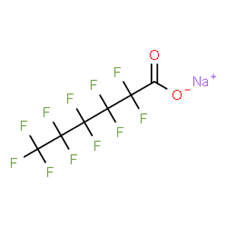
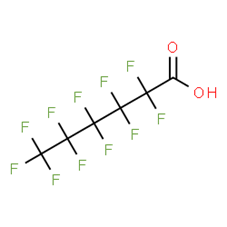
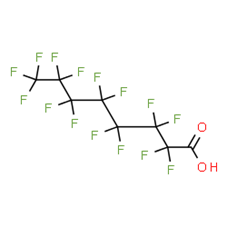
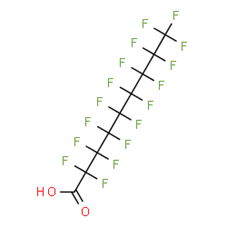
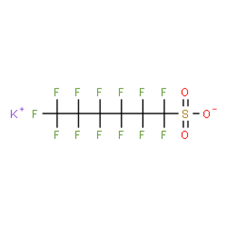Per- and polyfluoroalkyl substances: evaluation of thyroid effects using in vivo data (update) - PFAS/2023/04
Introduction, Background and Literature search
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Introduction
1. This paper is part of a series of papers supporting the COT assessment of the toxicology of per- and polyfluoroalkyl substances (PFAS). It provides the animal in vivo evidence on thyroid toxicity, with individual studies tabulated in Annex A, and updates the version provided to the COT PFAS subgroup in August 2023 (PFAS/2023/03).
2. A paper on evidence of thyroid toxicity based on in vitro toxicity studies is also presented at this meeting (PFAS/2023/05). Future papers will include human evidence for thyroid toxicity, and groups of papers covering other endpoints including developmental toxicity, liver toxicity and immunotoxicity.
Background
3. The COT has previously considered PFAS on a number of occasions (see summary in TOX/2022/53), and has recently a statement on the EFSA opinion. A paper summarising health-based guidance values (HBGV) was presented in December 2022 (TOX/2022/67) and following agreement in March 2023 the PFAS subgroup was established and an interim position published outlining future work.
Literature search
4. Search terms used previously by the European Food Safety Authority (EFSA) (2018 and 2020) were replicated. These search terms, the inclusion and exclusion criteria and the search results, are presented in Annex B to this paper.
5. A total of 34 published papers or reports were evaluated, some of which comprise more than one study and more than one PFAS. All papers and reports were evaluated for reliability using the ToxRTool (Klimisch et al., 1997) to determine data quality and reliability. As this report is an update to the paper presented in August 2023, all data, regardless of Klimisch scores, are presented in the tables below.
In vivo thyroid toxicity studies
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
6. For perfluorosulfonic acids (PFSAs), in vivo acute toxicity studies are available for perfluorooctane sulfonic acid (PFOS) and are presented in Annex A Table 3; for perfluoroalkyl carboxylic acids (PFCAs) in vivo acute toxicity studies are available for perfluorodecanoic acid (PFDA) and are presented in Annex A Table 4.
7. For PFSAs, repeated dose toxicity studies are available for perfluorobutane sulfonic acid (PFBS), perfluorohexanesulfonic acid (PFHxS) and PFOS presented in Annex A Table 5 to Table 7, and for PFCAs repeated dose studies are available for perfluorobutanoic acid (PFBA), perfluorohexanoic acid (PFHxA), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), PFDA, perfluorotetradecanoic acid (PFTeDA) and perfluorohexadecanoic acid (PFHxDA) and are presented in Annex A Table 8 to Table 13.
8. For PFSAs, developmental toxicity studies are available for PFBS, PFHxS and PFOS, and are presented in Annex A Table 15 to Table 17, and for PFCAs developmental toxicity studies are available for PFOA and are presented in Annex A Table 18.
9. From the 34 published sources, a total of 50 studies were carried out on 10 PFAS. Annex A Table 3 to Table 18 present no observed adverse effect levels (NOAELs) and lowest observed adverse effect level (LOAELs) based on thyroid effects.
10. The current paper considers effects in adult animals following exposure to PFAS by gavage, intraperitoneal (i.p) injection, diet or drinking water.
11. Data for 10 PFAS were identified, although most of the data relate to three PFAS: PFOS and PFHxS (PFSAs) and PFOA (PFCA).
12. Eight acute studies have been identified, for PFOS and PFDA.
13. Of the 26 repeated dose studies identified, 13 were carried out with PFSAs (PFHxS, PFOS) and 13 with PFCAs (PFBA, PFHxA, PFOA). Only one study was carried out with PFBS, PFNA, PFDA, PFHxDA and PFTeDA.
14. Of the 16 development toxicity studies identified, three were carried out on PFSA (PFBS, PFHxS, PFOS) and one on PFCA (PFOA). Only effects in the dam are discussed in the endpoint summaries below. Developmental effects in offspring, as a result of exposure during gestation and/or lactation, will be evaluated in subsequent papers.
15. The majority of acute and repeated dose studies were conducted in rats, with the exception of a single acute study in mice, and two acute studies and two repeated dose studies that were carried out in Cynomolgus monkeys. Developmental studies were carried out in mice and rats.
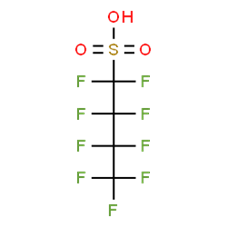
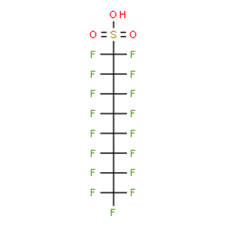
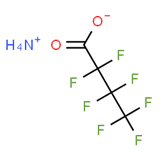
16. An overview of the PFAS chemical structure and molecular weight is presented in Annex C to this paper. Depending on the PFAS, studies have investigated the acid form, or a sodium, ammonium or potassium salt.
Endpoints investigated
17. Exposure to PFAS caused a number of thyroid effects in animals including effects on thyroid hormone (TH) levels, effects on thyroid histopathology and thyroid weight, impacts on gene transcription and associated process in the thyroid and other tissues.
18. Thirty-eight of the 50 studies (reported in the 34 published sources) measured THs, although not all THs were measured in each study, 25 included histopathology, 17 measured thyroid weight, and six included gene expression related to thyroid effects.
19. Observed effects at the LOAEL are based on statistically significant results. Effects seen at higher doses are not included. Abbreviations used in the tables are not spelled out.
Summary of results
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
20. Exposure to PFAS caused a number of thyroid effects in animals, including effects on TH levels (principally triiodothyronine (T3), thyroxine (T4) and thyroid stimulating hormone (TSH)), effects on thyroid histopathology and thyroid weight, and impacts on gene transcription and associated processes in the thyroid and other tissues.
Thyroid hormone levels
FT4
21. FT4 levels were measured in three of the eight acute studies, 14 of the 30 repeated dose studies, and eight of the 16 developmental studies.
22. In the acute studies, an increase in FT4 was seen in female rats following exposure to PFOS, although recovery was seen 24 hours after treatment (study 1 in Chang et al. (2008)). The authors concluded that the increase was transient and due to the ability of PFOS to compete with T4 for binding proteins. In contrast, no changes were seen in two acute studies in male and female Cynomolgus monkeys following exposure to PFOS (Chang et al., 2017).
23. In the repeated dose studies, FT4 was decreased in 10 of the 14 studies following exposure to PFBS (NTP, 2022b), PFHxS (NTP, 2022b), PFOS (NTP, 2022b; Thibodeaux et al., 2003) and PFBA (in the 28 day study by Butenhoff et al. (2012a)), PFHxA (NTP, 2022a), PFOA (Butenhoff et al., 2012a; NTP, 2022a)), PFNA (NTP, 2022a) and PFDA (NTP (2022a). All of these studies were conducted in rats.
24. In contrast, no effects were reported following long term exposure to PFOS in male and female monkeys (Seacat et al., 2002) and male rats (Yu et al., 2009a), in male monkeys following exposure to PFOA (Butenhoff et al., 2002), and in male and female rats following exposure to PFBA (in the 90 day study by Butenhoff et al. (2012a)).
25. Eleven out of the 14 repeated dose studies included males and females. Sex differences were seen in five studies, all of which were in rats, where decreases in FT4 were only seen in males following exposure to PFHxS (NTP, 2022b), PFBA (in the 28 day study by Butenhoff et al. (2012a)), PFHxA (NTP, 2022a), PFOA (NTP, 2022a) and PFDA (NTP, 2022a). No studies reported effects only in female animals.
26. No changes in FT4 were seen in developmental studies in either mice or rats.
Total T4
27. Total T4 (TT4) levels were measured in seven of the eight acute studies, 18 of the 30 repeated dose studies, and 12 of the 16 developmental studies.
28. In the acute studies, an increase in TT4 was seen in female mice following exposure to PFDA (Harris et al., 1989). Decreases were seen with PFDA in male rats (Langley & Pilcher, 1985; Van Rafelghem et al., 1987). Decreases were seen in three studies with PFOS, two studies in male and female rats both reported in Chang et al. (2008), and in male and female Cynomolgus monkeys (group 3 in Chang et al. (2017)). In contrast, no effects were reported in male and female Cynomolgus monkeys following exposure to a single dose of 9 mg/kg bw/day PFOS (group 2 in Chang et al. (2017)), but a decrease was seen following treatment to variable doses on three different occasions (13.3 and 14 mg/kg bw/day, male and female respectively) (group 2 in Chang et al. (2017)).
29. Three acute studies (Chang et al., 2017; Chang et al., 2008; Langley & Pilcher, 1985) included recovery groups or a recovery period for the treated animals, but no recovery of effects on TT4 was seen in any of the studies.
30. TT4 was decreased in 16 of the 18 repeated dose studies following exposure to PFBS (NTP, 2022b), PFHxS (NTP, 2022b), PFOS (Chang et al., 2008; Curran et al., 2008; NTP, 2022b; Thibodeaux et al., 2003; Yu et al., 2009a; Yu et al., 2011)), PFBA (in the 28- and 90-day studies by Butenhoff et al. (2012a)), PFHxA (NTP, 2022a), PFOA (Butenhoff et al., 2002; Butenhoff et al., 2012a; NTP, 2022a), PFNA (NTP, 2022a) and PFDA (NTP, 2022a). With the exception of a single study in Cynomolgus monkeys (PFOA, Butenhoff et al., 2002) all studies were carried out in rats.
31. In contrast, no effects on TT4 were reported in male and female Cynomolgus monkeys following exposure to PFOS (Seacat et al., 2002). In addition no effects on TT4 were seen in male and female rats following exposure to PFTeDA (Hirata-Koizumi, 2015).
32. Thirteen out of the 18 repeated dose studies included males and females. Sex differences were seen in five studies, all of which were in rats. Decreases in FT4 were seen only in males following exposure to PFBA in the 28- and 90-day studies by Butenhoff et al. (2012a), and to PFHxA (NTP, 2022a), PFOA (NTP, 2022a), and PFDA (NTP, 2022a). In no studies were effects seen only in female animals.
33. In developmental studies in mice, a decrease in TT4 was seen following exposure to PFBS (Feng et al., 2017). Conflicting results were reported for PFOS, as a decrease in TT4 was reported by Thibodeaux et al. (2003), but not Fuentes et al. (2006). No mouse developmental studies were conducted on PFCAs.
34. In developmental studies in rats, a decrease in TT4 was seen following exposure to PFHxS (Gilbert et al., 2021; Ramhøj et al., 2018) and the two studies by Ramhøj et al. (2018), to PFOS (Conley et al., 2022; Luebker et al., 2005; Thibodeaux et al., 2003; Wang et al., 2011)) and PFOA (Conley et al., 2022).
Free T3
35. Free T3 (FT3) was measured in one of the eight acute studies, two of the 30 repeated dose studies, and three of the 16 developmental studies.
36. In the acute study, no effect was reported following exposure to PFOS (Chang et al., 2008).
37. In the two repeated dose studies, FT3 was decreased in male and female Cynomolgus monkeys following exposure to PFOS (Seacat et al., 2002) but not in male Cynomolgus monkeys (females were not studied) following exposure to PFOA (Butenhoff et al., 2002).
38. In the developmental studies, PFOS exposure had no effect on FT3 levels in mice (Fuentes et al., 2006) or rats (Conley et al., 2022; Luebker et al., 2005), whereas PFOA decreased FT3 levels in rats (Conley et al., 2022) No mouse studies are available on PFOA, or other PFCAs.
Total T3
39. Total T3 (TT3) levels were measured in six of the eight acute studies, 15 of the 30 repeated dose studies, and nine of the 16 developmental studies.
40. In the acute studies, a decrease was seen in male rats following exposure to PFDA (Langley & Pilcher, 1985), with levels returning to be comparable to recovery group controls from study day four. No effect was seen following exposure to PFDA in female mice (Harris et al., 1989) and male and female rats (Van Rafelghem et al., 1987). No effect was also reported following exposure to PFOS in female rats (Chang et al., 2008) and two studies with male and female Cynomolgus monkeys (Chang et al., 2017).
41. In repeated dose studies, TT3 was decreased in eight of the 15 studies following exposure to PFBS (NTP, 2022b), PFHxS (NTP, 2022b), PFOS (Chang et al., 2008; Seacat et al., 2002; Thibodeaux et al., 2003), PFHxA (NTP, 2022a), PFOA (NTP, 2022a) and PFHxDA (Hirata-Koizumi, 2015). With the exception of the study in Cynomolgus monkeys by Seacat et al. (2002), all of the studies were in rats.
42. In contrast, no effects were reported following exposure to PFOS (Curran et al., 2008; NTP, 2022b; Yu et al., 2009a; Yu et al., 2011), PFOA (Butenhoff et al., 2002), PFNA (NTP, 2022a) and PFDA (NTP, 2022a). With the exception of the study in Cynomolgus monkeys by Butenhoff et al. (2002), all of the studies were in rats.
43. Ten out of the 15 repeated dose studies included males and females. Sex differences were seen in four studies, all of which were in rats. Decreases in TT3 were seen only in males following exposure to PFHxS (NTP, 2022b) and PFCAs (PFHxA (NTP, 2022a) and PFOA (NTP, 2022a)), and only in females following exposure to PFHxDA (Hirata-Koizumi, 2015).
44. In developmental studies in mice, no effect was reported on TT3 following exposure to PFOS (Fuentes et al., 2006; Thibodeaux et al., 2003), and a decrease was seen following exposure to PFBS (Feng et al., 2017). No mouse developmental studies were conducted on PFCAs.
45. In developmental studies in rats, PFOS exerted no effects (Conley et al., 2022; Luebker et al., 2005), but a decrease was seen with PFHxS (Gilbert et al., 2021; Ramhøj et al., 2020), PFOS (Thibodeaux et al., 2003) and PFOA (Conley et al., 2022).
TSH
46. TSH levels were measured in three of eight acute studies, 16 of the 30 repeated dose studies, and seven of the 16 developmental studies.
47. In the acute studies, a decrease was seen in female rats following exposure to PFOS (study 1 in Chang et al. (2008)), but no effect was seen in male and female Cynomolgus monkeys (Chang et al., 2017). TSH levels were comparable to controls in the study by Chang et al. (2008) at 24 hours post-treatment.
48. In repeated dose studies, TSH was decreased following exposure to PFOA (Butenhoff et al., 2012a), but increased in the study by NTP (2022a). Both studies were in rats, with the decrease seen in only in male rats and the increase seen only in female rats. Increases in TSH were also seen with PFOS in Cynomolgus monkeys and rats respectively (Seacat et al., 2002; Thibodeaux et al., 2003). No effects were seen with PFBS (NTP, 2022b), PFHxS (NTP, 2022b), PFOS (Chang et al., 2008; NTP, 2022b; Yu et al., 2009a), PFBA (in the 28 and 90 days studies by Butenhoff et al. (2012a)), PFHxA (NTP, 2022a), PFOA (Butenhoff et al., 2002), PFNA (NTP, 2022a), PFDA (NTP, 2022a) and PFTeDA (Hirata-Koizumi, 2015).
