood from the tissues is instantaneous and complete, blood flow is unidirectional, constant and non-pulsatile, and the presence of chemicals in the blood cells does not alter the blood flow rate. Any deviations from which should be justified and documented as recommended by the World Health Organisation (WHO) (WHO, 2010).
The WHO published the key principles and best practices for characterising and applying PBPK models in risk assessment (WHO, 2010). This was conducted within the International Programme on Chemical Safety (IPCS) project on the Harmonisation to Approaches to the Assessment of Risk from Exposure to Chemicals.
The WHO 2010 publication addressed model validation, which is required before a PBPK model can be used confidently as a tool in risk assessment. Validation is the “process by which the reliability and relevance of a particular model are established for a defined purpose” (IPCS, 2005). Validation should be conducted by considering the following factors (WHO, 2010):
i). The biological basis of the model structure and parameters;
ii). Closeness of the model simulations to actual pharmacokinetic data;
iii). Reliability of simulated dose metrics (as they relate to a specific purpose or application in risk assessment) and;
iv). Supplementary analyses of sensitivity, uncertainty and variability (might be important, depending upon the end use and extent of comparison with real life data).
The “dose metric” has been defined as the dose measure that is causally related to the toxic outcome (Andersen et al., 1987). It is more closely related with tissue response than external dose. When the direct measurement of a dose metric is unethical or not technically feasible, PBPK models can be used to simulate it.
The dose metric relates to the form of chemical (e.g. parent chemical or metabolite), its level (free or total concentration or amount), duration (instantaneous, daily, lifetime or a specific developmental period), intensity (maximum, average or integral), and the biological matrix (e.g. blood, target tissue) (US EPA, 2006).
For application to risk assessment, the model should be able to simulate the dose metric of relevance to the chemical’s mode of action (MoA). The dose metric that is estimated may correspond to an external reference dose such as the no-observed-adverse-effect- level (NOAEL) or benchmark dose level (BMDL) or another exposure scenario of interest to risk assessors. When there are several dose metrics, the appropriate one for use in risk assessment should be chosen on the basis of plausibility. The plausibility of a particular dose metric is determined by its consistency with available information on the chemical’s MoA as well as the dose-response information for the toxicological endpoint of concern (WHO, 2010).
A review by Sager et al., (2015) found that published PBPK models for pharmaceutical agents in humans (n=366 articles) were most commonly used for drug-drug interaction predictions (28%), followed by interindividual variability and general clinical pharmacokinetic predictions (23%), formulation or absorption modelling (12%), and predicting age-related changes in pharmacokinetics and disposition (10%).
PBPK models are generally complex, data and resource intensive, however, they can be highly informative, provide platforms for: integration and scaling of in vitro data, extrapolation across a wide variety of conditions (e.g. different exposure scenarios, disease states, changes with age and co-exposure to other chemicals) and enable prediction of pharmacokinetic endpoints.
PBPK modelling: Considerations of the COT
In 2003, the COT hosted a workshop on PBPK modelling. The presentations considered the use of PBPK models in risk assessment, and the requirements to allow for their incorporation in risk assessment. The presentations were followed by a general discussion which focused on the strengths and weaknesses of PBPK modelling, whether PBPK models could be integrated into risk assessments conducted by the COT, and how this might be achieved: (TOX/2003/40 Annex B).
A COT statement on PBPK modelling was published in 2003, where the COT considered PBPK modelling to be an established technique capable of predicting the in vivo behaviour of chemicals. PBPK modelling is widely used in the development and risk assessment of pharmaceutical products, where there is often sufficient human data available with which to validate the models. However, for many chemicals evaluated by COT, it was noted there are limited or no human pharmacokinetic data available that can be used for model validation. Members expressed their reservations in assessing a PBPK model that had not been validated in this way.
Furthermore, the COT considered that animal data can provide partial validation if it can be assumed, or there is evidence, that the chemical behaves similarly in animals and humans. Additionally, validation could be enhanced by mechanistic studies in experimental animals that show human relevance. However, there would be less confidence in the predictions of such models, and this would need to be expressed as a source of greater uncertainty in the risk assessment.
The Committee concluded that it would not be feasible to undertake PBPK modelling routinely for COT risk assessments because the generation and validation of a PBPK model is resource and time intensive. However, the COT agreed that relevant published PBPK models should be incorporated into risk assessments when possible, for example when submitted to support a risk assessment by industry.
In 2007, the COT held an open workshop on “Evolving Approaches to Chemical Risk Assessment”. A statement was published that summarises the presentations and Committee’s discussions. PBPK models were briefly discussed as part of the presentation on exploring uncertainty using sensitivity analysis: [ARCHIVED CONTENT] COT: COT statement on the COT workshop on evolving approaches to chemical risk assessment (nationalarchives.gov.uk).
