COT Evaluations 2021
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.The potential risk(s) of combined exposure to mycotoxins
1.1 The Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) has identified the potential risk(s) from combined exposure to mycotoxins as a possible concern during their review of mycotoxins in the diet of infants and young children.
1.2 Mycotoxins are secondary metabolites produced by plant fungi under particular climate and biological conditions and can cause adverse health effects in both humans and animals. Those of greatest concern to human health are produced by several groups of filamentous fungi, namely Aspergillus, Fusarium and Penicillium species.
1.3 Mycotoxins are stable, low-molecular weight chemicals and are often not affected by food processing (e.g., cooking).
1.4 Cereals (e.g. wheat, oats, rice, corn (maize), barley, sorghum, rye, and millet) are often the crops most severely affected; however, some nuts, fruits and spices can also be affected.
1.5 Advances in analytical techniques have allowed the simultaneous detection and quantification of multiple mycotoxins in both food and animal feed.
1.6 Climate change could have a significant impact on mycotoxin production. Changes in the climate are expected to affect levels of rainfall, humidity, temperature etc., which in turn, influence mycotoxin production, which varies for each individual pathogen species and/or strain.
1.7 Current government and industry regulations are usually based on assessing the risks from individual mycotoxins and, at most, group metabolites with the parent compound, but take no account of the varied dynamics and potential interactions between co-occurring groups of mycotoxins.
1.8 In light of this, new combinations of factors (mycotoxins/host plants and geographical location) will need to be considered when assessing the potential risk(s) from dietary exposure to mycotoxins.
1.9 Based on the available information, the COT was unable to complete a risk assessment on the potential risk(s) from combined exposure to mycotoxins for several reasons. These include:
- A lack of harmonisation of approaches/methodologies and data analysis/modelling for toxicological investigations.
- The underlying mechanisms of interactions between individual mycotoxins in different combination(s) have yet to be fully understood.
- There is little information on the potential toxic effect(s) of mycotoxin mixtures on the gut microbiota.
1.10 Considerations for possible co-exposures from breastmilk and weaning foods also need to be considered for infants and young children.
1.11 Co-occurrence data in food is scarce, and the available methods for multi-mycotoxin detection in food samples are still not harmonised for use in a regulatory setting. In addition to this the following need further consideration for a robust exposure assessment:
- The management data for which the true values are below the limit of detection and could not be accurately determined.
- The consistent and well-defined use of probabilistic models and methodologies for multi-biomarker studies that estimate levels of exposure to multiple mycotoxins in biological samples (e.g. urine).
1.12 The COT noted that there was a lack of UK specific data, particularly in biomonitoring; however, there were a number of studies ongoing and additional information will be available in the future. The Public Health England Secretariat informed COT Members that the UK will not be collecting new data for mycotoxins under the Human Biomonitoring for the European Union Initiative; however, in the future, more data could be obtained through Health Protection Research Units. The results of such research would be of potential value in the risk assessment of co-exposures to mycotoxins.
1.13 COT Members recommended that as a pragmatic first step, a review should be carried out of the mycotoxins that appeared to show a common effect on protein synthesis (i.e., DNA or RNA synthesis), assuming dose additivity, and that frequently co-occur in food commodities – an exposure estimate could be performed and the estimates compared with the recommended health-based guidance values to calculate the Margin of Exposure or the Hazard Index utilised, to determine whether there is any potential concern from co-exposure to these mycotoxins in UK consumers.
1.14 Depending on the outcome of this screening risk assessment, research may be needed on those mycotoxins affecting ribosomal protein synthesis, to determine whether they do in fact exhibit dose additivity in their effects, to help develop a reliable basis for their cumulative risk assessment.
The full COT statement, including references, can be found on the COT website: Statement on the potential risk(s) of combined exposure to mycotoxins 2021.
Overarching statement on the potential risks from exposure to microplastics
1.15 As part of horizon scanning, the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) identified the potential risks from microplastics as a topic it should consider. Upon review of the literature, it was decided that nanoplastics should also be included. An initial scoping paper was presented to the COT in October 2019 (TOX/2019/62). Since then, the topic and additional information has been discussed several times by COT with the final substantive discussion in December 2020.
1.15 The purpose of this overarching statement is to bring together these discussions, summarise the COT conclusions reached to date and provide a high-level overview of the current state of knowledge, data gaps and research needs with regards to this topic.