49. Twelve out of the 16 repeated dose studies included males and females. Sex differences were seen in two studies, both of which were in rats following exposure to PFOA. Decreases in TSH were seen only in male rats (Butenhoff et al., 2012a) and only in female rats (NTP, 2022a).
50. In developmental studies in mice, an increase in TSH was seen following treatment with PFBS (Feng et al., 2017), but no effect was reported with PFOS (Thibodeaux et al., 2003). No mouse developmental studies were conducted on PFCAs.
51. In developmental studies in rats, no effect on TSH was reported with PFHxS (Gilbert et al., 2021; Ramhøj et al., 2018) or PFOS (Chang et al., 2009; Luebker et al., 2005; Thibodeaux et al., 2003).
Recovery
52. In repeated dose studies, recovery was assessed in five studies, one of which was with a PFSA and four were with PFCAs.
53. Following the 182-day exposure to PFOS, THs that showed differences to controls in male and female Cynomolgus monkeys at 0.75 mg/kg bw/day PFOS (decreased TT3 and increased TSH in both sexes, decreased TT4 in males, and decreased FT3 in females) at the end of treatment were comparable to recovery group controls between days 33 to 61 in both sexes (Seacat et al., 2002).
54. Following the 28-day exposure to PFBA, THs that were decreased in male rats (TT4 and FT4) at 6 mg/kg bw/day PFBA were comparable to recovery group controls after a three week recovery period (Butenhoff et al., 2012a). However, at the highest dose tested (150 mg/kg bw/day) the observed decrease in TT4 did not show recovery.
55. Following the 90-day exposure to PFBA, the decreased TT4 seen in males at the end of treatment was subsequently increased relative to recovery group controls after the three-week recovery period (Butenhoff et al., 2012a).
56. Following the 28-day exposure to PFOA, THs that were decreased in female rats (TT4, FT4) at 30 mg/kg bw/day were comparable to recovery group controls after the 3-week recovery period (Butenhoff et al., 2012a). However, in male rats, of the THs that were decreased (TSH, TT4, FT4) at 30 mg/kg bw/day PFOA, both TT4 and FT4 remained significantly lower than recovery group controls whereas TSH returned to levels comparable to recovery group controls.
57. Following the 42-day exposure to PFHxDA, TH that were decreased in female rats (TT3) at 4 mg/kg bw/day were comparable to recovery group controls after the 14-day recovery period (Hirata-Koizumi, 2015). It should be noted that in the repeated dose part of this OECD 422 study, recovery group females were unmated and are therefore not directly comparable to treated females that were exposed for the same duration but through mating, gestation and to PND5. No effect on THs was seen in males at the end of the 42-day exposure up to the highest dose tested of 100 mg/kg bw/day, but a decrease in TT4 was seen at the end of the 14-day recovery period at this dose.
Relationship between T4, T3 and TSH
58. Overall, in repeated dose studies, consistent decreases in TH levels were observed with PFSAs (PFBS, PFHxS and PFOS) and PFCAs (PFBA, PFDA, PFOA and PFNA), mainly FT4, TT4, TT3. These reductions were not associated with compensatory increases in TSH in 19 studies (two acute studies, 12 repeated dose studies and five developmental studies). The exceptions to this, where increases in TSH were seen, were following exposure to PFOS in male and female Cynomologus monkeys (Seacat et al., 2002) and in female rats (Thibodeaux et al., 2003), to PFOA in female rats (NTP, 2022a) and to PFBS in pregnant mice (Feng et al., 2017). The results are therefore not generally indicative of a classical induced hypothyroid state, where decreased T4 and T3 levels would be associated with increased TSH. This is highlighted by NTP where the authors state that the reason for a lack of TSH response when a decrease in TH concentrations is seen is not clear, and is not consistent with a disruption in the hypothalamic-pituitary-thyroid axis (NTP, 2022a, 2022b).
59. Rats are uniquely sensitive to TH perturbation in association with induction of liver enzymes (Capen, 1997 cited in Loveless et al. (2009)). Circulating T3 and T4 bind to albumins in all species, but bind to globulins (thyroid-binding globulin (TBG)) with high affinity in primates and humans, which leads to an approximately 10-fold shorter half-life of THs in plasma in rodents compared to primates and humans, leading to a more rapid turnover of THs and potentially different effects arising from changes in TH levels in rodents and primates or humans (Alison et al., 1994), Consequently, maintaining homeostasis will be different between rodents and primates, including humans (Alison et al., 1994).
Thyroid histopathology
60. Histopathology was carried out in 25 of the 50 studies reviewed (two acute studies, 21 repeated dose studies, and two developmental studies).
61. In acute studies, no effects on thyroid histopathology were reported in male rats following exposure to PFOS (Elcombe et al., 2012a) or PFDA (Van Rafelghem et al., 1987).
62. Notable changes in thyroid histopathology were only identified in four out of 21 repeated dose studies.
63. An increased incidence of hypertrophy and hyperplasia of follicular epithelial cells was seen in male rats following exposure to PFHxS (Butenhoff et al., 2009a), PFBA (in the 90-day study by Butenhoff et al. (2012a)) and PFOA (Butenhoff et al., 2012a). However, no effect was reported in female rats treated with either PFBA (in the 90-day study by Butenhoff et al. (2012a)) and PFOA (Butenhoff et al., 2012a). An increased incidence of thyroid follicular epithelial hypertrophy was seen in male and female rats with PFHxA (Loveless et al., 2009).
64. No effects on thyroid histopathology were reported in 17 out of 21 studies, namely in seven studies conducted with PFSAs (PFBS (NTP, 2022b), PFHxS (NTP, 2022b) and PFOS (Butenhoff et al., 2012b; Curran et al., 2008; Elcombe et al., 2012a; Elcombe et al., 2012b; NTP, 2022b), and in 10 studies with PFCAs (PFBA in the 28-day study by Butenhoff et al. (2012a), PFHxA (NTP, 2022a), PFOA (Butenhoff et al., 2002; Butenhoff et al., 2012b; Griffith & Long, 1980; NTP, 2022a), PFNA (NTP, 2022a), PFDA (NTP, 2022a), PFHxDA (Hirata-Koizumi, 2015) and PFTeDA (Hirata-Koizumi, 2015)). Moreover, no changes were seen in dams in the two developmental studies on PFHxS (Ramhøj et al., 2020) and PFOS (Chang et al., 2009). With the exception of the study on PFOA by Butenhoff et al. (2002) in Cynomolgus monkeys, all studies were in rats.
65. Seventeen out of the 21 repeated dose studies included males and females. In the three studies in rats where an effect on thyroid histopathology was seen, sex differences were seen in two studies. An effect was seen only in males following exposure to PFBA in the 90-day study by Butenhoff et al. (2012a) and following exposure to PFOA in the 28-day study by Butenhoff et al. (2012a). In contrast, effects were seen in both sexes following exposure to PFHxA in the 90-day study by Loveless et al. (2009).
Recovery
66. Recovery was assessed in three of the four repeated dose studies where effects on thyroid histopathology were seen, all of which were with PFCAs.
67. For PFBA, the increased incidence of follicular hypertrophy/hyperplasia seen in male rats was comparable to recovery group controls after a 3-week recovery period (Butenhoff et al., 2012a). For PFHxA, the increased incidence of thyroid follicular epithelial hypertrophy seen in male and female rats did not show evidence of recovery on day 30. However, at the end of the recovery period on day 90, in female rats the incidence was comparable to controls. No recovery was seen in male rats (Loveless et al., 2009). For PFOA, the increased thyroid follicular epithelial cell height in male rats was comparable to controls following a 3-week recovery period, but the increased incidence of thyroid follicular hypertrophy/hyperplasia did not show evidence of recovery (Butenhoff et al., 2012a).
Thyroid weight
68. Thyroid weight was measured in 17 of the 50 studies reviewed (one acute study, 15 repeated dose studies, and one developmental study).
69. In the acute study, no effect was reported on thyroid weight in male rats treated with PFDA (Van Rafelghem et al., 1987).
70. In the repeated dose studies, an increase in thyroid weight was seen in only four of 15 repeated dose studies, all with PFCAs.
71. An increase in absolute thyroid weight was seen in male, but not female, rats treated with PFBA (Butenhoff et al., 2012a) and PFHxDA (Hirata-Koizumi, 2015), and conversely in female, but not male, rats treated with PFDA (NTP, 2022a).
72. No effects were seen on thyroid weight with PFBS (NTP, 2022b), PFHxS (NTP, 2022b), PFOS (Butenhoff et al., 2012b; Curran et al., 2008; NTP, 2022b; Seacat et al., 2002), PFHxA (NTP, 2022a), PFOA (Griffith & Long, 1980; NTP, 2022a), PFNA (NTP, 2022a) and PFTeDA (Hirata-Koizumi, 2015). With the exception of the study on PFOS in Cynomolgus monkeys (Seacat et al., 2002), all studies were carried out in rats.
73. No changes were seen in the developmental study in rats with PFHxS (Ramhøj et al., 2020).
74. All of the 15 repeated dose studies included males and females. In the four studies in rats where an effect on thyroid weight was seen, there appears to be no clear sex-specific effect following exposure to PFBA (in the 28-day study by Butenhoff et al. (2012a)), PFHxA (Loveless et al., 2009), PFDA (NTP, 2022a) and PFHxDA (Hirata-Koizumi, 2015).
Recovery
75. Recovery was assessed in three of the four repeated dose studies where effects on thyroid weight were seen, all of which were with PFCAs.
76. For PFBA, the increase in absolute thyroid weight seen in male rats was comparable to controls following the 3-week recovery period (Butenhoff et al., 2012a). For PFHxA, a delayed increase in thyroid weight (relative or absolute not specified) was seen in female rats, as no effects were seen at the end of treatment but a transient increase was seen during the recovery period (at 30 days) which had returned to control levels after 90 days (Loveless et al., 2009). Although no effect on thyroid weight was seen following treatment, the authors concluded that the increased weight observed during the recovery period was adverse and treatment related. For PFHxDA, the increase in relative thyroid weight seen in male rats was comparable to controls following the 14-day recovery period (Hirata-Koizumi, 2015).
Effects on gene expression
77. Thyroid-related gene expression was assessed in six studies (three repeated dose studies, and three developmental studies). Four studies (one in mice and three in rats) reported changes in thyroid-related gene expression. All studies were on PFSAs.
78. Three studies with PFOS reported effects in the liver (hepatic malic enzyme (ME), which responds to changes in THs, mRNA levels relating to hepatic T4 glucuronidation, and proteins associated with the hepatic uptake of T4) (Chang et al., 2008; Yu et al., 2009a; Yu et al., 2011), and one study with PFBS showed a transcriptional effect in rat hypothalamus (Feng et al., 2017).
79. Studies with PFHxS and PFOS (Chang et al., 2009; Ramhøj et al., 2020) reported no effect on gene expression at any dose tested.
Serum/plasma PFAS levels
80. Levels of PFAS in serum or plasma were measured in three acute studies with PFSAs, 10 repeated dose studies with PFSAs and seven with PFCAs, and in six developmental studies with PFSAs and one with PFCAs.
81. Levels of both PFSAs and PFCAs in males were typically higher than their female counterparts at the same dose levels, suggesting a sex-specific difference in plasma concentrations for certain PFAS and that males and females respond differently to exposure.
82. These results will be evaluated further in subsequent papers considering the toxicokinetics of PFAS.
Discussion
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
83. Ten PFAS are considered in this paper, comprising three PFSAs (PFBS, PFHxS and PFOS) and seven PFCAs (PFBA, PFHxA, PFOA, PFNA, PFDA, PFHxDA and PFTeDA).
84. Table 1 and Table 2 below present the lowest point of departure (POD) for PFSAs and PFCAs, respectively, based on thyroid effects. For PFBS, PFHxA and PFOA, only a LOAEL was determined, as effects were seen at the lowest dose tested.
85. THs were measured in a total of 38 studies in adult animals, being the most frequently studied thyroid-related endpoint and the most sensitive endpoint on which the majority of the N/LOAELs have been determined.
86. In repeated dose and developmental toxicity studies there were consistent decreases in TH levels observed with PFSAs (PFBS, PFHxS and PFOS) and PFCAs (PFBA, PFDA, PFOA and PFNA), mainly FT4, TT4, TT3. In general, these decreases were not associated with compensatory increases in TSH. Therefore, the results are not generally indicative of a classical induced hypothyroid state following exposure to PFAS, where decreased T4 and T3 levels would be associated with increased TSH. The five studies where recovery was assessed suggest that changes in TH levels are transient, and have the potential to return to control values, although this was not seen consistently.
Table 1 Lowest POD for PFAS based on thyroid effects - PFSAs
*Derived by contractor; NA – not applicable.
87. Thyroid histopathology was assessed in 25 studies. At lower PFAS doses, histopathological changes were seen less frequently than changes in TH levels. Notable changes in histopathology were identified at the LOAEL in only four studies, with PFHxS, PFBA, PFHxA and PFOA, and included increased incidence of hypertrophy and/or hyperplasia of follicular epithelial cells and increased thyroid follicular epithelial cell height. Loveless et al. (2009) noted that thyroid hypertrophy, as seen in their study with PFHxA, is a common finding in rats, associated with the induction of hepatic microsomal enzymes, leading to increased biliary excretion of T4 and elevation of TSH, which results in hypertrophy of follicular epithelial cells. It should be noted that this discussion by Loveless et al. (2009) is not supported by any measurements of THs in their study. In their 92/93-day study with PFHxA, thyroid follicular epithelial hypertrophy was seen only at doses that also produced liver hypertrophy. The authors also stated that due to the species-specific short half-life for T4 in rodents, rats are uniquely sensitive to thyroid hormone perturbation in association with induction of liver enzymes (Capen, 1997 cited in Loveless et al. (2009)), concluding that whilst the observed thyroid follicular cell hypertrophy is potentially adverse, it is unlikely that this effect is relevant to non-rodent species (Alison et al., 1994 cited in Loveless et al. (2009)). The three studies where recovery was assessed suggest that thyroid follicular hypertrophy may be transient, although this was not seen consistently.
88. Thyroid weight was measured in 17 studies, where an increased weight was seen at the LOAEL with PFBA, PFHxA, PFDA and PFHxDA. Increases were generally only seen at doses higher than those causing changes in THs. The three studies in which recovery was assessed suggest that changes in thyroid weight may be transient.
89. Sex-specific differences were seen with effects on THs, with changes more frequently seen in male than in female animals at comparable doses. No clear sex-specific effect was evident regarding histopathological changes or thyroid weight.
90. Serum/plasma PFAS levels will be evaluated further in subsequent papers considering the toxicokinetics of PFAS
91. It may be relevant to note the approach taken by two authoritative bodies, namely the ATSDR (ATSDR, 2021) and the United States Environmental Protection Agency (USEPA) (USEPA, 2023), in selecting thyroid effects as the basis for setting human health criteria values. These opinions will be explored in future papers.
92. Overall, the in vivo evidence indicates that low doses of PFSAs and PFCAs can produce adverse effects on levels of THs (typically without affecting TSH levels), and that higher doses can produce histopathological alterations in the thyroid and an increase in thyroid weight. However, some of these findings are inconsistent, and some endpoints appear to be sex-specific (with males being more sensitive than females).
93. Interpretation of the in vivo evidence with respect to adversity and human relevance is problematic and will be explored in future papers.
Questions on which the views of the Committee are sought
94. Members are invited to consider the following questions:
i). Are there any specific papers that the subgroup would like to review in more detail?
ii). Recovery is assessed in a minority of studies. Should the N/LOAEL be based on effects seen at the end of treatment or after the recovery period?
iii). Thyroid effects seen in developmental studies are presented for dams and offspring. The N/LOAELs are based on effects in the dam only. Does the subgroup agree with excluding effects seen in offspring, which will be reported in subsequent papers?