The COT’s overall conclusions were as follows: the need to more explicitly assess and describe the uncertainty in the available data, the use of more transparent and
reproducible methods (e.g. framework approaches and systematic rather than narrative reviews).
Additionally, new technologies should be carefully adopted, and only implemented if they offer a clear benefit in terms of improving the risk assessments by the Committee. Although, where appropriate, new approaches should be initially performed in parallel with existing methods, allowing for further investigation of divergent outcomes.
In 2009, the COT held a workshop on 21st century toxicology. The workshop addressed the US National Academy of Sciences report entitled Toxicity Testing in the 21st Century: A Vision and a Strategy | The National Academies Press. A statement was published: COT statement on the COT Workshop on 21st Century Toxicology where the COT welcomed the systematic approach of the strategy for the use of in vitro and in silico approaches to better understand toxicity.
More recently, in 2019, the COT reviewed PBPK modelling used for human health risk assessment (TOX/2019/34). The discussion of the Committee focused on ways to assess the reliability of human PBPK models in the absence of human pharmacokinetic data. Approaches that were considered to assess model reliability in this context included the use of the read-across approach (Read-across is a technique for predicting endpoint information for one substance (target substance), by using data from the same endpoint from (an)other substance(s); the source substance(s) and conducting interspecies extrapolations to animal species other than humans. The Committee agreed that it would be useful to have further information in the form of case studies, where in vitro data had been successfully extrapolated to in vivo, or cases where risk assessments considered in retrospect may have benefitted from PBPK modelling.
A follow-up paper (TOX/2019/73) was then presented in response to this request. The paper summarised a number of PBPK case studies that have been used in risk assessment (PFOS & PFOA, dioxins, bisphenol A, acrylamide & glycidamide, chloroform & carbon tetrachloride, vinyl acetate, methylene chloride, and vinyl chloride), in addition to cases where in vitro to in vivo extrapolation has been conducted (PFOS, triclosan, pyridaben & fluazinam, estragole, and trichloroethylene). Furthermore, examples involving 2-butoxyethanol, persistent organic pollutants, amphetamine analogues and electronic nicotine (and non-nicotine) delivery systems devices were described where the use of PBPK modelling may have facilitated their risk assessment.
The Committee noted that PBPK models have predominantly been developed and applied on a case-by-case basis, for example to assess exposures of chemicals with narrow margins of exposure or to fill in data gaps from more conventional approaches.
The Committee recognised that PBPK modelling is of current interest to regulators in the field of chemical risk assessment; however, it is still largely used more in research capacities to refine estimates of health risk. PBPK models are not routinely applied or assessed by regulatory bodies because they are generally complex and both labour and data intensive, for example in terms of the data required for model parameterisation.
However, despite the multitude of case-specific PBPK models, systems are being developed to enable generic PBPK models to be generated. Software platforms such as these may be used in conjunction with the read-across approach to assess human health risks without the need for animal testing.
In March 2020, the FSA COT held a workshop entitled “Exploring Dose Response” which was attended by scientists from regulatory agencies, government bodies, academia and industry. The workshop provided a platform from which to address and enable expert discussions on the latest in silico prediction models, new approach methodologies, PBPK modelling, future methodologies, integrated approaches to testing and assessment, as well as validation of methodology. Several case studies involving plastic particles, polymers, tropane alkaloids, and selective androgen receptor modulators were used to explore approaches fit for purpose in the context of future food safety assessment. Furthermore, possible future research initiatives were discussed, such as establishing points of departures using non-animal alternative models and improving use of exposure metrics in risk assessment.
The workshop report (TOX/2020/30) was discussed as reserved business in July 2020, as it is hoped to publish a peer reviewed paper. The key issues identified in respect to PBPK models would be further developed through the current workshop.
PBPK models
The below examples of PBPK models is not an exhaustive list but rather provides detail on some that are publicly available.
RVis
Rvis is a prototype, proof of concept application for the analysis of structure and performance of PBPK and other models, written in the free, open source syntax R or C++ developed by the Health Safety Laboratory with funding from the European Partnership for Alternative Approaches to Animal testing and the Health and Safety Executive. Further information is available on the Cefic website.
It is a general-purpose modelling platform, not just an in vitro and in vivo exposure predictor. The features of Rvis include the ability to load, run, visualise and plot graphical outputs from models. Model structures may be analysed using parameter elementary
effects screening and global sensitivity analysis for any species (e.g. human, rodent, farm animals), and parameter estimation using Markov Chain Monte Carlo simulation and Bayesian inference. The parameter estimation feature is used to perform “reverse dosimetry” to reconstruct human dose or exposure concentrations consistent with human biomonitoring data.