1.16 Future sub-statements, which will consider in detail the potential toxicological risks of exposure to microplastics via the oral and inhalation routes, are intended to provide supplementary material for this overarching statement. The Committee will review the potential risks from oral exposure of microplastics (resulting from their presence in food and bottled drinks). A review of the potential risks of microplastics via the inhalation route will be produced jointly with the Committee of Medical Effects of Air Pollutants (COMEAP) Secretariat at Public Health England. The need for additional reviews of other significant routes of exposure will also be considered.
1.17 Micro- and nanoplastics are widespread. They are either intentionally added to products or occur as a result of plastics being fragmented down into smaller sizes by natural processes such as wear, weathering and corrosion. There is no internationally agreed definition of what a microplastic is, however, the most widely used size range is 0.1 to 5,000 µm. Plastic particles that are smaller than the lower range are considered nanoplastics (i.e. 1 nm to 0.1 µm).
1.18 The COT noted that there is little data on the effects of microplastics on mammals (including humans) whether taken in orally or via inhalation. Some microplastics are excreted from the body (~>90%) but small amounts of others may remain in the gut (gastrointestinal tract; GIT) or move from the GIT into organs or tissues (via endocytosis by M cells and paracellular persorption). No epidemiological or controlled dose studies that evaluated the effects of orally ingested microplastics in humans were identified. There is a similar lack of information on inhaled microplastics.
1.15 As such, the COT concludes that based on the available data, it is not yet possible to perform a complete assessment for the potential risks from exposure to micro and nanoplastics via the oral and inhalation routes. However, the Committee concurs with the conclusions reached by other authoritative bodies (EFSA, 2016; WHO, 2019; SAPEA, 2019; SAM, 2020; ECCC and HC, 2020) that further research is required to better identify target tissues, threshold doses, and the toxic mode(s) of action for any toxicity observed.
1.17 The COT concluded that the literature data on exposure to particles from tyre wear would need separate consideration from microplastic exposure from food, since the particles were chemically quite different (in their polymeric nature). Risk assessment of such material was considered potentially outside the scope of the current exercise.
1.18 The most significant data gaps are the lack of appropriate and harmonised analytical methods for the detection of micro- and nanoplastics (together with suitable reference standards), as well as information on their toxicokinetic and toxicity profiles in/relevant to humans.
1.19 The COT highlighted that additional information will be needed from all exposure sources, which include indoor and outdoor air, dust and soil, before a risk assessment can be completed. The presence of MPs in food and water needs to be put into perspective with other sources of MPs such as atmospheric fallout.
1.20 Comprehensive assessment of microplastics and contaminant concentrations in different foods and the impact of cooking (on the release of and subsequent bioavailability of contaminants/leachates) need to be further investigated to better understand the implications for human health.
1.21 Current studies typically focus only on one type of particle/tissue interaction. As such, further research is necessary to explore the effects of the range of particle types in different tissues in silico, in vitro and in vivo. The range of particle types studied should also take account of emerging/novel plastic-based materials such as bioplastics.
The full COT statement, including references, can be found on the COT website: Microplastics Overarching Statement 2021.Page Break
Sub-statement on the potential risk(s) from exposure to microplastics: Oral route
1.22 The purpose of this sub-statement is to provide supplementary material to the overarching statement (COT Statement 2021/02) and to consider in detail the potential toxicological risks of exposure from microplastics ingested via the oral route (i.e. resulting from the presence of microplastics in food, drinking water and bottled drinks).
1.23 The COT noted that there are limited data regarding the toxicokinetic fate of orally ingested microplastics in mammalian species, and that microplastic particles can either translocate from the gastrointestinal tract (GIT) into organs or tissues (via endocytosis by M cells and paracellular persorption), and/or be excreted. The extent to which retention in the mammalian GIT tract is of concern, if at all, is not yet clear. No epidemiological or controlled dose studies in which the effects of orally ingested microplastics in humans have been evaluated were identified.
1.24 As such, the COT concludes that based on the available data, it is not yet possible to perform a complete assessment for the potential risks from exposure to micro and nanoplastics to humans via the oral route. It should be noted that the COT’s conclusions are consistent with those reached by other authoritative bodies, as described in the COT overarching statement on the potential risks from exposure to microplastics; COT Statement 2021/02; please refer to paragraphs 101-129).
1.25 The COT previously considered the extent to which exposure to tyre wear (a source of synthetic polymeric material) might contribute to the total burden of adverse effects of nano- and microplastics (NMPs) in humans (Annex B of TOX/2020/15). The COT concluded, however, that the literature data on exposure to particles from tyre wear would need separate consideration from microplastic exposure from food, since the particles were chemically quite different in their polymeric nature. Risk assessment of such material was considered to be outside the scope of the current exercise.