IEH Consulting under contract supporting the UKHSA COT Secretariat
December 2023
List of Abbreviations
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
|
125I |
Iodine-125 |
|
ANCOVA |
Analysis of covariance |
|
ATSDR |
Agency for Toxic Substances and Disease Registry |
|
BMD |
Benchmark dose |
|
CAR |
Constitutive androstane receptor |
|
CAS |
Chemical abstracts service |
|
CF |
Cross foster |
|
COT |
Committee on Toxicity |
|
CT |
Litters from treated dams fostered by control dams (pups only exposed postnatally) |
|
DIO1 |
Type 1 deiodinase, iodothyronine deiodinase type 1 |
|
EFSA |
European Food Safety Authority |
|
FDA |
Food and Drug Administration |
|
EtoH |
Ethyl alcohol |
|
FT3 |
Free triiodothyronine |
|
FT4 |
Free thyroxine |
|
FTI |
Free thyroxine index |
|
GD |
Gestational day |
|
GL |
Guideline |
|
GLP |
Good laboratory practice |
|
HBGV |
Health-based guidance value |
|
HPT |
Hypothalamic–pituitary–thyroid |
|
i.p. |
Intraperitoneal |
|
LD |
Lactation day |
|
LOAEL |
Lowest observed adverse effect level |
|
LOEL |
Lowest observed effect level |
|
ME |
Malic enzyme |
|
mRNA |
Messenger ribonucleic acid |
|
MRP2 |
Multidrug resistance–associated protein |
|
NA |
Not applicable |
|
NAM |
New approach methodology |
|
ND |
Not detected |
|
NIS |
Sodium-iodide symporter |
|
NOAEL |
No observed adverse effect level |
|
NR |
Not reported |
|
NTP |
National Toxicology Program |
|
OECD |
Organisation for Economic Co-operation and Development |
|
PFAS |
Per- and polyfluoroalkyl substances |
|
PFBA |
Perfluorobutanoate / Perfluorobutanoic acid |
|
PFBS |
Perfluorobutane sulfonate / Perfluorobutane sulfonic acid |
|
PFCA |
Perfluoroalkyl carboxylic acid |
|
PFDA |
Perfluorodecanoate / Perfluorodecanoic acid |
|
PFHpS |
Perfluoroheptanesulfonate / Perfluoroheptane sulfonic acid |
|
PFHxA |
Perfluorohexanoate / Perfluorohexanoic acid |
|
PFHxDA |
Perfluorohexadecanoic acid |
|
PFHxS |
Perfluorohexanesulfonate / Perfluorohexanesulfonic acid |
|
PFNA |
Perfluorononanoate / Perfluorononanoic acid |
|
PFOA |
Perfluorooctanoate / Perfluorooctanoic acid |
|
PFOS |
Perfluorooctane sulfonate / Perfluorooctane sulfonic acid |
|
PFPeS |
Perfluoropentanesulfonate |
|
PFPS |
Perfluoropropanesulfonate |
|
PFSA |
Perfluorosulfonic acids |
|
PFTeDA |
Perfluorotetradecanoate / Perfluorotetradecanoic acid |
|
PND |
Postnatal day |
|
POD |
Point of departure |
|
PPARα |
Peroxisome proliferator-activated receptor α |
|
PXR |
Pregnane X receptor |
|
QA |
Quality assurance |
|
RNA |
Ribonucleic acid |
|
rT3 |
Reverse triiodothyronine |
|
SD |
Standard deviation |
|
SE |
Standard error |
|
T3 |
Triiodothyronine |
|
T4 |
Thyroxine |
|
TC |
Litters from treated dams fostered by control dams (pups only exposed prenatally |
|
TT3 |
Total triiodothyronine |
|
TT4 |
Total thyroxine |
|
TH |
Thyroid hormone |
|
TG |
Thyroglobulin |
|
Trh |
Thyrotropin releasing hormone |
|
TSH |
Thyrotropin also thyroid stimulating hormone |
|
TSHR |
Thyroid stimulating hormone receptor |
|
TT |
Litters from treated dams fostered by other treated dams (pups exposed prenatally and postnatally) |
|
TTR |
Transthyretin |
|
UGT |
Uridine diphospho-glucuronosyl transferase |
|
USEPA |
United States Environmental Protection Agency |
References
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Alison, R. H., Capen, C. C., & Prentice, D. E. (1994). Neoplastic lesions of questionable significance to humans. Toxicol Pathol, 22(2), 179-186. https://doi.org/10.1177/019262339402200211
ATSDR. (2021). Toxicological Profile for Perfluoroalkyls.
Butenhoff, J., Costa, G., Elcombe, C., Farrar, D., Hansen, K., Iwai, H., Jung, R., Kennedy Jr, G., Lieder, P., Olsen, G., & Thomford, P. (2002). Toxicity of ammonium perfluorooctanoate in male cynomolgus monkeys after oral dosing for 6 months [Article]. Toxicological Sciences, 69(1), 244-257. https://doi.org/10.1093/toxsci/69.1.244
Butenhoff, J. L., Bjork, J. A., Chang, S. C., Ehresman, D. J., Parker, G. A., Das, K., Lau, C., Lieder, P. H., van Otterdijk, F. M., & Wallace, K. B. (2012a). Toxicological evaluation of ammonium perfluorobutyrate in rats: Twenty-eight-day and ninety-day oral gavage studies [Article]. Reproductive Toxicology, 33(4), 513-530. https://doi.org/10.1016/j.reprotox.2011.08.004
Butenhoff, J. L., Chang, S. C., Ehresman, D. J., & York, R. G. (2009a). Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats [Article]. Reproductive Toxicology, 27(3-4), 331-341. https://doi.org/10.1016/j.reprotox.2009.01.004
Butenhoff, J. L., Chang, S. C., Olsen, G. W., & Thomford, P. J. (2012b). Chronic dietary toxicity and carcinogenicity study with potassium perfluorooctanesulfonate in Sprague Dawley rats [Article]. Toxicology, 293(1-3), 1-15. https://doi.org/10.1016/j.tox.2012.01.003
Butenhoff, J. L., Ehresman, D. J., Chang, S. C., Parker, G. A., & Stump, D. G. (2009b). Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: Developmental neurotoxicity [Article]. Reproductive Toxicology, 27(3-4), 319-330. https://doi.org/10.1016/j.reprotox.2008.12.010
Butenhoff, J. L., Kennedy Jr, G. L., Chang, S. C., & Olsen, G. W. (2012c). Chronic dietary toxicity and carcinogenicity study with ammonium perfluorooctanoate in Sprague-Dawley rats [Article]. Toxicology, 298(1-3), 1-13. https://doi.org/10.1016/j.tox.2012.04.001
Chang, S., Allen, B. C., Andres, K. L., Ehresman, D. J., Falvo, R., Provencher, A., Olsen, G. W., & Butenhoff, J. L. (2017). Evaluation of serum lipid, thyroid, and hepatic clinical chemistries in association with serum perfluorooctanesulfonate (PFOS) in cynomolgus monkeys after oral dosing with potassium PFOS [Article]. Toxicological Sciences, 156(2), 387-401, Article kfw267. https://doi.org/10.1093/toxsci/kfw267
Chang, S. C., Ehresman, D. J., Bjork, J. A., Wallace, K. B., Parker, G. A., Stump, D. G., & Butenhoff, J. L. (2009). Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: Toxicokinetics, thyroid hormone status, and related gene expression [Article]. Reproductive Toxicology, 27(3-4), 387-399. https://doi.org/10.1016/j.reprotox.2009.01.005
Chang, S. C., Thibodeaux, J. R., Eastvold, M. L., Ehresman, D. J., Bjork, J. A., Froehlich, J. W., Lau, C., Singh, R. J., Wallace, K. B., & Butenhoff, J. L. (2008). Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS) [Article]. Toxicology, 243(3), 330-339. https://doi.org/10.1016/j.tox.2007.10.014
Conley, J. M., Lambright, C. S., Evans, N., Medlock-Kakaley, E., Dixon, A., Hill, D., McCord, J., Strynar, M. J., Ford, J., & Gray, L. E., Jr. (2022). Cumulative maternal and neonatal effects of combined exposure to a mixture of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) during pregnancy in the Sprague-Dawley rat [Article]. Environment International, 170, 107631. https://doi.org/10.1016/j.envint.2022.107631
Curran, I., Hierlihy, S. L., Liston, V., Pantazopoulos, P., Nunnikhoven, A., Tittlemier, S., Barker, M., Trick, K., & Bondy, G. (2008). Altered fatty acid homeostasis and related toxicologic sequelae in rats exposed to dietary potassium perfluorooctanesulfonate (PFOS) [Article]. Journal of Toxicology and Environmental Health - Part A: Current Issues, 71(23), 1526-1541. https://doi.org/10.1080/15287390802361763
EFSA. (2012). Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal, 10(30, 2579. https://doi.org/https://doi.org/10.2903/j.efsa.2012.2579
EFSA. (2018). Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA Journal, 16(12), 5194. https://doi.org/https://doi.org/10.2903/j.efsa.2018.5194
EFSA. (2020). Risk to human health related to the presence of perfluoroalkyl substances in food. EFSA Journal, 18(9), 6223. https://doi.org/https://doi.org/10.2903/j.efsa.2020.6223
Elcombe, C. R., Elcombe, B. M., Foster, J. R., Chang, S. C., Ehresman, D. J., & Butenhoff, J. L. (2012a). Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats from dietary exposure to potassium perfluorooctanesulfonate results from increased expression of xenosensor nuclear receptors PPARα and CAR/PXR [Article]. Toxicology, 293(1-3), 16-29. https://doi.org/10.1016/j.tox.2011.12.014
Elcombe, C. R., Elcombe, B. M., Foster, J. R., Chang, S. C., Ehresman, D. J., Noker, P. E., & Butenhoff, J. L. (2012b). Evaluation of hepatic and thyroid responses in male Sprague Dawley rats for up to eighty-four days following seven days of dietary exposure to potassium perfluorooctanesulfonate [Article]. Toxicology, 293(1-3), 30-40. https://doi.org/10.1016/j.tox.2011.12.015
Feng, X., Cao, X., Zhao, S., Wang, X., Hua, X., Chen, L., & Chen, L. (2017). Exposure of pregnant mice to perfluorobutanesulfonate causes hypothyroxinemia and developmental abnormalities in female offspring [Article]. Toxicological Sciences, 155(2), 409-419. https://doi.org/10.1093/toxsci/kfw219
Fuentes, S., Colomina, M. T., Rodriguez, J., Vicens, P., & Domingo, J. L. (2006). Interactions in developmental toxicology: Concurrent exposure to perfluorooctane sulfonate (PFOS) and stress in pregnant mice [Article]. Toxicology Letters, 164(1), 81-89. https://doi.org/10.1016/j.toxlet.2005.11.013
Gilbert, M. E., O'Shaughnessy, K. L., Thomas, S. E., Riutta, C., Wood, C. R., Smith, A., Oshiro, W. O., Ford, R. L., Hotchkiss, M. G., Hassan, I., & Ford, J. L. (2021). Thyroid Disruptors: Extrathyroidal Sites of Chemical Action and Neurodevelopmental Outcome-An Examination Using Triclosan and Perfluorohexane Sulfonate [Article]. Toxicological Sciences, 183(1), 195-213. https://doi.org/10.1093/toxsci/kfab080
Griffith, F. D., & Long, J. E. (1980). Animal toxicity studies with ammonium perfluorooctanoate [Article]. American Industrial Hygiene Association Journal, 41(8), 576-583. https://doi.org/10.1080/15298668091425301
Harris, M. W., Uraih, L. C., & Birnbaum, L. S. (1989). Acute toxicity of perfluorodecanoic acid in C57BL/6 mice differs from 2,3,7,8-tetrachlorodibenzo-p-dioxin [Article]. Toxicological Sciences, 13(4), 723-736. https://doi.org/10.1093/toxsci/13.4.723
Hirata-Koizumi, M., Fujii S, Hina K, Matsumoto M, Takahashi M, Ono A and Hirose A. (2015). Repeated dose and reproductive/developmental toxicity of long-chain perfluoroalkyl carboxylic acids in rats: perfluorohexadecanoic acid and perfluorotetradecanoic acid. . Fundamental Toxicological Sciences, 2, , 177–190.
Klimisch, H. J., Andreae, M., & Tillmann, U. (1997). A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol, 25(1), 1-5. https://doi.org/10.1006/rtph.1996.1076
Langley, A. E., & Pilcher, G. D. (1985). Thyroid, bradycardic and hypothermic effects of perfluoro-n-decanoic acid in rats [Article]. Journal of Toxicology and Environmental Health, 15(3-4), 485-491. https://doi.org/10.1080/15287398509530675
Lau, C., Thibodeaux, J. R., Hanson, R. G., Rogers, J. M., Grey, B. E., Stanton, M. E., Butenhoff, J. L., & Stevenson, L. A. (2003). Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci, 74(2), 382-392. https://doi.org/10.1093/toxsci/kfg122
Loveless, S. E., Slezak, B., Serex, T., Lewis, J., Mukerji, P., O'Connor, J. C., Donner, E. M., Frame, S. R., Korzeniowski, S. H., & Buck, R. C. (2009). Toxicological evaluation of sodium perfluorohexanoate [Article]. Toxicology, 264(1-2), 32-44. https://doi.org/10.1016/j.tox.2009.07.011
Luebker, D. J., York, R. G., Hansen, K. J., Moore, J. A., & Butenhoff, J. L. (2005). Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: Dose-response, and biochemical and pharamacokinetic parameters [Article]. Toxicology, 215(1-2), 149-169. https://doi.org/10.1016/j.tox.2005.07.019
NTP. (2022a). NTP Technical Report on the Toxicity Studies of Perfluoroalkyl Carboxylates (Perfluorohexanoic Acid, Perfluorooctanoic Acid, Perfluorononanoic Acid, and Perfluorodecanoic Acid) Administered by Gavage to Sprague Dawley (Hsd:Sprague Dawley SD) Rats (Revised). NTP TOX 97. August 2019. Revised July 2022.
NTP. (2022b). NTP Technical Report on the Toxicity Studies of Perfluoroalkyl Sulfonates (Perfluorobutane Sulfonic Acid, Perfluorohexane Sulfonate Potassium Salt, and Perfluorooctane Sulfonic Acid) Administered by Gavage to Sprague Dawley (Hsd:Sprague Dawley SD) Rats (Revised). NTP TOX 96. August 2019. Revised July 2022.
Ramhøj, L., Hass, U., Boberg, J., Scholze, M., Christiansen, S., Nielsen, F., & Axelstad, M. (2018). Perfluorohexane sulfonate (PFHxS) and a mixture of endocrine disrupters reduce thyroxine levels and cause antiandrogenic effects in rats [Article]. Toxicological Sciences, 163(2), 579-591. https://doi.org/10.1093/toxsci/kfy055
Ramhøj, L., Hass, U., Gilbert, M. E., Wood, C., Svingen, T., Usai, D., Vinggaard, A. M., Mandrup, K., & Axelstad, M. (2020). Evaluating thyroid hormone disruption: investigations of long-term neurodevelopmental effects in rats after perinatal exposure to perfluorohexane sulfonate (PFHxS) [Article]. Scientific Reports, 10(1), Article 2672. https://doi.org/10.1038/s41598-020-59354-z
Seacat, A. M., Thomford, P. J., Hansen, K. J., Olsen, G. W., Case, M. T., & Butenhoff, J. L. (2002). Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys [Article]. Toxicological Sciences, 68(1), 249-264. https://doi.org/10.1093/toxsci/68.1.249
Thibodeaux, J. R., Hanson, R. G., Rogers, J. M., Grey, B. E., Barbee, B. D., Richards, J. H., Butenhoff, J. L., Stevenson, L. A., & Lau, C. (2003). Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: Maternal and prenatal evaluations [Review]. Toxicological Sciences, 74(2), 369-381. https://doi.org/10.1093/toxsci/kfg121
USEPA. (2023). IRIS Toxicological Review of Perfluorohexanesulfonic Acid (PFHxS, CASRN 335-46-4) and Related Salts. EPA/635/R-23/148a. External Review Draft.