Rvis and a user-guide are available to download from the Rvis repository hosted on GitHub: GitHub - GMPtk/RVis: Open access PBPK modelling platform. Users can download the latest version and post issues that arise that should be addressed.
European Food Safety Authority (EFSA): A Web-based opensource tool for toxicokinetic (TK) and toxicodynamic (TD) modelling
An EFSA external report on a Web-based open source tool for TK and TD modelling has been recently published in November 2020 (Bossier et al., 2020). The software has been developed in R as a web-based tool that includes different components for the modelling of TK and TD processes in a structured workflow.
This workflow provides steps to perform TK-TD modelling for single chemicals involved in human health, animal health and ecological risk assessment. There are four main modules in the model, these are: chemical specific, physiological and life cycle trait, TK and TD modules.
In emerging approaches - Handbook 2021 Workshop
In this guide
In this guideCumulative Risk Assessment Approach for Mixtures
Pletz et al., (2020) investigated the suitability and limitations of generic PBPK models (IndusChemFate (ICF) tool Cefic website and High-Throughput Toxicokinetics (Httk) package US EPA website) in deriving biomonitoring equivalents for phenols (bisphenol A, Triclosan and benzophenone-3), phthalates (di-n-butyl phthalate and butylbenzyl phthalate) and parabens (methyl paraben, ethyl paraben, n-Propyl paraben, n-Butyl paraben) with a view to facilitating the use of human biomonitoring (HBM) data in the assessment of chemical mixtures at a screening level (i.e. establishing safe levels in urine or blood against which measure HBM values can be compared).
In brief, the methodology consisted of seven steps:
i). The selection of HBM data - Danish children and on Norwegian mothers and children;
ii). PBPK model selection – ICF and Httk package;
iii). Selection of chemicals to simulate including a literature search for health-based guidance values and selection of physiological parameters in the model;
iv). Forward dosimetry;
v). Evaluation of modelling results;
vi). Application in a case study for a single substance risk assessment and;
vii). Application in a case study for mixture risk assessment with outputs from the Httk analysis.
The authors noted both advantages and limitations of both PBPK models. For ICF, the main advantage was that the model included features for inclusion of metabolism, however, it required a substantial number of input parameters which were not readily available within the literature. For Httk, the advantage was that it had an in-built library of relevant parameters covering many chemicals and thus was considered to be more user- friendly, however, in the version tested (version 1.8), metabolism was only addressed via intrinsic clearance and thus predictions of metabolite concentrations could not be included.
It was concluded that the application of PBPK models provided a greater understanding and interpretation of HBM data. Although, the establishment of safety thresholds in urine for the compounds tested was difficult and complex. Model refinement was also recommended to reduce uncertainties (regarding metabolite concentrations) and improve predictions.
Current regulatory landscape - Handbook 2021 Workshop
In this guide
In this guideThe use of PBPK models has increased over the last several decades throughout various working sectors, including academia and industry. Particularly in conjunction with other emerging alternative methods to in vivo animal testing (e.g. in vitro studies and data- driven in silico quantitative-structure-activity-relationship (QSAR) predictions), where the generated data allows for increased confidence in models for chemicals without in vivo data for model calibration.
Despite this growing use and advantages offered by these applications, there remains some hesitation from public health and other regulatory agencies for the integration of these models for use in the risk assessment process due to lack of harmonised guidance, human data, or expertise in computational modelling (Paini et al., 2017).
In Europe, the European Union Reference Laboratory for Alternatives to Animal Testing (EURL-ECVAM) have recently published a status report on the Development, Validation and Regulatory Acceptance of Alternative Methods and Approaches in March 2020 (JRC news and updates - European Commission (europa.eu) in which the Rvis platform was mentioned. International cooperation with the Health and Environmental Sciences Institute (HESI) PBPK Models Committee (HESI website.) was also summarised. It was mentioned that there are two main efforts ongoing from this Committee. Firstly, the establishment of a harmonised template to report information, and provide recommendations to model reviewers to facilitate the uptake of PBK models in regulatory risk assessment and secondly, the development of a framework and decision tree on PBPK applications based on different degrees of data availability.