1.26 The most significant data gaps are the lack of appropriate and harmonised analytical methods for the detection and characterisation of micro- and nanoplastics (together with suitable reference standards), as well as information on their toxicokinetic and toxicity profiles in/relevant for humans.
1.27 The COT highlighted that additional information will be needed on all exposure sources, which include indoor and outdoor air, dust and soil before a holistic risk assessment can be completed. The presence of MPs in (sea)food and water needs to be put into perspective with other sources of MPs such as atmospheric fallout.
1.28 Comprehensive assessment of microplastics and contaminant concentrations in different foods and the impact of cooking on the desorption and subsequent bioavailability of contaminants/leachates, need to be further investigated to better understand the implications for human health.
1.29 Current studies typically focus on only one type of particle/tissue interaction, as such, further research is necessary to explore the effects of the range of particle types in different tissues in vitro and/or in vivo. These range of particle types should also take account of emerging/novel plastic-based materials such as bioplastics.
The full COT sub-statement can be found on the COT website: Sub-statement on the potential risk(s) from exposure to microplastics: Oral route 2021.
Consumption of plant-based drinks in children aged 6 months to 5 years of age
Introduction
1.30 The Department of Health and Social Care (DHSC), Public Health England (PHE) and the Food Standards Agency (FSA) are receiving an increasing number of enquiries regarding the use of plant-based drinks in the diets of infants and young children. Therefore, the COT was asked to consider the potential risks posed by soya, almond and oat drinks consumed in the diets of these age groups.
1.31 The UK government advises that first infant formula (which is usually based on cows’ milk) is the only suitable alternative to breast milk in the first 12 months of a baby’s life. Whole cows’ milk can be given as a main drink from the age of 1 year. From this age, unsweetened calcium-fortified plant-based drinks, such as soya, almond and oat drinks can also be given to children, as part of a healthy, balanced diet.
1.32 The main challenge in the assessment of the safety of these drinks is the lack of information regarding dietary intakes for infants and young children following dairy-free or plant-based diets.
1.33 Organisations providing recommendations for ensuring a balanced diet for vegan children under 5 were used to identify appropriate portion sizes and consumption frequency to develop representative intake scenarios for children following dairy-free or plant-based diets. These were then used to calculate daily intake figures for different age groups in order to calculate exposure to the chemicals of concern in the different drinks.
1.34 Although the exposure estimates made the best use of the available data, there was a high degree of uncertainty with regards to actual intakes. This was because these figures were based on recommendations to ensure that dietary requirements for infants and children of these ages were met. Actual intakes may be different.
1.35 The Committee agreed to use the previously adopted approach of assuming that a child’s consumption was exclusively of a single plant-based drink as it is possible that young children may develop a preference for one drink. This was regarded as the most cautious approach because it assumes the highest intakes.
1.36 The need for real-world consumption information for people following plant-based diets in all age groups was highlighted by the Committee, as the popularity of these diets is increasing and information on realistic dietary intakes would help inform future risk assessments.
Soya
1.37 Soya drinks are a popular alternative to dairy products and their use is becoming more widespread. Soya products contain phytoestrogens (in the form of isoflavones). Concerns about adverse effects from isoflavones in the diet of infants and young children relate principally to their ability to mimic the female hormone, oestrogen, and therefore their potential impact on development and reproduction.
1.38 The safety of phytoestrogens was considered by the COT in 2003 and 2013. In 2003, the Scientific Advisory Committee on Nutrition (SACN) considered the COT outputs and concluded that there was no scientific basis for changing the current government advice – namely, that there is no substantive medical need for, nor health benefit arising from the use of soya-based infant formula, and that it should be used only in exceptional circumstances to ensure adequate nutrition, such as for babies who have cows' milk allergy. In 2013 this was reconfirmed by the COT. Currently, soya formula should be used only if it has been recommended or prescribed by a health visitor or GP.
1.39 For this evaluation, the Committee reviewed data published since the 2013 evaluation. The Committee concluded that new animal studies did not add significantly to the overall database.
1.40 As with previous evaluations, although there was some indication of possible adverse effects in human studies, it was not possible to determine from the available data, whether sensitivity to phytoestrogens varies among different age groups.
1.41 The Committee concluded that the intakes of phytoestrogens from consumption of soya drinks in children aged 6 months to five years was no greater than the estimated maximum intake by infants aged 0 – 6 months consuming soya formula where medically necessary (see paragraph 9 above). This maximum level of phytoestrogen intake was estimated to be 9.5 mg/kg bw per day.