Van Rafelghem, M. J., Inhorn, S. L., & Peterson, R. E. (1987). Effects of perfluorodecanoic acid on thyroid status in rats [Article]. Toxicology and Applied Pharmacology, 87(3), 430-439. https://doi.org/10.1016/0041-008X(87)90248-1
Wang, F., Liu, W., Jin, Y., Dai, J., Zhao, H., Xie, Q., Liu, X., Yu, W., & Ma, J. (2011). Interaction of PFOS and BDE-47 co-exposure on thyroid hormone levels and TH-related gene and protein expression in developing rat brains [Article]. Toxicological Sciences, 121(2), 279-291. https://doi.org/10.1093/toxsci/kfr068
Yu, W. G., Liu, W., & Jin, Y. H. (2009a). Effects of perfluorooctane sulfonate on RAT thyroid hormone biosynthesis and metabolism [Article]. Environmental Toxicology and Chemistry, 28(5), 990-996. https://doi.org/10.1897/08-345.1
Yu, W. G., Liu, W., Jin, Y. H., Liu, X. H., Wang, F. Q., Liu, L., & Nakayama, S. F. (2009b). Prenatal and postnatal impact of perfluorooctane sulfonate (PFOS) on rat development: a cross-foster study on chemical burden and thyroid hormone system. Environ Sci Technol, 43(21), 8416-8422. https://doi.org/10.1021/es901602d
Yu, W. G., Liu, W., Liu, L., & Jin, Y. H. (2011). Perfluorooctane sulfonate increased hepatic expression of OAPT2 and MRP2 in rats [Article]. Archives of Toxicology, 85(6), 613-621. https://doi.org/10.1007/s00204-010-0613-x
Annex A
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Table 3 Acute toxicity studies for PFSAs - PFOS
*Derived by contractor; NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw) / vehicle / route of admin / duration / Guideline (GL) study / Good Laboratory Practice (GLP) status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups). Recovery (controls vs treated groups) |
Published NOAEL / LOAEL (mg/kg bw) |
Study author conclusions |
Comments |
|
PFOS (potassium salt). CAS No. not given 86.9%. Chang et al. (2008) Study 1: effects of time and PFOS on serum thyroid hormones. |
Sprague-Dawley rats Female
|
0 or 15. 0.5% Tween 20® in distilled water. Gavage Single dose, followed by sacrifice 2, 6 and 24 hr post-treatment Non-GL study GLP not stated.
|
At 15 mg/kg bw 66.90 ± 8.00 at 6 hr, 61.58 ± 8.81 at 24 hr. |
Females (mean ± SE):
Liver (mean ± SE): ↑ ME mRNA (ME transcript per 18s rRNA copies) at 2 hr (7.14 ± 1.05 vs 11.30 ± 1.28), but not at 6 hr or 24 hr. Recovery assessed as reported above over 24 hr period. |
Females: NA / 15.
|
PFOS caused transiently increased tissue availability of THs and turnover of T4 with a resulting reduction in serum TT4. Transient elevations of FT4 was hypothesised to be due to ability of PFOS to compete with T4 for binding proteins. A classic hypothyroid state was not induced under the conditions of this study.
|
K1 Only female animals used. Only two dose groups, i.e. control and single treatment group. Low purity. No details of impurities given. No effect on FT3 and TT3 at LOAEL. mRNA transcripts for hepatic ME, respond to changes in THs. Study 2 is presented below.
Authors are affiliated to 3M Company. |
|
PFOS (potassium salt) CAS No. not given 86.9%.
Chang et al. (2008) Study 2: effects of PFOS on 125I elimination. |
Sprague-Dawley rats Male and female 4/dose (male), 5/dose (female).
|
0 or 15. 0.5% Tween 20® in distilled water. Gavage Single dose, followed by sacrifice 24 hr post-treatment. Non-GL study GLP not stated. |
NR. |
Males and females: ↓ TT4 at 24 hr (data only reported in figures). Recovery not assessed. |
Males: NA / 15*. Females: NA / 15*.
|
The decrease in TT4 is due to increased turnover and elimination. |
K1 Only two dose groups, i.e., control and single treatment group. Low purity. No details of impurities given. Study investigated 125I elimination, therefore only TT4 measured. Study 1 is presented above. Authors are affiliated to 3M Company. |
|
PFOS (potassium salt). CAS No. not given 88.9%. Chang et al. (2017). |
Cynomolgus monkeys Male and female 6/sex/dose.
|
Group 1 and 2: 0 or 9 0.5% Tween 20® ± 5% EtoH. Gavage Single dose, animals not sacrificed Non-GL study GLP not stated. Recovery period: 294 days. |
At 9 mg/kg bw in males on day 113 (mean ± SD) Serum: 67.7 ± 7.5. At 9 mg/kg bw in females on day 113 (mean ± SD): Serum: 68.8 ± 2.5. |
Males and females: No effects seen on thyroid hormones (TSH, FT4, TT3, TT4) (data only reported in figures). Recovery: No effects seen on THs (TSH, FT4, TT3, TT4) (data only reported in figures). |
Males: 9* / NA. Females: 9* / NA.
|
No toxicologically meaningful or clinically relevant changes in TH. |
K1 Only two dose groups, i.e., control and single treatment group. Low purity. Impurities include 3.2% PFHxS, 1.2% PFHpS, 1.1% PFPeS, 0.97% PFBS and 0.74% PFPS. Study funded by 3M Company. (See below for study group 3). |
|
PFOS (potassium salt) CAS No. not given 88.9%. Chang et al. (2017). |
Cynomolgus monkeys Male and female 4-6/sex/dose. |
Group 1 and 3: 0, 14, 14.8 / 17.2 (male/female) or 11 0.5% Tween 20® ± 5% EtoH. Gavage Single doses on days 43, 288 and 358, animals not sacrificed Non-GL study. GLP not stated. Recovery period: 294 days.
|
At 14 mg/kg bw in males on day 50 (mean ± SD) Serum: 104.8 ± 502. At 14 mg/kg bw in females on day 50 (mean ± SD): Serum: 96.5 ± 6.2. At 14.8 mg/kg bw in males on day 288 (mean ± SD) Serum: 141.0 ± 13.1.
At 17.2 mg/kg bw in females on day 288 (mean ± SD) Serum: 147.6 ± 17.5. At 11 mg/kg bw in males on day 365 (mean ± SD) Serum: 160.8 ± 14.2. At 11 mg/kg bw in females on day 365 (mean ± SD) Serum: 165.0 ± 6.7. |
Males and females: ↓ TT4 (data only reported in figures). Recovery: ↓ TT4 (data only reported in figures) TSH, FT4, TT3 comparable to controls.
|
Males: NA / 13.3* (average dose). Females: Recovery Males: NA / 13.3* (average dose). Females:
|
No toxicologically meaningful or clinically relevant changes in TH at serum PFOS concentrations up to 165 µg/ml. Measurement of TT4 reflected >99% of the biologically inactive thyroxine, and therefore is not considered a clinically relevant endpoint for interpreting thyroid function. Decrease in TT4 while remaining within the normal range, appeared to be associated with treatment. |
K1 Only two dose groups i.e. control and single treatment group, with variable dosing of 14, 14.8/17.2 or 11 mg/kg bw/day, varying across treatment days, and between sexes on study day 288 (aiming to achieve serum concentrations ~ 100 µg/ml (on Study Day 43), 150 µg/ml (on Study Day 288), and 170 µg/ml (on Study Day 358), Low purity. Low purity. Impurities include 3.2% PFHxS, 1.2% PFHpS, 1.1% PFPeS, 0.97% PFBS and 0.74% PFPS. THs (TSH, FT4, TT3, TT4) measured on days -7, 1, 8, 22, 43, 50, 64, 85, 106, 113, 127, 148, 169, 176, 190, 211, 232, 253, 274, 288, 295, 309, 330, 358, 365, 379 and 400. Study funded by 3M Company |
|
PFOS (potassium salt) CAS No. not stated 86.9%. Elcombe et al. (2012a). |
Sprague-Dawley rats Male 10/dose (total 30/dose, sacrificed on days 2, 8 and 29). Recovery group: 10/dose.
|
0, 20 or 100 ppm in diet equivalent to 0, 2.12 or 11.05. RMI powdered diet. Diet Single dose, followed by sacrifice 2 days post-treatment. Non-GL study GLP not stated. Recovery group: 0, 20 or 100 ppm in diet equivalent to 0, 2.12 or 11.05, 8 and 29 days. |
NR. |
Males: No effects seen. Recovery: Thyroid histopathology, cell apoptosis and cell proliferation comparable to controls. |
Males: 11.05* / NA. |
No specific comments on thyroid toxicity. |
K1 Only male animals used. Three dose groups, including control. Low purity. No details of impurities given. Principal objective to investigate the activation of the xenosensor nuclear receptors PPARα and CAR/PXR as aetiological factors in PFOS-induced hepatomegaly and hepatic tumorigenesis in rats. Thyroid histopathology, cell apoptosis or cell proliferation measured. Study funded by 3M Company |
Table 4 Acute toxicity studies for PFCAs - PFDA
*Derived by contractor; NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups)
Recovery (controls vs treated groups) |
Published NOAEL / LOAEL (mg/kg bw) |
Study author conclusions |
Comments |
|
PFDA CAS No. 335-76-2 96%. Harris et al. (1989). |
C57BL/6N mice. Female 10/dose.
|
0, 20, 40, 80, 160 or 320 Corn oil. Gavage Single dose, followed by sacrifice 30 days post-treatment. Non-GL study GLP not stated. |
NR. |
Females (mean ± SE) ↑ TT4 (3.6 ± 0.2 µg/dL vs 5.1 ± 0.4 µg/dL)#. Recovery not assessed (THs only measured 30 days post-treatment).
|
Females: NA / 20*. |
All mice at 160 and 320 mg/kg bw died by 14-days post-exposure. PFDA appears to cause a rise in TT4. A similar rise was also seen in the congenic C57BL/6J strain (data NR).
|
K1 Only females used. TSH, FT3 and FT4 not measured. No effect on TT3 at LOAEL. THs only measured once, 30 days post-treatment, therefore levels at other time points are unknown. #THs measured in 4-10 animals per dose. No details of funding or conflicts of interest given. |
|
PFDA CAS No. not given. Purity not given. Langley and Pilcher (1985).
|
Wistar rats Male 5/dose-time point (total 30/dose, 5 sacrificed on each of days 0.5, 1, 2, 4, 6 and 8 days post-treatment).
|
0 or 75. Propylene glycol i.p. Single dose, followed by sacrifice 0.5 days post-treatment. Non-GL study GLP not stated. Recovery group: 0 or 75. 1, 2, 4, 6 and 8 days.
|
NR. |
Males: ↓ TT4 (all time points, data only reported in figures).
Recovery: ↓ TT4 (days 1, 2, 4, 6 and 8, data only reported in figures). TT3 levels comparable to controls on days 4, 6 and 8. |
Males: NA / 75*. Recovery Males: NA / 75*.
|
Effects on the thyroid axis may be an early primary response to PFDA, and some subsequent effects may be secondary to the change in TH levels. Decreased THs not solely a result of starvation. |
K1 i.p. method of administration. Only male animals used. Only two dose groups, i.e. control and single treatment group, however multiple recovery times used. Purity not given. TSH, FT3 and FT4 not measured. No details of funding or conflicts of interest given.
|
|
PFDA CAS No. not given 96%.
Van Rafelghem et al. (1987). |
Sprague-Dawley rats Male 8 -16/dose.
|
0, 20, 40 or 80 Propylene glycol-water i.p. Single dose, followed by sacrifice 7 days post-treatment. Non-GL study GLP not stated. |
NR. |
Males (mean ± SE): ↓ TT4 (4.41 ± 0.19 µg/dL vs 2.81 ± 0.19 µg/dL). Recovery not assessed. |
Males: NA / 20*. |
Despite alterations in plasma TH levels, no consistent effect on hypo / hyperthyroidism. |
K1 i.p. method of administration. Only male animals used. No effect on thyroid weight or histopathology at the LOAEL. TSH, FT3 and FT4 not measured. No effect on TT3 at LOAEL. Dose response for TT4. Study funded by the Air Force Office of Scientific Research. |
Table 5 Repeated dose toxicity studies for PFSAs – PFBS
*Derived by contractor; **calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups)
Recovery (controls vs treated groups) |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author comments |
Comments |
|
PFBS CAS No. 375-73-5 >97%. NTP (2022b). |
Sprague-Dawley rats Male and female 10/sex/dose.
|
0, 62.6, 125, 250, 500 or 1000. 2% Tween® 80 in deionized water. Gavage 28 days NTP protocol GLP study (FDA GLP Regs).
|
At 62.6 mg/kg bw/day in males on day 29 (mean ± SE) Plasma: 2.222 ± 0.477. At 62.6 mg/kg bw/day in females on day 29 (mean ± SE) Plasma: 0.154 ± 0.048.
|
Males (mean ± SE): ↓ TT4 (3.34 ± 0.18 µg/dL vs 0.90 ± 0.009 µg/dL)#. ↓ FT4 (2.09 ± 0.09 ng/dL vs 0.64 ± 0.04 ng/dL) #. ↓ TT3 (117.76 ± 8.31 ng/dL vs 87.85 ± 5.00 ng/dL) #. Females (mean ± SE): ↓ TT4 (3.10 ± 0.15 µg/dL vs 1.48 ± 0.09 µg/dL) #. ↓ FT4 (1.54 ± 0.08 ng/dL vs 0.72 ± 0.05 ng/dL) #. ↓ TT3 (89.29 ± 5.57 ng/dL vs 61.81 ± 3.34 ng/dL) #. Recovery not assessed. |
Males: NA / 62.6*. Females: NA / 62.6*.
|
The reason for a lack of TSH response in the face of substantially low TH concentrations is not clear and not consistent with a disruption in the HPT axis. |
K1 FT3 not measured. No effect on thyroid or parathyroid histopathology, or thyroid weight at LOAEL. TSH levels unaffected by treatment. Significant decreases in TT4, FT4 and TT3 at all doses but no clear dose response for males. THs not measured in surviving animals at 1000 mg/kg bw/day. #THs measured in 9 control males and 9 treated males at 500 mg/kg bw/day. THs measured in 9 female animals at 250 and 500 mg/kg bw/day. Government funded study. Study was audited retrospectively by an independent QA contractor. |
Table 6 Repeated dose toxicity studies for PFSAs – PFHxS
*Derived by contractor; **calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups)
Recovery (controls vs treated groups) |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author comments |
Comments |
|
PFHxS (potassium salt) CAS No. not given 99.98%. Butenhoff et al. (2009). |
Sprague-Dawley rat Male 15/dose.
|
0, 0.3, 1, 3 or 10 0.5% Carboxymethylcellulo. Gavage Males: 42 days beginning 14 days prior mating to sacrifice on study day 44. Females: 14 days prior to mating to PND22 OECD 422 (with modifications) GLP not stated.
|
At 1 mg/kg bw/day in F0 males on day 42 (mean ± SD) Serum: 89.12 ± 0.80. At 3 mg/kg bw/day in F0 males on day 42 (mean ± SD) Serum: 128.67 ± 10.30. |
Males: ↑ hyperplasia of thyroid follicular cells in F0 males (total incidence 2 vs 4). Recovery not assessed.
|
Males: 1 / 3.
|
THs not measured. Changes in thyroid consisted of hypertrophy and hyperplasia of follicular epithelial cells. Changes were consistent with known effects of compounds that cause microsomal enzyme induction where the hepatocellular hypertrophy results in a compensatory hyperplasia and hypertrophy of the thyroid due to increased plasma turnover of T4 and associated stimulation of TSH in rats. |
K1 Only male animals used for repeated dosing segment of the OECD 422 study. While this is a repeat dose with reproductive/developmental toxicity study, results are presented here as they relate to effects (in males only). Study funded by 3M Company. |
|
PFHxS |
Sprague-Dawley rats Male and female 10/sex/dose.