The proposed PBPK model reporting template has now been published (Tan et al., 2020). In brief, the authors expanded the existing guidance designed for pharmaceutical applications (WHO, 2010; US FDA, 2018; EMA, 2019) by recommending additional elements that are relevant to environmental chemicals. There are 8 main sections which includes 3 to 8 sub-sections:
i). Executive summary;
ii). Background/Introduction – chemical’s physicochemical, PK and PD properties; known exposure, toxicity and efficacy; PBPK-related regulatory history; cross- referencing other PBPK efforts; relevant data used for model calibration; relevant data used for model evaluation;
iii). Model purpose;
iv). Materials and methods – modeling strategy; summary of data for model development and evaluation; model development and structure; model equations; model parameters; model simulations; software;
v). Results – model evaluation; sensitivity, uncertainty, and variability analyses; model applicability;
vi). Discussions and conclusions;
vii). Electronic files and Supporting Documents and;
viii). Appendices.
The authors note that the template can be adapted and customised, as well as serving as a general guidance for submitting PBPK-related studies for publication in journals or other modeling sharing purposes. The authors hoped that the use of the template will help in standardising PBPK model reporting and communication and thereby enhance their application and regulatory acceptance.
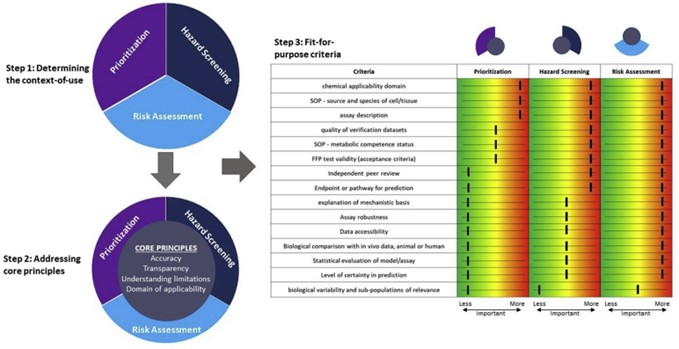
Considering the bigger picture, PBPK modelling is part of new approach methodologies (NAMs) for human health and safety assessment. An evaluation framework guideline for evaluating NAMs has been published by Parish et al., (2020), which is comprised of three steps. These are: determining the context of use (i.e. will the NAM be used in prioritization, hazard screening or risk assessment), addressing the core principles (which must be addressed, irrespective of the context-of-use) and the fit for purpose criteria. Figure 2 provides a schematic representation of the framework.
Figure 2: a schematic representation of the three steps of the evaluation framework for new approach methodologies (NAMs) as recommended by Parish et al., (2020) (reproduced from Parish et al., 2020).
The authors note that, their recommendations do not constitute regulatory guidance and are not meant to supersede or supplant any existing regulatory policy or address how NAMs could be implemented. The framework contextualizes the importance of the derived criteria from regulatory guidance (e.g. the Organisation for Economic Cooperation and Development (OECD) Guidance Document No.34 (OECD, 2005)), in such a way that NAM evaluations can be performed based on their level of importance (i.e. high or low), which is driven by the context-of-use. There is emphasis on ensuring that a NAM is fit for its intended purpose, as determined by problem formulation.
In this context, the application of PBPK models are considered under the ‘explanation of mechanistic basis’ criteria, where there are current efforts on supporting the integration of toxicokinetics into in vitro evaluations of toxicodynamics. Methods that enable in vitro to in vivo extrapolation (IVIVE) are necessary to accurately estimate relevant human exposures that correspond to observed in vitro bioactivity. The use of IVIVE approaches with PBPK modelling was suggested by the authors to quantitatively bridge in vitro and in vivo data and to explore the key mechanisms dictating the pharmacokinetics. Combining in vitro methods with appropriate exposure data will improve applicability in a risk assessment framework and thus allow specific consideration with regard to route of exposure, target-specificity, and the potential for human extrapolation.
Questions put forward for the discussion sessions - Handbook 2021 Workshop
In this guide
In this guideLimitations / Ensuring fit for purpose
- What are (if there are any), the limitations of using PBPK modelling in an agrochemical/pharmacological setting?
- ·Can PBPK models fully replace animal testing, or are there some cases where animal studies may still be required?
- Are there any circumstances where we can use simpler in silico compartmental models versus PBPK?
Potential applications
- Can PBPK models be used to provide relevant substances to benchmark against known human biomonitoring data?
- Exploration into intracellular dosing.
- Could PBPK modelling be used to convert estimates of external exposure into an estimate of internal exposure at the site where toxicity occurs to refine estimates of risk?
- PBPK modelling provides a way to incorporate kinetics into consideration in animal-free, in vitro based safety/risk assessment and to relative in vitro toxicity assay findings to human safe exposure estimates.
- Can PBPK models lower the reliance on default uncertainty factors and would it reduce this uncertainty?
Validation for regulatory application
- Are there any harmonised guidelines available for regulators?