1.42 The Committee agreed that, based on the available information, exposure to phytoestrogens from other soya-based products in the diets of children aged 6 months to 5 years of age was lower than that from soya drinks, and therefore of less concern. It was, however, noted that when exposure to phytoestrogens from all sources of soya in the diet was considered, the exposure came much closer to the maximum level of 9.5 mg/kg bw per day.
1.43 Members agreed that, in addition to potential toxicological concerns, consideration of nutritional issues would also be required to assess whether it was necessary to issue additional advice on the consumption of soya-based drinks in children aged 6 months to 5 years of age.
Oats
1.44 Oat drinks can be given to children following plant based or dairy- free diets, as an alternative to cows’ milk. Oats can be contaminated with mycotoxins, notably the trichothecene mycotoxins T-2 and HT-2, deoxynivalenol (DON), and Ochratoxin A (OTA). Mycotoxins are naturally occurring toxins produced by certain moulds. As such, they are unavoidable contaminants in certain foods, like oats. International standards are in place to limit exposures to mycotoxins to the lowest possible levels. The COT evaluated the available data and considered the estimated exposures to the above contaminants.
T2 and HT-2
1.45 The European Food Safety Authority (EFSA) considered the safety of T-2 and HT-2 in 2017. Health-based guidance values were established for emetic effects (causing vomiting) following acute (short term or single) exposure, and for immune- and hepatotoxicity effects (toxic effects on the liver) following long-term exposure. After reviewing UK intake data, COT concluded that in terms of acute exposure to the sum of HT-2 and T-2, consumption of a large quantity of oat drink (minimum of 5.4L/ day) was required to exceed the Acute Reference Doses (ARfD). Thus, acute exposure to HT-2 & T-2 from the consumption of oat drink was considered to be of low risk.
1.46 Generally, all long term exposures for T-2, HT-2 were below the respective TDI, with the exception of minor exceedances observed in children aged 1-2 years old for T-2 and HT-2. The assessment of total exposure from oat drinks combined with the general diet was considered conservative (i.e., high compared with likely reality) and as the exceedances were minor and transient in nature, it was concluded that there would be no chronic health effects in respect to T-2 and HT-2.
DON
1.47 For DON, a group Tolerable Daily Intake (TDI) was established for the sum of DON, and its related compounds, 3-Ac-DON, 15-Ac-DON and DON-3-glucoside based on animal studies in which body weight gain was reduced. Vomiting was identified as the critical effect following acute exposure in humans.
1.48 COT concluded that in terms of acute exposure to DON, consumption of a large quantity of oat drink (minimum 28L/d) was required to exceed the Acute Reference Dose (ARfD). Thus, acute exposure to DON was considered to be of low risk.
1.49 Generally, all long term exposures for T-2 and HT-2 were below the TDI, with the exception of minor exceedances observed in children aged 1-5 years old. The assessment of total exposure from oat drinks combined with that from the general diet was considered conservative and as the exceedances were minor and transient in nature, it was concluded that there would be no chronic health effects in respect to DON.
OTA
1.50 For OTA, EFSA in 2020 established a Margin of Exposure (MOE) approach for neoplastic and non- neoplastic effects (kidney tumours and microscopic kidney lesions, respectively) to assess the risk posed by OTA. The MOE is a measure that is used to determine the level of exposure at which there starts to be a safety concern. For genotoxic carcinogens, MOEs ≥10, 000 indicate low concern. For other effects, an MOE ≥100 indicates low concern. It is not clear whether OTA can cause kidney tumours by directly interacting with the DNA (genotoxic carcinogen), or via a different mechanism.
1.51 It was noted that there were many uncertainties in the cancer endpoint used for risk characterisation, and furthermore, it was unclear whether or not OTA was a genotoxic carcinogen and thus which MOE threshold value would be applicable. The Committee noted that the MOE of ≥10,000 for substances that are directly genotoxic and carcinogenic may not be appropriate in this case because there is some evidence that OTA does not interact directly with DNA. Some age groups had MOEs lower than desirable for non-neoplastic changes while all age groups had MOEs lower than 10,000 for cancer effects. The uncertainty in the assessment was considered to be high, especially considering the lack of analytical information on the presence of these contaminants in oat drinks and the assumptions used in the exposure assessment. It was noted that it is likely that the risk was being overestimated.
1.52 In respect of OTA, the Committee was unable to conclude whether the exposure estimates indicated a potential health concern. It was agreed that assessments of actual exposure are needed for adults as well as young children, to establish whether there were potential health concerns for the general population.