|
0, 0.625, 1.25, 2.5, 5 or 10 (males) 0, 3.12, 6.25, 12.5, 25 or 50 (females) 2% Tween® 80 in deionized water. Gavage 28 days. NTP protocol GLP study (FDA GLP Regs).
|
At 0.625 mg/kg bw/day in males (mean ± SE) Plasma: 66.76 ± 3.518. At 3.12 mg/kg bw/day in females (mean ± SE) Plasma: 37.030 ± 1.651. At 6.25 mg/kg bw/day in females (mean ± SE): Plasma 50.410 ± 1.552.
|
Males (mean ± SE): ↓ TT4 (4.24 ± 0.23 µg/dL vs 2.39 ± 0.08 µg/dL). ↓ FT4 (1.74 ± 0.10 ng/dL vs 0.82 ± 0.07 ng/dL). ↓ TT3 (85.18 ± 5.74 ng/dL vs 66.21 ± 4.20 ng/dL). Females (mean ± SE): ↓ TT4 (3.99 ± 0.19 µg/dL vs 3.37 ± 0.17 µg/dL). Recovery not assessed. |
Males: NA / 0.625*. Females: 3.12 / 6.25. |
The reason for a lack of TSH response (an increase) in the face of substantially low TH concentrations is not clear and not consistent with a disruption in the HPT axis. Female rats displayed much lower plasma PFAS concentrations than males. |
K1 FT3 not measured. No effect on thyroid or parathyroid histopathology, or thyroid weight, at LOAEL. Significant decreases in TT4, FT4 and TT3 at all doses but no clear dose response for males. Government funded study. Study was audited retrospectively by an independent QA contractor. |
Table 7 Repeated dose toxicity studies for PFSAs – PFOS
*Derived by contractor; **calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups)
Recovery (controls vs treated groups) |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author comments |
Comments |
|
PFOS (potassium salt) CAS No. not given 86.9%. Butenhoff et al. (2012b). |
Sprague-Dawley rats Male and female 60-70/dose. Recovery group: 40/dose.
|
0, 0.5, 2, 5 or 20 ppm in diet equivalent to 0, 0.024, 0.098, 0.242 or 0.984 (males) and 0, 0.029, 0.120, 0.299 or 1.251 (females). Rodent Diet 5002 meal Diet 104 weeks Non-GL study GLP not stated. Recovery group: 20 ppm in diet equivalent to 1.144 (males) or 1.385 (females) 52 weeks treatment followed by control diet to 104 weeks. |
At 0.984 mg/kg bw/day in males at 105 weeks (mean ± SD) Serum: 69.3 ± 57.9. At 0.984 mg/kg bw/day in females at 105 weeks (mean ± SD) Serum: 233.0 ± 124.0. |
Males and females: No effects seen# (NOAEL is the highest dose tested for both sexes). Recovery: No effects seen during treatment or recovery. |
Males: 0.984* / NA. Females: 1.251* / NA. |
No anatomical indications of a response of the thyroid, including thyroid weight and microscopic histological changes. No treatment-related findings for thyroid tissue. In males, there was a significant increase in incidence of thyroid follicular cell adenoma, and incidence of combined thyroid follicular cell tumours (adenoma and carcinoma), at 1.144 mg/kg bw/day in the recovery group, compared with controls. Although the increased incidence of thyroid follicular cell tumours was outside the range of historical control values, there was no other microscopic evidence of thyroid abnormality. The increased incidence of thyroid follicular cell adenoma in the 1.144 mg/kg bw/day recovery group, without similar increases at 0.984 mg/kg bw/day in males and/or at 1.251 mg/kg bw/day in females during treatment, is contradictory and may represent a chance occurrence. In females, there was a significant increase in combined thyroid follicular cell adenoma and carcinoma at 0.299 mg/kg bw/day, compared with controls. The authors noted these tumours are known to occur in historical controls. |
K1 Low purity. Impurities include 4.73% PFHxS, 0.71% perfluorinated carboxylic acids (C4, C5, and C8), 1.45% metals, 0.59% inorganic fluoride. In the basic study, thyroids were only removed from top dose animals at scheduled necropsy at week 53. #Thyroid weight and histopathology measured in males at 11-25/dose, and in females at 12-24/dose. Recovery group had no comparable controls; animals were observed for reversibility, persistence, or delayed occurrence of toxic effects for 52 weeks post-treatment. Study funded by 3M Company. |
|
PFOS (potassium salt) CAS No. not given 86.9%. Chang et al. (2008). |
Sprague-Dawley rats Male 6/dose.
|
0 or 3, 0.5% Tween 20® in distilled water. Gavage 7 days Non-GL study GLP not stated. |
NR. |
Males: ↓ TT4 (data only reported in figures). ↓ TT3 (data only reported in figures). Recovery not assessed. |
Males: NA / 3*. |
PFOS did not appear to alter the function of the HPT hypothalamic axis.
|
K1 Only male animals used. Only two dose groups. Low purity. No details of impurities given. Study aim to investigate pituitary function (Trh-mediated pituitary release of TSH). FT3 and FT4 not measured. No effect on TSH at LOAEL. Authors are affiliated to 3M Company. No details of funding given. |
|
PFOS (potassium salt) CAS No. 2795-39-3 ≥98%. Curran et al. (2008). |
Sprague-Dawley rats Male and female 15/sex/dose
|
0, 2, 20, 50 or 100 ppm in diet equivalent to 0, 0.14, 1.33, 3.21 or 6.3 (males) and 0, 0.15, 1.43, 3.73 or 7.58 (females.) AIN-93G purified diet. Diet 28 days Non-GL study GLP not stated. |
At 0.14 mg/kg bw/day in males (mean ± SD) Serum: 0.95 ± 0.13. At 1.33 mg/kg bw/day in males (mean ± SD) Serum: 13.45 ± 1. 48. At 0.15 mg/kg bw/day in females (mean ± SD) Serum: 1.50 ± 0.23. At 1.43 mg/kg bw/day in females (mean ± SD) Serum: 15.40 ± 0.56. |
Males (mean ± SD): ↓ TT4 (80.94 ± 11.83 nmol/L vs 14.36 ± 4.18 nmol/L). Females (mean ± SD): ↓ TT4 (37.71 ± 15.41 nmol/L vs 19.62 ± 2.49 nmol/L). Recovery not assessed. |
Males: Females: |
No specific comments on thyroid toxicity. Decreased TT4 and TT3 occurred concurrently with hepatic changes indicative of peroxisome proliferation. At doses above the LOAEL significant treatment-related changes in thyroid weight relative to body weight, were suggestive of altered endocrine functions. |
K1 Authors derived a LOEL (not LOAEL). No effect on TT3 levels at LOEL, absolute or relative thyroid weight, or thyroid:brain weight. No histopathological changes in thyroid tissue. Significant decrease in TT4 at all doses above the LOAEL but no clear dose response in either males or females. TSH, FT3 and FT4 not measured. There was a dose related increase in PFOS serum levels; PFOS in other tissues also showed a dose related response. No details of funding or conflicts of interest given. |
|
PFOS (potassium salt). CAS No. not given 86.9%. Elcombe et al. (2012b). |
Sprague-Dawley rats Male 10/dose (total 40/dose, 10 sacrificed on days 1, 28, 56 and 84 days post-treatment). Recovery group: 10/dose.
|
0, 20 or 100 ppm in diet equivalent to 0, 1.93 or 9.65. RMI powdered diet. Diet 7 days Non-GL study GLP not stated. Recovery groups: 0, 20 or 100 ppm in diet equivalent to 0, 1.93 or 9.65. Sacrificed on days 28, 56, and 84. |
At 1.93 mg/kg bw/day on days 1, 28, 56 and 84 (mean ± SD) |
Males: No effects seen (NOAEL is highest dose tested). ↓ body weight on recovery days 21 and 28 (mean ± SD): 412.2 ± 46.8 g vs 384.8 ± 46.8 g and 428.2 ± 50.9 g vs 397.0 ± 51.4g respectively). Recovery: Effects comparable to controls. |
Males: 9.65* / NA. |
Thyroid parameters (thyroid status (histology, follicular epithelial apoptosis, follicular epithelial cell proliferation) were unaffected at all time points. |
K1 Only male animals used. Only three dose groups. Low purity. No details of impurities given. Authors are affiliated to 3M Company. No details of funding given. |
|
PFOS (potassium salt) CAS No. not stated 86.9%. Elcombe et al. (2012a). |
Sprague-Dawley rats Male 10/dose (total 30/dose, 10 sacrificed at three time points).
|
0, 20 or 100 ppm in diet equivalent to 0, 1.66 or 7.90. RMI powdered diet Diet 7 or 28 days Non-GL study GLP not stated. |
NR. |
Males: No effects seen (NOAEL is highest dose tested) in both 7-day or 28-day studies. Recovery not assessed. |
Males: 7.90* / NA.
|
No specific comments on thyroid toxicity. |
K1 Only male animals used. Only three dose groups. Low purity. No details of impurities given. Principal objective to investigate the activation of the xenosensor nuclear receptors PPARα and CAR/PXR as etiological factors in PFOS-induced hepatomegaly and hepatic tumorigenesis in rats. Thyroid histopathology, cell apoptosis and cell proliferation measured. Study funded by 3M Company. No details of funding given. |
|
PFOS CAS No. 1763-23-1 >96%. NTP (2022b). |
Sprague-Dawley rats Male and female 10/sex/dose.
|
0, 0.312, 0.625, 1.25, 2.5 or 5 2% Tween® 80 in deionized water. Gavage 28 days NTP protocol GLP study (FDA GLP Regs).
|
At 0.312 mg/kg bw/day in males (mean ± SE) Plasma: 23.73 ± 1.114. At 0.312 mg/kg bw/day in females (mean ± SE) Plasma: 30.53 ± 0.918. |
Males (mean ± SE): ↓ TT4 (3.51 ± 0.30 µg/dL vs 1.33 ± 0.19 µg/dL). ↓ FT4 (2.53 ± 0.22 ng/dL vs 0.95 ± 0.10 ng/dL). Females (mean ± SE): ↓ TT4 (2.21 ± 0.24 µg/dL vs 1.11 ± 0.12 µg/dL) #. ↓ FT4 (1.74 ± 0.23 ng/dL vs 1.07 ± 0.09 ng/dL) #. Recovery not assessed. |
Males: NA / 0.312. Females: NA / 0.312.
|
The reason for a lack of TSH response in the face of substantially low TH concentrations is not clear and not consistent with a disruption in the HPT axis. |
K1 No effect on TT3, thyroid weight, thyroid or parathyroid histopathology at LOAEL. Significant decreases in TT4 and FT4 at all doses but no clear dose response for TT4 for either males or females. #THs measured in 9 females at 5 mg/kg bw/day. Government funded study. Study was audited retrospectively by an independent QA contractor.
|
|
PFOS (potassium salt). CAS No. 2795-39-3 86.9%. Seacat et al. (2002). |
Cynomolgus monkeys Male and female 4-6/dose. Recovery group: (2/group).
|
0, 0.03, 0.15 or 0.75 Lactose. Gavage 182 days Non-GL study GLP not stated. Recovery group: 0, 0.15 or 0.75 1 year.
|
At 0.15 mg/kg bw/day in males on day 183 (mean ± SD). At 0.15 mg/kg bw/day in females on day 183 (mean ± SD) 66.8 ± 10.8. At 0.75 mg/kg bw/day in males on day 183 (mean ± SD) At 0.75 mg/kg bw/day in females on day 183 (mean ± SD) Serum: |
Males on day 184 (mean ± SD): ↓ TT3 (146 ± 19.8 ng/dL vs 76 ± 22 ng/dL). ↓ FT3 (4.21 ± 0.85 pg/mL vs 2.45 ± 0.80 pg/mL). ↑ mortality (0 vs 2).
Females on day 184 (mean ± SD): ↓ TT3 (148 ± 21.6 ng/dL vs 99 ± 16.8 ng/dL). ↓ FT3 (4.05 ± 0.98 pg/ml vs 2.82 ± 0.29 pg/ml). Recovery: all TH levels returned to control levels between days 33 to 61 in both sexes (NOAEL is highest dose tested). |
Males: 0.15 / 0.75 Females: 0.15 / 0.75. Recovery Males: 0.75 / NA. Females: 0.75 / NA.
|
Variations in TT4 levels were not consistent with respect to dose response or over time. No evidence of hypothyroidism. Clinical relevance of the decreased TT3 values was not apparent since there was no indication of a clinical hypothyroid response. Decrease in TT3 consistent with the slight (approximately 2-fold) compensatory increase in TSH. |
K1 Low purity. Impurities include 8.4% lower chain length homologues of PFOS, 1.4% sodium, 0.6% inorganic fluoride, 0.3% perfluorooctanoate, 0.3% nonafluoropentanoic acid, 0.1% heptafluorobutyric acid. No effect on FT4, TT4, or thyroid/parathyroid weight at LOAEL. No clear dose response for TSH in females. Serum PFOS levels similar in both sexes. Authors are affiliated to 3M Company. No details of funding given. |
|
PFOS (potassium salt). CAS No. not given 91%. Thibodeaux et al. (2003). |
Sprague-Dawley rats Female 6-8 /dose.
|
0, 3 or 5 0.5% Tween® 20. Gavage 20 days Non-GL study. GLP not stated. |
NR. |
Females: ↓ TT4 on day 3 – 20 (data only reported in figures). ↓ FT4 on day 3 – 20 (data only reported in figures). Recovery not assessed |
Females: NA / 3*. |
T3 and T4 results largely substantiated other findings in pregnant rats, discounting potential confounding effects of pregnancy. |
K1 Only female animals used. Only three dose groups. No details of impurities. FT3 not measured. Study funded primarily by US EPA, analytic chemistry support from 3M Company. See Table 15 for developmental studies also reported in this paper. |
|
PFOS (potassium salt). CAS No 2795-39-3 98%. Yu et al. (2011). |
Wistar rats Female 12-13/dose. |
0, 0.2, 1.0 or 3.0 0.5 % Tween 20®. Gavage 5 days Non-GL study. GLP not stated. |
At 0.2 mg/kg bw/day (mean ± SE) Serum: 1.09 ± 0.12. At 1.0 mg/kg bw/day (mean ± SE) Serum: 8.20 ± 0.13.
|
Females: ↓ TT4 (data only reported in figures)#. ↑ relative expression MRP2 mRNA. Recovery not assessed.
|
Females: 0.2 / 1. |
MRP2, a member of the ATP-binding cassette transporters, plays an important role in regulating T4 hepatic efflux. PFOS increased hepatic expression of OAPT2 (at higher doses), which could possibly enhance hepatic uptake and metabolism of T4 in rats. PFOS-induced TT4 deficiency is mainly due to the extrathyroidal metabolism of T4, which is probably different from the classic goitrogen, propylthiouracil. |
K1 Only female animals used. No effect on TT3 at LOAEL.TSH, FT3 and FT4 not measured. #THs measured in 6-7animals per dose. The aim of the study was to further identify the major factors contributing to a reduction in circulating TT4 in rats following PFOS exposure. Parameters examined were serum concentrations of TTR and TG as well as transcripts of transporters involved in hepatic T4 uptake and efflux. Study funded by the National Nature Science Foundation of China, Scientific Research Fund of Liaoning Provincial Education Department, and the Program for Changjiang Scholars and Innovative Research Team in University. |
|
PFOS (potassium salt). CAS No. 2795-39-3 >98%. Yu et al. (2009a).
|
Sprague-Dawley rats Male 8-10/dose. |
0, 1.7, 5 or 15 mg/L in drinking water equivalent to 0, 0.15, 0.45 or 1.35**. Drinking water 91 days. Non-GL study GLP not stated. |
At 0.15 mg/kg bw/day (mean ± SE) Serum:5.0 ± 0.3. At 0.45 mg/kg bw/day (mean ± SE) Serum: 33.6 ± 2.1.