- Have there been any cases where there has been a human PBPK model developed without human data? If so, how was the model validated (if at all)?
- What are some of the hurdles to PBPK modelling being used more widely by scientists, and accepted by regulators?
Duties as regulators
- What aspects of the model do regulators have to check before it can be used in risk assessment?
- Are regulators expected to use PBPK models (for example, to double-check calculations, to examine the source code) or can regulators just take simulation results at face value?
- How could PBPK modelling be used more extensively in food safety assessment?
- Is the integration of PBPK models into current human health assessment methodologies a risk worth embracing?
Speakers biosketches - Handbook 2021 Workshop
In this guide
In this guideProfessor Alan Boobis OBE
Alan Boobis is emeritus Professor of Toxicology, Imperial College London. He retired from his position at the College as Professor of Biochemical Pharmacology ad Director of Public Health England/Department of Health-supported Toxicology Unit in June 2017, after over 40 years. His main research interests lie in in mechanistic toxicology, drug metabolism, mode of action and chemical risk assessment. He has over 470 publications (H-factor 80). He is or has been a member of several national and international advisory committees; including former member of the UK Committee on Carcinogenicity, vice-chair of the EFSA Panel on Plant Protection Products and their Residues, and member of the EFSA Panel on Contaminants; current chair of the UK Committee on Toxicity, member of the UK Committee on Medical Effects of Air Pollutants, member of the WHO Study group on Tobacco Product Regulation (TobReg), member and sometime chair of FAO/WHO JECFA (veterinary residues) and member and sometime chair of FAO/WHO JMPR (pesticide residues). He is a member and previous chair of the Board of Trustees of ILSI (International Life Sciences Institutes), member and previous president of ILSI Europe and a member and previous chair of the HESI Board of Trustees. He is involved in several ILSI Europe projects. He has been elected fellow of several learned societies, including honorary fellow of the British Toxicology Society (BTS) and has received a number of awards recognising his contributions to toxicological sciences, including the BTS John Barnes Prize Lectureship, Royal Society of Chemistry Toxicology Award, the Arnold J Lehman Award of the US Society of Toxicology, the EuroTox Merit Award, the Toxicology Forum Philippe Shubik Distinguished Scientist Award and the civilian award of Officer of the British Empire (OBE).
Dr Melvin Ernest Andersen PhD, CIH, DABT, ATS
Now semi-retired, Dr Andersen (Mel) is Senior Fellow at ScitoVation LLC, Durham, NC. He has worked in toxicology and risk assessment since 1971. His career has primarily focused on developing biologically realistic models of the uptake, distribution, metabolism, and biological effects of various chemicals and applying these models in safety assessments and quantitative health risk assessments. His primary area of work was physiologically based pharmacokinetic modeling (PBPK). Over his career, he has worked with a remarkably capable group of colleagues and collaborators and with them has produced over 500 papers and book chapters. He has received two career achievement awards - the Mildred S. Christian Award (Academy of Toxicological Sciences, 2016) and the Merit Award (2016) from the Society of Toxicology. From 2004 through 2007, he was a member of a US National Academy of Sciences Committee on toxicity testing of environmental agents, outlining future directions to move to testing based on new alternative methods (NAMs) rather than reliance on intact animal models. Mel still pursues research using gene expression analysis and pharmacokinetics to better understand modes of action of compounds both in intact animals and in cells in vitro.
Professor Mark Cronin
Mark Cronin is Professor of Predictive Toxicology at the School of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Liverpool. He has over 30 years’ experience in the application of in silico approaches to predict the toxicity and fate of chemicals; in addition to development of strategies to develop alternatives to whole animal testing for toxicity. His current research includes the application of chemical grouping and read-across to assess human health and environmental endpoints, particularly the linking of Adverse Outcome Pathways (AOPs) to category information. This research effort has resulted in four books and over 270 publications in all areas of the use of (Q)SARs, expert systems and read-across to predict toxicity. He has worked in numerous projects in this area including more than ten EU framework projects, as well as assisting in the uptake of in silico methods for regulatory purposes.
Professor Amin Rostami-Hodjegan PhD, FCP, FAAPS, FJSSX
Amin is the Director of the Centre for Applied Pharmacokinetic Research (CAPKR) at the University of Manchester and a Professor of Systems Pharmacology with an active program of training PhD students in proteomics, PBPK, clinical PKPD and precision dosing. Graduates from his team hold positions in the pharmaceutical industry or academia.