1.53 Overall, it was concluded that for the sum of DON and T-2 and HT-2, based on the available data there was no risk to health. However due to the uncertainties in the available dataset, the risk from exposure to OTA could not be determined.
Almonds
1.55 Almond drinks have a lower nutritional value than soya or oat drinks, however they can be given to children as an alternative to cows’ milk. The mycotoxin, aflatoxin B1 was identified as a possible chemical contaminant in almonds, which could be potentially transferred to almond drinks. Aflatoxin B1 is a genotoxic carcinogen, so the EU sets a legal limit for the amount of aflatoxin which can be present; this is called the maximum level and uses the ‘as low as reasonably achievable’ (ALARA) principle. This is to ensure that exposure to such compounds is at the lowest possible level. As no more reliable data on aflatoxin levels were available, it was assumed that the almonds contained aflatoxin at the legal maximum level.
1.56 The lack of analytical information on the effect that processing of almonds during almond drink manufacture has on the levels of aflatoxins, as well as the lack of information on the levels in almond drinks themselves, was considered the main limitation in assessing the risk to health. Considering the above limitations, it was concluded that undertaking a risk assessment based on the Maximum Levels set by EFSA was highly uncertain and was likely to lead to an overestimation of risk and therefore was not appropriate. The risk to health from exposure to AFB1 could not be determined.
1.57 Almonds also contain cyanogenic glycosides, which can be released when the almond is physically broken down by chewing or processing. When this happens, they may interact with the enzyme ß-glucosidase, also present in almonds. This enzyme breaks down the cyanogenic glycosides and can yield hydrogen cyanide. Exposure to large amounts of hydrogen cyanide can lead to convulsions, loss of consciousness, dizziness, weakness, mental confusion and heart failure.
1.58 High levels of glycosides are present in bitter almond varieties, whereas there is very little present in sweet varieties. The quantity of cyanogenic glycosides present in almond drinks is uncertain, but only low levels of cyanide have been detected on analysis. Available information indicates that bitter almond varieties are not grown in commercial almond orchards and although the inadvertent use of bitter almonds in almond milk drinks cannot be completely ruled out, bitter almonds would not be deliberately used as they would be unpalatable, imparting a strong ‘marzipan’ flavour to the drink. Overall, Members agreed that there were no specific concerns for acute toxicity from cyanogenic compounds in almond drinks.
Position paper on the alternatives to conventional plastics for food & drinks packaging
1.59 In conjunction with pressure from environmentally aware consumers and the strategy to reach net zero to mitigate the effects of climate change recent years have seen a major global increase in the development and use of alternative biobased materials to conventional plastics for food and drinks packaging.
1.60 These alternatives are a diverse, complex set of materials and blends. The materials are usually derived from living matter (animal, plant or fungal biomass) and are partially or wholly made of substances that are naturally available or are synthesised from biomass, such as sugarcane, corn, and algae. Some examples include, but are not limited to, wheat straws; beeswax wraps to replace clingfilm; and bamboo/rice husk for paper coffee cups.
1.61 The alternative materials are usually classified into three main groups: bio-based plastics, biodegradable plastics and compostable materials.
Advice on biobased food contact materials (BBFCMs) has been increasingly requested from the Food Standards Agency (FSA) so it was therefore considered timely for the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) to review the available toxicological information on BBFCMs.
1.62 Several papers have been presented to the COT, which included discussion of the following topics: the limited research that has been undertaken into the development of BBFCMs and the associated potential risks to the consumer; relevant market data and reports; a table of enquiries received from the FSA Food Contact Material (FCM) Policy Team - these included Non-intentionally added substances (NIAS) such as the presence of formaldehyde in bamboo cups and the allergic potential of material such as chitin and wheat; as well as a detailed discussion paper focussing on the immunogenicity and allergenicity of chitin and chitosan-based BBFCMs.
1.63 The COT acknowledged the challenges and complexities associated with BBFCMs as well as highlighting several limitations and knowledge gaps on BBFCMs research and regulation. These included labelling, composition (including biodegradability), contaminants and standardisation. Members noted that quantitative information was needed on contamination, degradation, migration of chemicals and allergens during the manufacture and use of commercial BBFCMs, as well as environmental impacts after disposal, such as the formation of micro/nanoparticles upon entering landfill or from energy-from-waste processes. It was noted that only limited evidence exists to demonstrate BBCFMs in direct food-contact applications meet similar standards of safety as conventional plastics.