|
Males (mean ± SE): ↓ TT4 (40.9 ± 1.8 µg/L vs 23.9 ± 1.3 µg/L) #. Upregulation of hepatic mRNA UGT1A1. Recovery not assessed.
|
Males: 0.15 / 0.45. |
No effect on thyroid weight or TSH levels at any dose. Consistent with previous reports, PFOS decreased serum TT4 levels in rats without significant alteration in TSH. Hepatic UGT1A1, but not UGT1A6, mRNA was up-regulated at 5.0 and 15.0 mg/L (i.e. 0.45 and 1.35 mg/kg bw/day). Treatment lowered hepatic DIO1 mRNA at 15.0 mg/L but increased thyroidal DIO1 mRNA dose dependently. The activity of TPO, NIS, and TSHR mRNA in thyroid were unaffected. These results indicate that increased hepatic T4 glucuronidation via UGT1A1 and increased thyroidal conversion of T4 to T3 via DIO1 were responsible in part for PFOS-induced hypothyroxinemia in rats. |
K1 Only male animals used. No effect on TSH, FT4 or TT3 at LOAEL. FT3 not measured. Significant decrease in TT4 at all doses, with a dose response. #THs measured in 5-6 animals per dose. EFSA. (2012) dose conversion factor of 0.09 for subchronic studies used. EFSA. (2018) present doses as equivalent to 0, 0.09, 0.25 or 0.75 mg/kg bw/day, assumed to be based on a drinking water conversion factor of 0.05 for chronic studies. UGT1A and UGT1A6 in liver used to determine the effect of PFOS on T4 metabolism. Study funded by the National Nature Science Foundation of China. |
Table 8 Repeated dose toxicity studies for PFCAs – PFBA
*Derived by contractor; **calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups). Recovery (controls vs treated groups) |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author conclusions |
Comments |
|
PFBA (ammonium salt). CAS No. not given 28.9% solution in distilled water. Butenhoff et al. (2012a).
|
Sprague-Dawley rats Male and female 10/sex/dose. Recovery group: Male and female 10/sex/dose.
|
0, 6, 30 or 150 (actual dose 0, 5.3, 25.4 or 130.2). Milli-Q or Milli-U water Gavage 28 days. Non-GL study. GLP not stated. Recovery group: 0, 6, 30 or 150 (actual dose 0, 5.3, 25.4 or 130.2) 3 weeks.
|
At 6 mg/kg bw/day in males at end of treatment (mean ± SD) Serum: 24.65 ± 17.63. At 6 mg/kg bw/day in males at end of recovery (mean ± SD) Serum: 0.06 ± 0.02. At 30 mg/kg bw/day in males at end of treatment (mean ± SD) Serum: 38.04 ± 23.15. At 30 mg/kg bw/day in males at end of recovery (mean ± SD) Serum: 0.20 ± 0.13. At 150 mg/kg bw/day in females at end of treatment (mean ± SD) Serum: 10.30 ± 4.50. At 150 mg/kg bw/day in females at end of recovery (mean ± SD) Serum: 0.234 ± 0.165. |
Males (mean ± SD): ↓ TT4 (3.09 ± 0.82 µg/dL vs 1.26 ± 0.26 µg/dL). ↓ FT4 (1.83 ± 0.53 ng/dL vs 0.98 ± 0.34 ng/dL). ↑ absolute thyroid weight (0.011g vs 0.023 g). Females: No effects seen (NOAEL is highest dose tested). Recovery: TT4, FT4, TSH and thyroid weight comparable to controls after a 3-week recovery period, at 6 mg/kg bw/day (but no recovery of TT4 at 150 mg/kg bw/day).
|
Males: NA / 6*. Females: 150 / NA. Recovery Males: 6 / 30. Females: 150 / NA.
|
NOAELs were 6 and 150 mg/kg bw/day for male and female rats respectively, based on reversibility of effects on THs (reported at 6 mg/kg bw/day in males following treatment) after 3 weeks. Male rats appeared more sensitive. Due to lack of dose-response, known difficulties in obtaining thyroid weights, and lack of consistent histological correlations, the thyroid weight increases observed in males at 6 and 30 mg/kg bw/day (but not observed at 150 mg/kg bw/day) were not considered toxicologically significant.
|
K1 No effect on TSH at LOAEL, TT3 and FT3 not measured. No dose response for thyroid weight. Recovery of TT4 and FT4 at 6 and 30 mg/kg bw/day occurred, but the decrease in TT4 seen at the end of treatment at the highest dose tested of 150 mg/kg bw/day did not show recovery. Study funded by US EPA. Authors are affiliated to 3M Company.
|
|
PFBA (ammonium salt). CAS No. not given 28.9% solution in distilled water. Butenhoff et al. (2012a). |
Sprague-Dawley rats Male and female 10/sex/dose. Recovery group: Male and female 10/sex/dose.
|
0, 1.2, 6 or 30 (actual dose 0, 1.4, 6.9 or 32.4) Milli-Q or Milli-U water. Gavage 90 days. Non-GL study. GLP not stated. Recovery group: 0 and 30 (actual doses 0 and 32.4) 3 weeks. |
At 6 mg/kg bw/day in males at end of treatment (mean ± SD) Serum: 13.63 ± 9.12 (no data for end of recovery period). At 30 mg/kg bw/day in males at end of treatment (mean ± SD) Serum: 52.22 ± 24.89. At 30 mg/kg bw/day in males at end of recovery (mean ± SD) Serum: 0.51 ± 0.31. At 30 mg/kg bw/day in females at end of treatment (mean ± SD) Serum: 5.15 ± 3.29. At 30 mg/kg bw/day in females at end of recovery (mean ± SD) Serum: 0.11 ± 0.0 |
Males (mean ± SD): ↓ TT4 (5.27 ± 0.71 µg/dL vs 3.23 ± 0.55 µg/dL) ↑ incidence thyroid follicular hypertrophy/ hyperplasia (40% vs 90%)
Females: No effects seen (NOAEL is highest dose tested Recovery: Males (mean ± SD): ↑ TT4 (5.14 ± 0.33 µg/dL vs 6.37 ± 0.76 µg/dL). FT4 and TSH comparable to controls Incidence of thyroid follicular hypertrophy/ hyperplasia comparable to controls (43% vs 50%)#. |
Males: 6 / 30
Females: 30 / NA
Recovery NA (effects seen in males). |
NOAELs were 6 and >30 mg/kg bw/day for male and female rats respectively. Male rats appeared more sensitive. Insufficient serum for measurement of FT4 not available for control males at the end of treatment. A decrease in mean FT4 was evident in 30 mg/kg bw/day males at the end of treatment, based on a statistically significant reduction when compared to the mean value for the 1.2 mg/kg bw/day group males. |
K1 No effect on TSH at LOAEL, TT3 and FT3 not measured. Thyroid weight not measured. No dose response for hypertrophy/hyperplasia incidence. Male (and female) treatment and recovery groups for 1.2 and 6 mg/kg bw/day not included in study design for PFAS serum concentrations. # Histological observations in 7 control animals and 6 treated animals. Study funded by US EPA. Authors are affiliated to 3M Company. |
Table 9 Repeated dose toxicity studies for PFCAs – PFHxA
*Derived by contractor; **calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups). Recovery (controls vs treated groups) |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author conclusions |
Comments |
|
PFHxA (sodium salt) CAS No. 2923-26-4 100%. Loveless et al. (2009). |
Crl:Cd Sprague-Dawley rats Male and female 10/sex/dose. Recovery group: 10/sex/dose for 30-day recovery period, and 5/sex/dose for 90-day recovery period.
|
0, 20, 100 or 500 NANOpure® water. Gavage 92/93 days (males/females) OECD 408 GLP not stated. Recovery groups: 0 or 500, followed by recovery for 30 days (to day 122/123). 0, 20 or 100, followed by recovery for 90 days (to day 182/183). |
NR. |
Males: ↓ body weight (transient on, days 42 to 105, data only reported in figures) ↑ thyroid follicular epithelial hypertrophy (day 92, 2/10 animals). Females: ↑ thyroid follicular epithelial hypertrophy (day 92, 4/10 animals). Recovery: Males: ↑ incidence thyroid follicular epithelial hypertrophy on recovery days 30 and 90 (3/10 and 2/10 animals respectively). Females: ↑ thyroid follicular epithelial hypertrophy on recovery day 30 (6/10 animals). Comparable to controls on recovery day 90 ↑ thyroid weight on recovery day 30. Comparable to controls on recovery day 90. Data NR. |
Males: 100 / 500. Females: 100 / 500. Recovery Males: 100 / 500. Females: 500 / NA.
|
Increased female thyroid weights in the 30-day recovery group may be adverse and treatment related. Thyroid follicular cell hypertrophy is potentially adverse (although may not be relevant to non-rodent species) albeit minimal and likely to be secondary and related to liver hypertrophy and induction of metabolic liver enzymes. Increase in thyroid weight in female rats at 500 mg/kg bw/day in the 30-day recovery group were not seen at the end of the dosing period, nor after 90 days recovery, or in male rats at any time point. |
K1 OECD 408 study. Authors affiliated to the DuPont Company. No details of funding given. |
|
PFHxA CAS No. 307-24-4 >99% NTP (2022a). |
Sprague-Dawley rats Male and female 10/sex/dose.
|
0, 62.6, 125, 250, 500 or 1000. 2% Tween® 80 in deionized water. Gavage 28 days NTP protocol GLP study (FDA GLP Regs). |
At 62.6 mg/kg bw/day in males (mean ± SE) Plasma: 0.378 ± 0.178. At 1000 mg/kg bw/day in females (mean ± SE) Plasma: 6.712 ± 0.841
|
Males (mean ± SE): ↓ TT4 (4.26 ± 0.15 µg/dL vs 3.40 ± 0.23 µg/dL) ↓ FT4 (2.88 ± 0.09 ng/dL vs 2.16 ± 0.17 ng/dL) ↓ TT3 (84.17 ± 5.25 ng/mL vs 68.88 ± 3.76 ng/mL) Females: No effects seen (NOAEL is highest dose tested). Recovery not assessed. |
Males: NA / 62.6
Females: 1000 / NA
|
In general, the effects in male and female rats administered PFHxA were of lower magnitude or not apparent compared with effects following exposure to PFNA or PFDA. The reason for a lack of TSH response (an increase) in the face of substantially low TH concentrations is not clear and not consistent with a disruption in the HPT axis. |
K1 No effect on thyroid weight, thyroid or parathyroid histopathology at LOAEL. Significant decreases in TT4, FT4 and TT3 at all doses, but no clear dose response for TT3. Government funded study. Study was audited retrospectively by an independent QA contractor. |
Table 10 Repeated dose toxicity studies for PFCAs – PFOA
*Derived by contractor; **calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls bs treated groups)
Recovery (controls vs treated groups) |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author conclusions |
Comments |
|
PFOA (ammonium salt). CAS No. not given 97.99%. Butenhoff et al. (2012a).
|
Sprague-Dawley rats Male and female 10/sex/dose. Recovery group: Male and female 10/sex/dose.
|
0 or 30 (actual dose 0 or 27.8). Milli-Q or Milli-U water. Gavage 28 days Non-GL study. GLP not stated. Recovery group: 0 or 30 (actual dose 0 or 27.8) 3 weeks.
|
At 30 mg/kg bw/day in males at end of treatment (mean ± SD) Serum: 145.60 ± 28.25. At 30 mg/kg bw/day in males at end of recovery (mean ± SD) Serum: 14.67 ± 5.30. At 30 mg/kg bw/day in females at end of treatment (mean ± SD) Serum: 7.98 ± 4.03. At 30 mg/kg bw/day in females at end of recovery (mean ± SD) Serum: 0.03 ± 0.02. |
Males (mean ± SD): ↓ body weight gain (data NR). ↓ food consumption (data NR). ↓ TSH (4.88 ± 3.30 ng/dL vs 2.59 ± 0.96 ng/dL). ↓ TT4 (3.09 ± 0.82 µg/dL vs 0.52 ± 0.15 µg/dL). ↓ FT4 (1.83 ± 0.53 ng/dL vs 0.94 ± 0.34 ng/dL). ↑ thyroid follicular epithelial cell height (10.6 ± 2.3 µm vs 14.8 ± 2.4 µm). ↑ incidence thyroid follicular hypertrophy/ hyperplasia (30% vs 100%). Females (mean ± SD): ↓ TT4 (2.06 ± 0.74 µg/dL vs 0.62 ± 0.42 µg/dL). ↓ FT4 (2.00 ± 0.68 ng/dL vs 1.14 ± 0.35 ng/dL). Recovery: Males (mean ± SD): ↓ body weight gain (data NR). ↓ TT4 (3.52 ± 0.56 µg/dL vs 1.40 ± 0.39 µg/dL). ↓ FT4 (1.98 ± 0.43 ng/dL vs 1.26 ± 0.29 ng/dL). ↑ incidence thyroid follicular hypertrophy/ hyperplasia (40% vs 80%). TSH comparable to recovery group controls Thyroid follicular epithelial cell height comparable to recovery group controls. Females: THs comparable to recovery group controls (NOAEL is highest dose tested). |
Males: NA / 30*. Females: NA / 30*. Recovery Males: NA / 30*. Females: 30* / NA.
|
PFOS was used as a comparator to PFBA. No specific discussion of thyroid toxicity.
|
K1 Only two dose groups. TT3 and FT3 not measured. TH effects, and thyroid follicular hypertrophy/ hyperplasia in males were not reversible. Study funded by US EPA. Authors are affiliated to 3M Company.
|
|
PFOA (ammonium salt) CAS No. 3825-26-1 95.2%. Butenhoff et al. (2002) |
Cynomolgus monkeys. Male 4-6/dose. Recovery group: 2/dose.
|
0, 3, 10 or 30 (highest dose suspended on day 12 and reduced to 20 from day 22). No vehicle. Gelatin capsules 26 weeks (182 days). Non-GL study. GLP not stated. Recovery group: 0, 3, 10 or 30 90 days. |
At 3 mg/kg bw/day at week 6 (mean ± SD) Serum: 77 ± 39. |
Males: At week 27 / 183 days (mean ± SD): ↓ TT4 (3.84 ± 0.77 µg/dL vs 2.58 ± 0.17 µg/dL). ↑ TSH (0.40 ± 0.23 µg/dL vs 0.65 ± 0.17 µg/dL). Recovery not assessed for THs.
|
Males: NA / 3*. |
No effects on thyroid histology. There were no clear changes in TH homeostasis. All TH values (TSH, FT3, TT3, FT4, TT4) were within normal range, and there did not appear to be any relevant histological changes nor relevant changes in TT4 or TSH. Serum concentrations of PFOA did not increase in a linear dose-dependent manner. |
K1 Only male animals used. TH levels not reported in the recovery groups. Significant decrease in TT4 at all doses, with no clear dose response. TSH increased at 3 and 10 mg/kg bw/day but not at highest dose. Study funded by 3M Company and member companies of the Association of Plastic Manufacturers of Europe. |
|
PFOA (ammonium salt) CAS No. 3825-26-1 97.2%. Butenhoff et al. (2012c). |
Sprague-Dawley rats Male and female 50-65/sex/dose. |
0, 30 or 300 ppm in diet equivalent to 0, 1.3 or 14.2 (males) and 0, 1.6 or 16.1 (females). Certified Purina Laboratory Chow Diet 2 years Non-GL study. GLP not stated |
NR. |
Males and females: No effects seen (NOAEL is highest dose tested). Recovery not assessed. |
Males: 14.2* / NA. Females: 16.1* / NA.
|
No specific comments on non-neoplastic microscopic findings in the thyroid.
|
K1 No effects on incidence of hyperplasia (C-cell or follicular cell) in males or females. Study funded by 3M Company.