Professor Rostami has authored/co-authored over 280 highly cited articles (>15,500 citations, H-Factor = 68). In 2017, he was listed by ISI as one of the world’s most highly cited researchers (under ‘Pharmacology & Toxicology’). He was a founding editor of Pharmacometrics and System Pharmacology and serves on the Editorial Boards of several other journals (e.g. BDD, CDM, CPDD, DMPK). Professor Rostami is renowned for his contribution to translational modelling (e.g. PBPK) and has been an invited speaker at over 200 international and national meetings, in addition to leading numerous workshops in the area of IVIVE- PBPK linked models.
Amin is also the Senior Vice President of Research & Development and Chief Scientific Officer at Certara, where he ensures that various pharmaceutical companies incorporate the latest scientific advances in the field of biosimulation into their drug development efforts.
Dr Alexander J. Stevens BSc, MSc, PhD
Alex Steven’s roles in industry have focused on establishing concentration-effect relationships via the application of pharmacokinetics (and where possible integrated pharamacodynamic studies) to aid in the translation to the human situation to better inform on human health risk assessments. His expertise was developed and applied within preclinical and clinical drug development settings and also within the agrochemical industry where he has worked for Syngenta for the past 11 years.
He currently works in Crop Protection Research providing Product Safety input (both human and environmental safety) as part of multidisciplinary Research Portfolio Teams who manage and guide the research projects to bring forward candidate compounds for development. Prior to this, he was responsible for the ADME and Toxicokinetics skillset working in support of discovery and regulatory projects across the research portfolios. Previous positions held include: over 8 years at GlaxosmithKline in pre-clinical DMPK within the Immunoinflammation and the Neurology & Gastrointestinal Centres of Excellence for Drug Discovery and 3 years as a pharmacokineticist at Medeval Ltd. where he was responsible for primary pharmacokinetic, adverse event and statistical analysis of Phase I Clinical Studies for the pharmaceutical industry. This was preceded by 6 years in the pharmacokinetics group in the Department of Pharmacy, University of Manchester where he obtained MSc and PhD degrees and 3 years as a clinical biochemist at Glenfield General Hospital, Leicester where he was involved in therapeutic drug monitoring. All of the above was built upon the foundation provided by a BSc degree in Pharmacology obtained from Sunderland Polytechnic graduating in 1986.
Dr Sheila Annie Peters
Sheila Annie Peters is currently the Head of Translational Quantitative Pharmacology Group at Merck KGaA, Darmstadt, where she has contributed to the R&D strategy 2023 at Merck and is currently part of the strategy implementation team. Her areas of expertise include physiologically-based pharmacokinetic (PBPK) drug absorption, translational PK/PD, clinical pharmacology and drug-drug interactions. She is the European Federation of Pharmaceutical Industries and Associations (EFPIA) Topic leader for the ICH (International Council for Harmonisation) M12 group focused on harmonising drug- drug interaction (DDI) guidelines.
Previously, she worked for AstraZeneca, Mölndal, where she has developed a generic whole-body PBPK model in MATLAB® which she used to support several drug discovery and early development projects across different R&D sites with innovative approaches to identifying potential limitations to drug disposition. She successfully implemented Model- based drug discovery (MBDDx) strategy in Respiratory Inflammation and Autoimmunity iMed through cross-functional collaborations. She wone the 2013 IMED (Innovative Medicines) Science Award at AstraZeneca for the “Design and Development of LungSim Simulation tool for Inhalation PK modelling”.
She has published several papers in high impact journals as well as a book on PBPK modelling. As part of the IQ Consortium, she co-authored a White Paper on PBPK and continues to work with the on various topics of interest in DMPK and Clinical Pharmacology.
Dr George Loizou PhD
George Loizou is a computational toxicologist with over 36 years’ experience in quantitative, mechanistic, chemical toxicology. For the past 23 years, George has been engaged in strategic research for the Health & Safety Executive (HSE) and external customers investigating whether computational tools can be designed to exploit new technologies and mathematical modelling to provide a biologically based, quantitative chemical risk assessment.
This work had focused on the use of physiologically-based pharmacokinetic (PBPK) modelling to analyse, quantify and explain toxicological data with the ultimate aim of replacing the current slow, inefficient and expensive animal-based chemical risk assessment paradigm. For the past 4 years, George had also been investigating developments of personalised medicine where data obtained in people may potentially be appropriate for occupational and environmental toxicology. The use of gene expression (transcriptomics), metabolite (metabolomics) data and bioinformatics could lead to the development of a ‘next generation’ approach to chemical risk assessment based on human data.
Dr Judith Madden
Dr Judith Madden is a Reader in Molecular Design in the School of Pharmacy and Biomolecular Sciences at Liverpool John Moores University (LJMU, UK). She undertook a Ph D in computer-aided drug development (at LJMU) and post-doctoral research in the area of pharmacokinetics (University of Manchester). Her research interests are in the application of in silico methods to predict the effects of chemicals, on humans and environmental species. Research is directed towards predicting, both the interaction of a chemical with its biological target and the potential of a chemical to reach site of action.