1.64 Members agreed that there was a general lack of information on the presence of nanomaterials in BBFCMs. Therefore, overall, information on specific migration of all the possible migrating substances (nanofillers, plasticizers, antimicrobial additives, micron and nano sized plastic particles etc.) under different testing conditions would improve identification of potential hazards and enable an estimation of possible exposure. This would allow better demonstration that these novel biodegradable packaging materials meet comparable requirements. Additional toxicity studies or approaches to enable assessment of long term risk may be needed for a more comprehensive risk assessment.
1.65 The COT agreed a priority list of BBFCMs for health risk assessment based on their potential health hazards, extent of usage, and UK policy interest. The prioritised materials to be reviewed are: polylactic acid (PLA), starches, bamboo biocomposites and polyhydroxyalkanoates (PHA). This was not a closed list, other priority BBFCMs could be added as necessary based on the same criteria.
Health risk assessments of the prioritised BBFCMs should be considered within the context of life cycle assessment studies, which include environmental hazards to address indirect impacts on human health. However, this was not all within the remit of the COT. It was noted that the Department for Environment, Food and Rural Affairs (DEFRA) (and its expert scientific committee, the Hazardous Substances Advisory Committee, HSAC), the Organisation for Economic Cooperation and Development (OECD), and the Environment Agency were assessing the wider environmental impacts. These impacts should be monitored to identify additional potential hazards to human health.
1.66 Further assessments of intelligent packaging (also known as smart packaging) and nanomaterials used within food packaging will be undertaken as policy priorities and resources permit as part of the Committee’s work and would include bio sensors as well as nano coatings.
The full COT statement can be found on the COT website: Position paper on the alternatives to conventional plastics for food & drinks packaging.
Review of the EFSA opinion on dioxins
1.67 The COT reviewed the scientific basis and implications for risk management of the new EFSA tolerable weekly intake (TWI) for dioxins and considered that there were substantial uncertainties over the derivation of the TWI and possible inconsistencies between the animal and human data. Given the implications for risk management, the Committee felt that the rationales for the choices of key studies were not sufficiently clear in the published opinion, which made it difficult to evaluate the strength of the evidence. These concerns meant that the COT was unable to endorse the opinion and considered it necessary to reconsider the evidence base and set its own tolerable intake.
1.68 EFSA established a new TWI of 2 pg/TEQ/kg bw, which is 7-fold lower than its previous tolerable intake, based on data from a Russian Children’s study, identifying semen quality, following pre- and postnatal exposure, as the critical effect. The COT noted this study appeared inconsistent with the findings in a second study and considered the Russian study to provide only a weak data set. The studies on experimental animals (rodents) included in the EFSA evaluation confirmed that developmental effects occurred at body burdens similar to those used as the basis for the previous risk assessment. However, the COT considered there were inconsistencies in the animal data presented in the EFSA opinion and was unclear, in particular, regarding the rationale for the selection of the study to evaluate the critical body burdens. The COT had raised specific concerns about their reliability in 2001 and later FSA commissioned studies to address these concerns, which failed to replicate the specific findings but found other reproductive effects at similar body burdens. Overall, the data presented in EFSA’s opinion implied that humans were more sensitive to dioxins than rats. However, this would be inconsistent with the existing body of data on dioxins and knowledge on the relative sensitivity of the human and rat aryl hydrocarbon receptor (AHR). Due to these uncertainties, the COT did not agree with the newly established TWI and the 7-fold reduction in the TWI appeared too conservative for the database overall. The Committee was unable to comment on the dietary exposures and whether they should be compared to the new TWI.
1.69 The European Commission (EC) has not yet adopted EFSA’s new TWI due to ongoing work at the international level to review the basis and values of the WHO toxic equivalent factors (TEFs). The review of the TEFs and a finalised assessment by the EC are not expected until 2022, at the earliest. The COT noted that this also presupposes that the effects of concern are mediated via the AHR.
1.70 The Committee acknowledged that a further review of dioxins would be an extensive and lengthy undertaking. However, even if the current HBGV were immediately reduced, it would take decades to reduce body burden in the population, due to the nature of dioxins, especially their long half-life in humans. The current COT TDI was based on the most sensitive endpoint in the animal studies and is intended to protect the most sensitive population group, hence it would also be protective for all population groups and for other less sensitive effects.
1.71 Thus, while the re-assessment of dioxins is a necessary and important piece of work going forward, the COT did not consider it necessary in the meantime to alter its existing advice on dioxins. The COT considered that their current TDI of 2 pg/kg bw per day is protective for effects on the developing male fetus, that this was supported by later studies on this endpoint and was consistent with their consideration of the WHO-TEF concept.