|
|
PFOA (ammonium salt). CAS No. not given Purity not given. Griffith and Long (1980). |
ChR-CD albino rats Male and female 5/sex/dose. |
0, 10, 30, 100, 300 or 1000 ppm in diet equivalent to 0, 0.9, 2.7, 9, 27 or 90**. Purina laboratory chow Diet 90 days Non-GL study GLP not stated. |
NR. |
Males and females: No effects seen (NOAEL is highest dose tested). Recovery not assessed. |
Males: 90* / NA. Females: 90* / NA. |
No specific comments on thyroid toxicity. |
K2 Purity not given. No methodology for histopathology. Thyroid and parathyroid weight and histopathology assessed. EFSA. (2012) dose conversion factor of 0.09 for subchronic studies used. Study funded by 3M Company. |
|
PFOA CAS No. 335-67-1 >98%. NTP (2022a). |
Sprague-Dawley rats Male and female 10/dose.
|
0, 0.625, 1.25, 2.5, 5 or 10 (males) or 0, 6.25, 12.5, 25, 50, or 100 (females) 2% Tween® 80 in deionized water. Gavage 28 days NTP protocol GLP study (FDA GLP Regs).
|
At 0.625 mg/kg bw/day in males (mean ± SE) Plasma: 50.690 ± 2.207. At 6.25 mg/kg bw/day in females (mean ± SE) Plasma: 0.491 ± 0.072. |
Males (mean ± SE): ↓ TT4 (2.34 ± 0.24 µg/dL vs 0.21 ± 0.05 µg/dL). ↓ FT4 (2.14 ± 0.13 ng/dL vs 0.44 ± 0.04 ng/dL). ↓ TT3 (88.55 ± 5.58 ng/mL vs 53.52 ± 1.45 ng/mL). Females (mean ± SE): ↑ TSH (10.05 ± 0.81 ng/mL vs 14.08 ± 1.17 ng/mL)#. Recovery not assessed. |
Males: NA / 0.625. Females: NA / 6.25.
|
The reason for a lack of TSH response in the face of substantially low TH concentrations is not clear and not consistent with a disruption in the HPT axis. |
K1 No effect on thyroid weight or thyroid histopathology at LOAEL. FT3 not measured. Significant decrease in TT4, FT4 and TT3 at all doses in males, but no clear dose response. Significant increase in TSH in females at all doses, but no clear dose response. #THs measured in 9 female animals at 100 mg/kg bw/day. Government funded study. Study was audited retrospectively by an independent QA contractor. |
Table 11 Repeated dose toxicity studies for PFCAs – PFNA
*Derived by contractor; **calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups) Recovery (controls vs treated groups) |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author conclusions |
Comments |
|
PFNA |
Sprague-Dawley rats Male and female 10/dose.
|
0, 0.625, 1.25, 2.5, 5 or 10 (males) 0, 1.56, 3.12, 6.25, 12.5 or 25 (females) 2% Tween® 80 in deionized water. Gavage 28 days NTP protocol GLP study (FDA GLP Regs).
|
At 0.625 mg/kg bw/day in males (mean ± SE) Plasma: 56.730 ± 1.878. At 1.56 mg/kg bw/day in females (mean ± SE) Plasma: 26.400 ± 1.085. At 3.12 mg/kg bw/day in females (mean ± SE) Plasma: 54.360 ± 2.486.
|
Males (mean ± SE): ↓ TT4 (2.36 ± 0.27 µg/dL vs 0.21 ± 0.07 µg/dL). ↓ FT4 (2.16 ± 0.15 ng/dL vs 0.55 ± 0.02 ng/dL). Females (mean ± SE): ↓ TT4 (4.37 ± 0.41 µg/dL vs 2.81 ± 0.17 µg/dL). ↓ FT4 1.70 ± 0.20 ng/dL vs 1.10 ± 0.10 ng/dL). Recovery not assessed. |
Males: NA / 0.625. Females: 1.56 / 3.12. |
The reason for a lack of TSH response in the face of substantially low TH concentrations is not clear and not consistent with a disruption in the HPT axis. |
K1 No effect on thyroid weight, thyroid or parathyroid histopathology at LOAEL. FT3 not measured. Significant decrease in TT4 and FT4 at all doses in males, but no clear dose response for TT4. THs not measured in any animals at the top two doses due to mortality. Government funded study. Study was audited retrospectively by an independent QA contractor. |
Table 12 Repeated dose toxicity studies for PFCAs – PFDA
*Derived by contractor; **calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups). Recovery (controls vs treated groups). |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author conclusions |
Comments |
|
PFDA |
Sprague-Dawley rats / Male and female 10/dose.
|
0, 0.156, 0.312, 0.625, 1.25, or 2.5 2% Tween® 80 in deionized water. Gavage 28 days NTP protocol GLP study (FDA GLP Regs).
|
At 0.156 mg/kg bw/day in males (mean ± SE) Plasma: 8.51 ± 0.59. At 0.156 mg/kg bw/day in females (mean ± SE) Plasma: 11.21 ± 0.43. At 0.312 mg/kg bw/day in males (mean ± SE) Plasma: 23.03 ± 1.77. At 0.312 mg/kg bw/day in females (mean ± SE) Plasma: 25.70 ± 1.05. |
Males (mean ± SE): ↓ TT4 (4.36 ± 0.32 µg/dL vs 3.24 ± 0.18 µg/dL). ↓ FT4 (2.02 ± 0.21 ng/dL vs 1.17 ± 0.11 ng/dL). Females (mean ± SE): ↑ absolute thyroid weight (0.0253 ± 0.0010 g vs 0.0336 ± 0.0016 g) ↑ relative thyroid weight (0.11 ± 0.00 g vs 0.14 ± 0.01 g). Recovery not assessed. |
Males: 0.156 / 0.312. Females: 0.156 / 0.312.
|
The reason for a lack of TSH response in the face of substantially low TH concentrations is not clear and not consistent with a disruption in the HPT axis. |
K1 No effect on thyroid histopathology at LOAEL. FT3 not measured. Decrease in TT4 only seen at 0.312 mg/kg bw/day in males with no clear dose response. Government funded study. Study was audited retrospectively by an independent QA contractor. |
Table 13 Repeated dose toxicity studies for PFCAs - PFTeDA
*Derived by contractor; **calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups). Recovery (controls vs treated groups). |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author conclusions |
Comments |
|
PFTeDA CAS No. not given 96.5%. Hirata-Koizumi (2015).
|
Crl:CD (SD) rats Males and females 7-12/sex/dose. Recovery group: Males and females 5/sex/dose.
|
0, 1, 3 or 10 0.5% water solution of carboxymethylcellulose sodium. Gavage Males: 42 days beginning 14 days prior mating, females: 14 days prior to mating, gestation and to PND5 OECD 422 GLP. Recovery group: 0 or 10 14 days. |
NR. |
Males: No effects seen# (NOAEL is highest dose tested). Females: No effects seen# (NOAEL is highest dose tested). Recovery:Effects on thyroid weight and histopathology comparable to controls.
|
Males: 10* / NA. Females: 10* / NA.
|
Increased incidence of thyroid follicular cell hypertrophy was observed in males at 3 and 10 mg/kg bw/day during treatment and in males at 10 mg/kg bw/day following recovery, but this was not significantly different to controls. THs not measured because no change in thyroid weight.
|
K2 Only 7 males/dose in the main group. OECD 422 recommends that each group be started with at least 10 males and 12-13 females. Results of repeated dose part of OECD 422. Thyroid weight and histopathology assessed. #Thyroid weight measured in 7/sex/ dose. Funded by the Ministry of Health, Labour and Welfare, Japan, and supported by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare, Japan. |
Table 14 Repeated dose toxicity studies for PFCAs - PFHxDA
*Derived by contractor; **calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups). Recovery (controls vs treated groups). |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author conclusions |
Comments |
|
PFHxDA CAS No. not given 95.3%. Hirata-Koizumi (2015).
|
Crl:CD (SD) rats Males and females 7-12/sex/dose. Recovery group: Males and unmated females 5/sex/dose.
|
0, 4, 20 or 100 0.5% water solution of carboxymethylcellulose sodium. Gavage Males: 42 days beginning 14 days prior mating. Females: 14 days prior to mating, gestation and to PND5 OECD 422 GLP. Recovery group: 0 or 100. Males: 14 days Females: 14 days (recovery group females were not mated). |
NR. |
Males: ↑ relative thyroid weight (mean ± SD): 18.94 ± 1.6 mg vs 24.26 ± 4.28 mg). No effect at 100 mg/kg bw/day (see recovery)#. Females: ↓ TT3 (mean ± SD: 0.734 ± 0.023 ng/mL vs 0.606 ± 0.036 ng/mL)#. Recovery: Males at 100 mg/kg bw/day (mean ± SD): ↓ TT4 (117.50 ± 15.00 ng/mL vs 89.25 ± 11.87 ng/mL). Thyroid weight comparable to controls. Females: THs and thyroid weight comparable to controls. |
Males: 4 / 20. Females: NA / 4*. Recovery Males: 20 / 100*. Females: 100* / NA.
|
Effects on the thyroid (THs TT3, FT4 and TT4, histopathology and weight) were not consistent between sexes and lacked clear dose-dependency. Results indicate that PFHxDA may slightly affect the thyroid system through a similar mechanism to PFTeDA and PFDA based on other studies. NOAEL concluded to be 4 mg/kg bw/day for repeated dose toxicity (sex not specified). |
K2 Only 7 males/dose in the main group. OECD 422 recommends that each group be started with at least 10 males and 12-13 females. Results of repeated dose part of OECD 422. No effects on TSH in treatment or recovery groups. No effect on thyroid histopathology. FT3 not measured. Increase in thyroid weight in males shows no dose response. #THs and thyroid weight measured in 7/sex/ dose. Effects on TT4 seen in males at 100 mg/kg bw/day following the recovery period, but not during treatment. Effects on TT3 seen in females reported at 4 mg/kg bw/day during treatment, but no effect following the recovery period. Recovery group females were administered PFHxDA for 42 days without mating. Funded by the Ministry of Health, Labour and Welfare, Japan, and supported by a Health and Labour Sciences Research Grant from the Ministry of Health, Labour and Welfare, Japan. |
Table 15 Developmental toxicity studies for PFSAs – PFBS
*Derived by contractor; ** calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups). Recovery (controls vs treated groups). |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author conclusions |
Comments |
|
PFBS (potassium salt) CAS No. 9420-49-3 98%. Feng et al. (2017). |
ICR mice Female (pregnant) 10/dose.
|
0, 50, 200 or 500 0.1% carboxymethyl cellulose. Gavage GD1-GD20 Non-GL study GLP not stated. |
At 50 mg/kg bw/day in dams at GD20 (mean ± SE). At 200 mg/kg bw/day in dams at GD20 (mean ± SE). At 50 / 200 mg/kg bw/day in F1 pups Serum: NR. |
Maternal effects (mean ± SE): ↓ TT3 (90.63 ± 3.22 ng/dL vs 75.55 ± 3.99 ng/dL). ↓ FT4 (16.81 ± 0.70 pg/ml vs 14.74 ± 0.51 pg/ml). Offspring effects: (female) (mean ± SE): ↓ TT4 and TT3 on PND1, PND30 and PND60 (data only reported in figures). Recovery not assessed. |
Maternal: F1 pups: |
Prenatal exposure causes permanent hypothyroxinemia in female mice.
|
K1 FT3 not measured in dams or offspring. Trh mRNA in hypothalmus not measured in dams. Decreases in TT4, TT3 and FT4 in dams and decreases in TT4 and TT3 in pups do not show a clear dose response. Increases in TSH in dams and pups do not show a clear dose response. Increase in Trh mRNA does not show a clear dose response. THs and mRNA measured in 30, 10 and 10 offspring at PND1, PND30 and PND60, respectively. Funded by the National 973 Program and the National Natural Science Foundation. |
Table 16 Developmental toxicity studies for PFSAs – PFHxS
*Derived by contractor; ** calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups). Recovery (controls vs treated groups). |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author conclusions |
Comments |
|
PFHxS (potassium salt) CAS No. 3871-99-6 ≥98%. Gilbert et al. (2021). |
Long-Evans rats Female (pregnant) 6-9/group.
|
0 or 50 2% Tween-20® and deiodinized Water. Gavage GD6-PND21 Non-GL study GLP not stated |
NR |
Maternal effects: ↓ TT3 in serum (data only reported in figures) ↓ FT4 in serum (data only reported in figures) Offspring effects: ↓ TT3 in serum (data only reported in figures) ↓ FT4 in serum (data only reported in figures) Recovery not assessed. |
Maternal: NA / 50*. F1 pups: NA / 50*. |
No effect on expression of TH-response genes in offspring cortex. Reliance on serum THs as prescriptive of specific neurodevelopmental outcomes may be too simplistic. Mapping the relationships between serum and brain tissue THs may more accurately identify xenobiotics of neurotoxicological concern. Thyroid gland transcripts involved in hormone synthesis (Tpo) and responsive to thyroid stimulating hormone (Nis) were not significantly altered in thyroid glands collected from dams on PND22 or pups on PND0, PND6, or PND14. Despite significant reductions in serum and brain T4 in the early neonatal period, the few effects observed were not consistent with what would be expected based on serum THs. |
K2 Only two dose groups. Some basic methodology is not given, but the study is well reported. No effect on serum TSH at LOAEL. No effect on brain TT3 at LOAEL. FT3 not measured. THs measured in 8 or 10 offspring at 0 or 50 mg/kg bw/day, respectively. Authors declared no conflicts of interest. No details of funding given. |
|
PFHxS (potassium salt) CAS No. 3871-99-6 >98%. Ramhøj et al. (2018). |
Wistar rats Female (pregnant) 8/dose.
|
0, 25 or 45 Corn oil. Gavage GD7-PND22 Non-GL study (range finding study for main study -see below) GLP not stated. |
At 25 mg/kg bw/day in dams (mean ± SD) |
Maternal effects: Offspring effects: ↓ TT4 (data only reported in figures). Recovery not assessed. |
Maternal: NA / 25. F1 pups: NA / 25. |
No author conclusions for the range finding study and thyroid effects. |
K1 Only three dose groups. Range finding study. Only TT4 measured. No specific discussion of thyroid toxicity. Funded by the Danish Centre on Endocrine Disruptors and the Environmental Protection Agency, Ministry of Environment and Food of Denmark. |
|
PFHxS (potassium salt) CAS No. 3871-99-6 >98%. Ramhøj et al. (2018). |
Wistar rats Female (pregnant) 16-20/dose.
|
0, 0.05, 5 or 25 Corn oil. Gavage GD7-PND22 Non-GL study GLP not stated |
NR |
Maternal effects: Offspring effects: ↓ TT4 (data only reported in figures). Recovery not assessed. |
Maternal: 0.05 / 5. F1 pups: 0.05 / 5.
|
A marked effect on TT4 levels was seen in both dams and offspring. PFHxS is an effective TH disruptor in rats. The observed postnatal TT4 decreases in offspring, seen at PND16/17, were likely due to lactational transfer of PFHxS. |
K1 Only TT4 reported. #THs measured in 13-20 animals /dose in dams, and in one male and one female per litter (where the litter comprises both sexes), from 14-18 litters/ dose. Funded by the Danish Centre on Endocrine Disruptors and the Environmental Protection Agency, Ministry of Environment and Food of Denmark. |
|
PFHxS (potassium salt) CAS No. 3871-99-6 >98%. Ramhøj et al. (2020). |
Wistar rats Female (pregnant) 16-20/dose.