Hence, her research encompasses the use and evaluation of in silico tools (such as (quantitative) structure-activity relationships ((Q)SARS) and read-across to predict biological activity/toxicity, pharmacokinetic/toxicokinetic-relevant properties and methods to inform the development of physiologically based kinetic (PBK) models.
Dr Harvey J. Clewell III PhD, DABT, FATS
Harvey Clewell is currently a Principal Consultant with Ramboll US Consulting, Inc. He was previously the Director of the Center for Human Health Assessment at The Hamner Institutes for Health Sciences. He received a master’s degree in chemistry from Washington University, St. Louis, and a PhD in Toxicology from the University of Utrecht, the Netherlands. He is a Diplomate of the American Board of Toxicology and a Fellow of the Academy of Toxicological Sciences. He has more than forty-five years of research experience in environmental transport, toxicology and chemical risk assessment, and has authored more than 200 peer-reviewed scientific publications and book chapters. He played a seminal role in the incorporation of pharmacokinetic and mode of action information in chemical risk assessments, having contributed to the first applications of physiologically based pharmacokinetic (PBPK) modeling in risk assessments by the USEPA, USFDA, ATSDR, OSHA and Health Canada. In 2007, the Society of Toxicology recognized Clewell with the Arnold J. Lehman Award for his major contributions to chemical safety and risk assessment. Dr Clewell is currently a member of the Chemical Assessment Advisory Committee of the USEPA’s Scientific Advisory Board. He also served as a member of the ECVAM Scientific Advisory Committee from 2012 to 2016.
COT Members and Secretariat - Handbook 2021 Workshop
In this guide
In this guideCOT Members
Professor Alan Boobis (Chair)
Dr Phil Botham
Ms Jane Case
Dr Stella Cochrane
Dr James Coulson
Dr René Crevel
Professor John Foster
Dr Caroline Harris
Professor Gary Hutchinson
Dr Sarah Judge
Professor Gunther Kunhle
Dr David Lovell
Dr Mac Provan
Ms Juliet Rix
Dr Michael Routledge
Dr Cheryl Scudamore
Dr Natalie Thatcher
Professor Mireille Toledano
Professor Faith Williams
Professor Philippe Wilson
Professor Matthew
Wright Professor Maged Younes
COT Secretariat
Dr David Gott
Ms Cath Mulholland
Dr Alexander Cooper
Dr Barbara Doerr
Ms Jocelyn-Frimpong Manso
Dr Douglas Hedley
Ms Frances Hill
Ms Cleanncy Hoppie
Mr Barry Maycock
Dr Olivia Osborne
Ms Claire Potter
Dr Joseph Shavila
Ms Chloe Thomas
Ms Sabrina Thomas
Ms Chara Tsoulli
Ms Frederique Uy
Abbreviations and References - Handbook 2021 Workshop
In this guide
In this guideAbbreviations
|
ADME |
Absorption, Distribution, Metabolism and Excretion |
|
AOP |
Adverse Outcome Pathway |
|
ATSDR |
Agency for Toxic Substances and Disease Registry |
|
BMDL |
Benchmark Dose Level |
|
COT |
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment |
|
DDI |
Drug-drug interaction |
|
EFPIA |
European Federation of Pharmaceutical Industries and Associations |
|
EFSA |
European Food Safety Authority |
|
EMA |
European Medicines Agency |
|
EURL-ECVAM |
European Union Reference Laboratory for Alternatives to Animal Testing |
|
FSA |
Food Standards Agency |
|
HBM |
Human Biomonitoring |
|
HESI |
Health and Environmental Sciences Institute |
|
Httk |
High-Throughput Toxicokinetics |
|
ICF |
IndusChemFate |
|
ICH |
International Council for Harmonisation |
|
ILSI |
International Life Sciences Institute |
|
IMED |
Innovative Medicines |
|
IPCS |
International Programme on Chemical Safety |
|
ISI |
The Institute for Scientific Information |
|
JECFA |
Joint Food and Agriculture Organisation/World Health Expert Committee on Food Additives |
|
JMPR |
Joint Food and Agriculture Organisation/World Health Expert Committee on Pesticide Residues |
|
LJMU |
Liverpool John Moores University |
|
MoA |
Mode of Action |
|
NAMs |
New Approach Methodologies |
|
NOAEL |
No-observed-adverse-effect-level |
|
OECD |
Organisation for Economic Cooperation and Development OSHA |
|
OECD |
Organisation for Economic Cooperation and Development OSHA |
|
PBPK |
Physiologically based Pharmacokinetic Modelling |
|
PFOA |
Perfluorooctanoic acid |
|
(Q)SAR |
(Quantitative-) Structure Activity Relationship SERD |
|
TD |
Toxicodynamic |
|
TK |
Toxicokinetic |
|
US EPA |
United States Environmental Protection Agency US FDA |
|
TD |
Toxicodynamic |
|
WHO |
World Health Organisation |
References
Andersen, M. E., Clewell III, H. J., Gargas, M. L., Smith, F. A. and Reitz, R. H. (1987) Physiologically based pharmacokinetics and the risk assessment process for methylene chloride. Toxicology and Applied Pharmacology 87(2), pp. 185-205.