COT principles for assessing risks from less than lifetime exposure or variable exposure over a lifetime
1.73 Dietary exposures to chemicals are typically compared to a health-based guidance value (HBGV), for example a tolerable daily intake (TDI), that has been established to be safe for long term exposure. Such values set a level of exposure that is considered acceptable if continued throughout a normal lifetime, i.e., it is an upper amount to which an individual can be exposed daily over a lifetime without a significant risk to health.
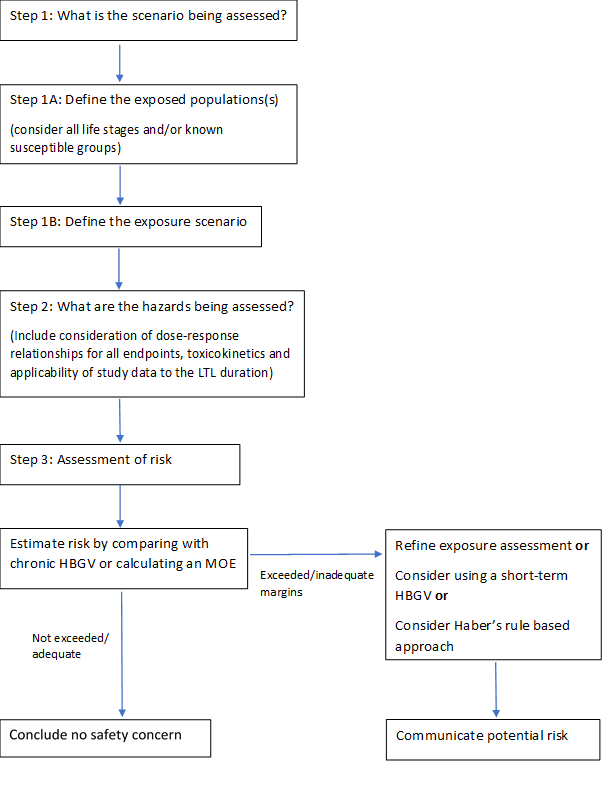
1.74 Sometimes people may be exposed to chemicals at a higher level for a shorter period of time. The COT produced a statement containing COT recommendations on possible ways of refining the risk assessment for such less-than-lifetime exposures. The statement includes a flowchart to illustrate the process, which is reproduced in Figure 1, below.
The full COT statement can be found on the COT website: Statement on COT principles for assessing risks from less than lifetime exposure or variable exposure over a lifetime.
Figure 1: Flowchart to illustrate the process of assessing risks from less than lifetime or variable (LTLV) exposures. Where appropriate, toxicokinetic or toxicodynamic modelling could be applied to refine any of the steps.
Development of Human Biomonitoring Guidance Values in the HBM4EU project
1.75 The Committee were asked to comment on the methodology for the derivation of human biomonitoring guidance values by the European Human Biomonitoring Initiative, referred to as HBM4EU, which is a project designed to develop a harmonised and systematic strategy for the derivation of human biomonitoring guidance values (HBM-GVs).
1.76 Members considered other types of human biomonitoring guidance values to allow comparison with established methods and discussed the potential application of the HBM4EU strategy and values, as well as their relevance to the UK.
1.77 There were two aspects that needed to be considered: the generation of the human biomonitoring guidance values and the application of these values to the population. It was also noted that, similar to determining any guidance value, the derivation of the human biomonitoring guidance values would depend on the type of data available and on establishing the relationship between the exposure and the effect. UK specific biomonitoring data would be useful for risk assessment and more information (such as appropriate auxiliary data) would be required before being able to use these values for this purpose.
1.78 In terms of the methodology for deriving the human biomonitoring guidance values, the values would need to be validated from a toxicological perspective. Ideally, exposure could be correlated to environmental levels in combination with human biomonitoring data, for example by collaborating with the agencies such as the Environment Agency or Defra to collect environmental biomonitoring exposure data. Correlation of National Diet and Nutrition Survey (NDNS) data with environmental biomonitoring data would be useful to refine exposures.
1.79 There may be insufficient toxicological data to establish human biomonitoring guidance values and a continuation project with targeted studies to allow for the generation of suitable data may be necessary.
1.80 On occasion, both external and internal guidance values will be needed - for example in cases where there is variability in the exposure depending on the product, and therefore monitoring of both product levels and internal levels in humans would be needed; this would need to be done on a case by case basis. Human biomonitoring guidance values are not often used stand alone, but they add value when they can be used in combination with other approaches
1.81 Further information would be useful on the pharmacokinetic requirements needed to establish a biomonitoring equivalent and it was noted that the sampling and exposure scenarios needed to fit sampling time. Requirements for marker substances were not included in the paper. Appropriate data on dermal exposure would also be important in ensuring the assumptions made were correct.
1.81 The Committee agreed that the strategy developed by HBM4EU was robust and scientifically valid, depending on kinetics information and data availability. In principle, the use of HBM-GVs derived by the HBM4EU in the UK would be possible. In practice, and in line with any other guidance value, detailed evaluation of the human biomonitoring value would be needed to determine whether the critical endpoint was appropriate for the UK population.
1.82 Going forward, the use of human biomonitoring guidance values in risk assessment could be helpful to the FSA and the Committee was content to review future case studies and offer their perspective. However, if endorsement of these values was needed, the Committee would have to perform a detailed evaluation to offer their perspective.
1.83 This topic has also been discussed by the COC (see paragraph 3.1 below)
First draft non-technical statement on how the Committees evaluate the relevance and reliability of data when assessing a chemical of concern
1.84 This topic was brought to the COT by the COC Secretariat.
1.85 Guidance aimed at a lay audience had been prepared, providing clarity on how the expert committees evaluate data with respect to consideration of biological relevance and statistical significance.
1.86 The topic arose during COC horizon scanning activities and the draft guidance for a number of years. the draft guidance been revised following review by lay members of the COC, COT and COM.
1.87 The COT considered the guidance was largely appropriate for the purpose of describing the mechanisms of ascribing biological and statistical significance to the assessment of the risk posed to the consumer by a chemical, but acknowledged that the statistical methods described were potentially overly complex for a lay readership. However, any simplification of the definition of concepts, such as the null hypothesis and p-value, should ensure that their meaning was lost.
1.88 The Committee noted that information on the workings of the sister committees should be included on the Committee website. However, further information was needed on some aspects, for example, how a particular chemical or issue was added to the agenda, how the risks to the consumer from it were assessed, and the basis of the conclusions reached. However, some of these aspects are covered in the Committee Code of Practice, albeit briefly.
1.89 The Committee made a number of additional minor suggestions for amendments.
Review of EFSA Scientific opinion on the safety assessment of titanium dioxide as a food additive (E171)
1.90 The COT was asked to comment on the “Scientific opinion on the safety assessment of titanium dioxide as a food additive (E171) “ published by EFSA in May of 2021. In this opinion, the EFSA panel concluded that on the basis of the currently available evidence along with all the uncertainties, in particular the fact that the concern regarding genotoxicity could not be resolved, that E171 can no longer be considered as safe when used as a food additive.
1.91 The EFSA Opinion had also been presented to the COM for comments (see paragraph 2.33).
1.92 The Committee note the COM’s preliminary comments, regarding the quality of the data and the difficulties in evaluating it adequately from the description given in the opinion. The lack of a good dataset and a well-defined test compound (due to the poorly defined specifications) are also considered as severe limitations. The COM consider the mechanism of genotoxicity appears to be indirect and probably has a threshold and, that the positive effects observed in the genotoxicity studies could be attributed to the nano-fraction of titanium dioxide.
1.93 The COT agree with the COM view and note the large discrepancy between the underlying dataset and the conclusions drawn by EFSA. On the genotoxicity of nanoparticles, it was noted that this could either be a concentration effect leading to oxidative damage or a stress effect, however, it was unclear as the results in different cell lines were equivocal and inconsistent. It was also noted that in some tests titanium dioxide had shown less reactivity.
1.94 In several parts of the Opinion, published papers are presented at face value, and there is no discussion of the results nor the Weight of Evidence to support the conclusions being made. There are also discrepancies and conflicts between the results of the studies reported and the overall conclusions.
1.95 On balance, the Committee considers that the weight of evidence does not support the conclusions drawn by EFSA. The Committee also agree with the comments of the COM with regards to risk communication that “As it stands the conclusion is highly risk adverse based on the weak evidence available, and it might create unnecessary concern to the public.” Care should be taken when expressing such conclusions in a binary manner given the extensive uncertainties in the dataset.
1.96 The COT suggested that the COM should independently review the database on genotoxicity and apply the COM’s Guidance on determining thresholds.
1.97 EFSA’s concluded that no differentiation could be made with regards to size/form of titanium dioxide and different aspects of toxicity, however, it seems likely that nanoparticles may be driving the toxicity.
1.98 It was decided that an interim position paper, capturing the COT’s view and the proposed next steps should be published. This can be found at: COT position paper on titanium dioxide