Bossier, H., Chau, J., Ndour, C., Varewyck, M., Verbeke, T. and Vergucht, S. (2020) A Web-based open source tool for Toxicokinetic and Toxicodynamic modelling. EFSA Supporting Publication: EN-1926. Available at: A Web‐based open source tool for Toxicokinetic and Toxicodynamic modelling | EFSA (europa.eu). Accessed: 17/11/2020.
IPCS. (2005) Principles of characterizing and applying human exposure models. Geneva, World Health Organisation, International Programme on Chemical Safety. Harmonisation Project Document No. 3; pp.67. Available at: Principles of characterizing and applying human exposure models (who.int) Accessed: 28/10/2020.
OECD. (2005) Guidance document on the validation and international acceptance of new or updated test methods for hazard assessment. OECD Series on Testing and Assessment Number 34. OECD Guidance Document 34: Validation and International Acceptance of New or Updated Internationally Acceptable Test Methods for Hazard Assessment (nih.gov) Accessed: 17/11/2020.
Paini, A., Leonard, J. A., Kliment, T., Tan, Y-M. and Worth, A. (2017) Investigating the state of physiologically based kinetic modelling practices and challenges associated with gaining regulatory acceptance of model applications. Regulatory Toxicology and Pharmacology 90, pp. 104-115.
Parish, S. T., Aschner, M., Casey, W., Corvaro, M., Embry, M. R., Fitzpatrick, S., Kidd, D., Kleinstreuer, N. C., Lima, B, S., Settivari, R. S., Wolf, D. C., Yamazaki, D. and Boobis, A. (2020) An evaluation framework for new approach methodologies (NAMs) for human health safety assessment. Regulatory Toxicology and Pharmacology 111, 104592.
Pletz, J., Blakeman, S., Paini, A., Parissis, N., Worth, A., Andersson, A-M., Frederiksen, H., Sakhi, A. K., Thomsen, C. and Bopp, S. K. (2020) Physiologically based kinetic (PBK) modelling and human biomonitoring data for mixture risk assessment. Environment International 143, 105978.
Rietjens, I. M. C. M., Louisse, J. and Punt, A. (2011) Tutorial on physiologically based kinetic modelling in molecular nutrition and food research. Molecular Nutrition and Food Research 55(6), pp. 941-956.
Sager, J. E., Yu, J., Raguneneau-Majlessi, I. and Isoherran, N. (2015) Physiologically based pharmacokinetic (PBPK) modelling and simulation approaches: A systematic review of published models, applications, and model verification. Drug Metabolism and Disposition 34; pp. 1823-1837.
Tan, Y-M., Chan, M., Chukwudebe, A., Domoradzki, J., Fisher, J., Hack, C. E., Hinderliter, P., Hirasawa, K., Leonard, J., Lumen, A., Paini, A., Qian, H., Ruiz, P., Wambaugh, J., Zhang, F. and Embry. M. (2020) PBPK Model reporting template for chemical risk assessment applications. Regulatory Toxicology and Pharmacology 115, 104691.
Teorell, T. (1937) Kinetics of distribution of substances administered to the body. I & II. Archives internationales de pharmacodynamie et de therapie 57, pp. 205-240. ISSN 0003-9780.
US EPA. (2006) Approaches for the Application of Physiologically Based Pharmacokinetic (PBPK) Models and Supporting Data in Risk Assessment (Final Report). U.S. Environmental Protection Agency, Washington, D.C., EPA/600/R-05/043F, 2006: Approaches For the Application of Physiologically Based Pharmacokinetic (PBPK) Models and Supporting Data In Risk Assessment (Final Report) | Risk Assessment Portal | US EPA Accessed: 28/10/2020.
WHO. (2010) Guidance on principles of characterizing and applying PBPK models in risk assessment: Characterization and application of physiologically based pharmacokinetic models in risk assessment (who.int) Accessed: 26/10/2020.