|
0, 0.05, 5 or 25 Corn oil. Gavage GD7-PND22 Non-GL study GLP not stated |
NR |
Maternal effects: ↓ TT3 (data only reported in figures) #. Offspring effects: Recovery not assessed. |
Maternal: 5* / 25*. F1 pups: 0.05* / 5*. |
TH levels decreased in a dose dependent manner, with no effect on TSH levels. HPT axis not activated. No evidence of TH-mediated neurobehavioral disruption in offspring, but current rodent models are not sufficiently sensitive to detect adverse neurodevelopmental effects of maternal and perinatal hypothyroxinemia. |
K1 TT4 levels reported in Ramhøj et al. (2018). No effect on TSH or thyroid histopathology in dams or offspring at the LOAEL. #THs measured in 15-20 animals/dose in dams. TT3 and TT4 measured in up to one male and one female per litter, from 14-18 litters/dose. Thyroid weight measured in 11-16 offspring/dose. Funded by the Danish Centre on Endocrine Disruptors and the Environmental Protection Agency, Ministry of Environment and Food of Denmark. |
Table 17 Developmental toxicity studies for PFSAs – PFOS
*Derived by contractor; ** calculated according to EFSA. (2012); NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/mL) |
Observed effects at LOAEL (controls vs treated groups). Recovery (controls vs treated groups). |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author comments |
Comments |
|
PFOS (potassium salt) CAS No. 2795-39-3 ≥98%. Conley et al. (2022). |
Sprague-Dawley rats Female (pregnant) 5/dose.
|
0, 0.1, 0.3, 1, 2 or 5 0.5 % Tween-80®. Gavage GD8-PND 2 Non-GL study GLP not stated.
|
At 0.1 mg/kg bw/day in dams on PND2 (mean ± SE). Serum: 2.2 ± 0.1. At 0.3 mg/kg bw/day in pups (mean ± SE) Serum: NR. |
Maternal effects (mean ± SE): ↓ TT4 on PND2 (32.5 ± 2.5 ng/mL vs 25.8 ± 2.3 ng/mL). Offspring effects (mean ± SE): ↓ TT4 on PND2 (6.38 ± 2.8 ng/mL vs 4.92 ± 0.59 ng/mL). Recovery not assessed. |
Maternal: NA / 0.1*. F1 pups: 0.1/ 0.3*. |
PFOS did not decrease maternal FT3 at any dose. Decreases in serum THs are potentially involved in the observed reductions in pup body weights and growth (at higher doses), which requires additional investigation.
|
K1 No effect on TT3 at LOAEL.TSH not measured. #THs measured in 4-5 animals /dose in dams. THs measured in 4-5 animals /dose in offspring. No measurements at 5 mg/kg bw/day due to complete litter loss. Study funded by the US EPA Research and Development and Office of Water.
|
|
PFOS (potassium salt) CAS No. 2795-39-3 86.9%. Chang et al. (2009). Butenhoff et al. (2009b) for maternal body weight data and test guidelines.
|
Sprague-Dawley rats Female (pregnant) 25/dose.
|
0, 0.1, 0.3 or 1.0 0.5% Tween-20® in water. Gavage GD0-PND20 EPA OPPTS 870.6300 and OECD 426 GLP not stated.
|
At 1.0 mg/kg bw/day in dams on GD20, PND4 and PND21 (mean ± SE). Serum: 26.63 ± 3.94, 34.32 ± 31.15 and 30.48 ± 1.29. At 1.0 mg/kg bw/day in female fetuses or pups on GD20 (fetus), PND4, PND21 and PND72 (mean ± SE) Serum: 31.463 ± 1.032, 22.440 ± 0.723, 18.010 ± 0.744 and 1.993 ± 0.293. |
Maternal effects: Offspring effects (mean ± SE): Recovery not assessed. |
Maternal: F1 fetus: |
Interpretation of the toxicological significance of fetal cell proliferation is problematic without further studies. No clear adverse effect on thyroid status (TSH, morphology, hormone homeostasis, proliferation, and liver gene expression). In PND21 male offspring, follicular epithelial cell height was significantly higher than controls, although this difference was considered spurious by the authors because of the low result in the male control group as compared to the female control group. For the significant increase in number of Ki-67-positive thyroid follicular epithelial cells seen in fetus at 1.0 mg/kg bw/day. Due to the range in corresponding control values, the authors were unable to determine the toxicological significance of this finding. |
K1 OECD 426 study. Low purity. No details of impurities given. TSH is the only TH measured. Thyroid follicular epithelial cell counts based on positive staining for Ki-67 as evidence of cell proliferation only measured at 0 and 1 mg/kg bw/day. Thyroid histopathology in 6-10/ dose in offspring. NOAEL (maternal) is highest dose tested. Study funded by 3M Company.
|
|
PFOS (potassium salt) CAS No. not given Purity not given. Fuentes et al. (2006). |
CD-1 mice Female (pregnant) 10-11/dose.
|
0, 1.5, 3 or 6 0.5% Tween-20®. Gavage GD6-GD18 Non-GL study GLP not stated.
|
NR. |
Maternal effects: No effects seen (NOAEL is highest dose tested). Offspring effects: NR. Recovery not assessed. |
Maternal: 6 / NA.
|
Serum THs in pregnant mice at term tended to reduce circulating TT3, FT3, TT4 or FT4 levels. However, no significant differences between dams exposed and non-exposed to PFOS could be noted. |
K2 Purity not given. Information on housing and feeding conditions not given. TT3, FT3, TT4 and FT4 measured. TSH not measured. No details of funding or conflicts of interest given. |
|
PFOS (potassium salt) CAS No. not given 91%. Lau et al. (2003). |
Sprague-Dawley rats Female (pregnant) 17-28/dose.
|
0, 1, 2, 3, 5 or 10 0.5% Tween-20®. Gavage GD2-GD21 Non-GL study GLP not stated.
|
Data only reported in figures. |
Maternal effects: NR. Offspring effects: ↓ FT4 (data only reported in figures). Recovery not assessed. |
Maternal: NR. Offspring NA / 1*.
|
Hypothyroxinemia was detected in treated neonates as early as PND2. Rats are more sensitive than the mouse to PFOS (see entry below). |
K1 No details of impurities given. No effect on TSH or TT3 at LOAEL. FT3 not measured. Dose response in TT4 and TT3 in pups unclear in figures. THs measured in 1 animal/sex/litter (3-8 measurements) in offspring. Maternal and prenatal data reported in Thibodeaux et al. (2003). Study funded primarily by US EPA, analytic chemistry support from 3M Company. |
|
PFOS (potassium salt) CAS No. not given 91%. Lau et al. (2003). |
CD-1 mice Female (pregnant) 21-22/dose.
|
0, 1, 5, 10, 15 or 20 0.5% Tween-20®. Gavage GD1-GD17 Non-GL study GLP not stated.
|
NR. |
Maternal effects: NR. Offspring effects:
Recovery not assessed. |
Maternal: NR. Offspring: 10 / NA.
|
No specific comments on thyroid toxicity. Most offspring exposed to 15 or 20 mg/kg bw/day did not survive for 24 h after birth. 100% mortality from PND3 at both doses. |
K1 No details of impurities given. TT4, FT4, TT3, TSH measured. FT3 not measured. THs measured in 1/sex/litter (3-7 measurements) in offspring. Maternal and prenatal data reported in Thibodeaux et al. (2003). Study funded primarily by US EPA, analytic chemistry support from 3M Company. |
|
PFOS (potassium salt) CAS No. not given 86.9%. Luebker et al. (2005). |
Sprague-Dawley rats Female 8-20/dose.
|
0, 0.4, 0.8, 1.0, 1.2, 1.6 or 2. 0.5% Tween-80®. Gavage 42 days prior to and throughout mating, lactation to GD20 or LD4. Non-GL study. GLP not stated.
|
At 0.4 mg/kg bw/day in dams on GD21, GD7, GD15 and GD21 (mean ± SD). Serum: 40.7 ± 4.46, 40.9 ± 5.89, 41.4 ± 4.80 and 26.2 ± 16.1. At 0.4 mg/kg bw/day in pups. Serum: NR. |
Maternal effects (mean ± SD). Offspring effects (mean ± SD): Recovery not assessed. |
Maternal:
F1 pups: |
Results did not suggest a hypothyroid state in pups. The lack of a major increase in TSH, and that liver malic enzyme (a marker for TH response) concentrations were comparable to controls, suggest that pups were in a normal (euthyroid) state. No histopathological changes in pup thyroids. |
K3 Low purity. Impurities include 8.4% lesser homologues of PFOS (C4-C7), 1.9% unspecified impurities, 1.5% metals, 0.6% inorganic fluoride, 0.3% perfluorooctanoic acid, 0.3% nonafluoropentanoic acid and 0.1% heptafluorobutyric acid. No histopathology methodology and number animals/endpoint either low or not given. No effect on TSH, FT3 or TT3 at LOAEL. Decrease in TT4 shows a dose response in dams, but no clear dose response in pups. #THs measured in 6-20 animals/dose in dams, and in 1-13 animals /dose in offspring. Authors affiliated to 3M Company. No details of funding given. |
|
PFOS (potassium salt) CAS No. not given 91%. Thibodeaux et al. (2003). |
Sprague-Dawley rats Female (pregnant) 25-50/dose.
|
0, 1, 2, 3, 5 or 10 0.5% Tween-20®. Gavage GD2-GD20 Non-GL study. GLP not stated.
|
NR. |
Maternal effects: ↓ TT4 on GD7, GD14 and GD21 (data only reported in figures) #. ↓ FT4 on GD7, GD14 and GD21 (data only reported in figures) #. Offspring effects: NR in this paper. Recovery not assessed. |
Maternal: NA NA / 1*. BMDL5/BMD5 (based on maternal serum TT4 on GD7) 0.046 / 0.234. F1 pups: NR. |
Despite a decrease in THs, a feedback elevation of TSH through activation of the HPT axis was not apparent.
|
K1 No details of impurities given. No effect on TSH at LOAEL. Offspring data reported in Lau et al. (2003). #THs measured in 9-14/dose. Study authors do not specify why a 5% response was selected for BMD modelling. Study funded primarily by US EPA, analytic chemistry support from 3M Company. |
|
PFOS (potassium salt) CAS No. not given 91%. Thibodeaux et al. (2003).
|
CD mice Female (pregnant) 60-80/dose.
|
0, 1, 5, 10, 15 or 20 0.5% Tween-20®. Gavage GD1-GD17 Non-GL study. GLP not stated.
|
Data primarily only reported in figures. No data reported at LOAEL. At 10 mg/kg bw/day in dams on GD18 (mean ± SE) Serum: 179 ± 7. |
Maternal effects: ↓ TT4 on GD6 (data only reported in figures). Offspring effects: NR in this paper. Recovery not assessed. |
Maternal: 15 / 20. BMDL5/BMD5 (based on maternal serum TT4 on GD7) 0.352 / 0.513. F1 pups: NR. |
Similar pattern of decreased TT4 to rats. Reductions of TT3 and TT4 without a compensatory elevation of TSH, though puzzling, is not unique. Generally, the mouse appeared to be a less sensitive species than the rat. |
K1 No details of impurities given. Number of animals for TH analysis not given. No effect on TSH, TT3 or FT4 at LOAEL. Dose response for decrease in TT4 unclear from figures. Study authors do not specify why a 5% response was selected for BMD modelling. Offspring data reported in Lau et al. (2003) Study funded primarily by US EPA, analytic chemistry support from 3M Company. |
|
PFOS (potassium salt) CAS No. 2795-39-3 >98%. Wang et al. (2011). |
Wistar rats Female (pregnant) 4-9/dose.
|
0, 3.2 and 32 ppm in diet equivalent to 0.38 or 3.8**. 2% Tween-20® in deionized water mixed with diet power. Diet GD1-PND14 Non-GL study GLP not stated.
|
At 0.38 mg/kg bw/day in dams on PND1, PND7 and PND14 (mean ± SE). At 0.38 mg/kg bw/day in pups on PND1, PND7 and PND14 (mean ± SE). Serum: 5.85 ± 0.33, At 3.8 mg/kg bw/day in pups on PND1, PND7 and PND14 (mean ± SE). Serum: 32.9 ± 0.81, 21.3 ± 1.06 and 25.2 ± 1.27. |
Maternal effects (mean ± SE): ↓ TT4 on PND1 (43.2 ± 1.6 ng/mL vs 29.5 ± 0.8 ng/mL) and PND7 (42.9 ± 1.8 ng/mL vs 30.9 ± 1.0 ng/mL#. Offspring effects (mean ± SE): ↓ TT4 on PND7 (40.3 ± 0.5 ng/mL vs 24.8 ± 1.2 ng/mL) and PND14 (73.7 ± 2.9 ng/mL vs 53.5 ± 1.4 ng/mL). Recovery not assessed. |
Maternal: NA / 0.38*. F1 pups: NA / 0.38*.
|
Exposure- and time-dependent alterations in TH concentrations. Results suggest a complex TH-mediated gene and protein response to PFOS exposure that seems little related to TH homeostasis. |
K1 Only three dose groups. TSH, FT4 and FT3 not measured. Decrease in TT4 in dams and pups does not show a clear dose response. #THs measured in 3-9 animals /dose in dams, and in 4-12 animals /dose in offspring. EFSA. (2012) dose conversion factor of 0.12 for subacute studies used. Funded by National Natural Science Foundation of China. |
|
PFOS (potassium salt) CAS No. 2795-39-3 98%. Yu et al. (2009b).
|
Wistar rats Female (pregnant) 20/dose. CF study: Wistar rats Male and female pups 5/dose.
|
0 or 3.2 mg/kg diet equivalent to 0.29** 0.5 %. Tween-20® mixed with diet powder Diet. Through gestation and lactation. Non-GL study. GLP not stated. CF study: 0 or 0.29 / diet / GD0 to weaning at PND21 or to PND35. Pups exposed prenatally only (TC) postnatally only (CT) prenatally and postnatally (TT). |
At 0.29 mg/kg bw/day in TC male pups on PND21 and PND35 (mean ± SE). Serum: 2.41 ± 0.17 and 0.41 ± 0.02. At 0.29 mg/kg bw/day in TC female pups on PND21 and PND35 (mean ± SE). Serum: 2.40 ± 0.08 and 1.02 ± 0.08. At 0.29 mg/kg bw/day in CT male pups on PND21 and PND35 (mean ± SE). Serum: 4.80 ± 0.22 and 6.64 ± 0.35. At 0.29 mg/kg bw/day in CT female pups on PND35 (mean ± SE) Serum: 7.04 ± 0.59. At 0.29 mg/kg bw/day in TT male pups on PND21 and PND35 (mean ± SE). Serum: 8.95 ± 0.16 and 10.60 ± 0.53. At 0.29 mg/kg bw/day in TT female pups on PND21 and PND35 (mean ± SE) Serum: 8.89 ± 0.07 and 11.53 ± 0.28. |
Maternal effects: NR. Offspring effects (mean ± SE): ↓ TT4 in TC group on PND21 (58.1 ± 3.1 ng/mL vs 46.3 ± 2.7 ng/mL) and PND35 (67.5 ± 3.5 ng/mL vs 54.4 ± 3.3 ng/mL). ↓ TT4 in CT group on PND21 (58.1 ± 3.1 ng/mL vs 41.5 ± 2.6 ng/mL). and PND35 (67.5 ± 3.5 ng/mL vs 43.3 ± 3.0 ng/mL). ↓ TT4 in TT group on PND14 (67.8 ± 3.5 ng/mL vs 42.9 ± 1.7 ng/mL), PND21 (58.1 ± 3.1 ng/mL vs 43.8 ± 2.4 ng/mL) and PND35 (67.5 ± 3.5 ng/mL vs 42.3 ± 2.2 ng/mL). ↑ TTR (TT, PND21, male and female combined; data only reported in figures). Recovery not assessed. |
Maternal: NR. F1 pups (TC, CC, TT): NA / 0.29*. |
Prenatal and postnatal exposure induced hypothyroxinemia in rat pups. No alteration of mRNA expression observed for selected genes important for TT4 deiodination, glucuronidation and TH receptors. |
K1 Only two dose groups. No effect on TT3 or rT3 at LOAEL. TSH not measured. THs measured in 1 male and 1 female (where possible) per nursing dam. EFSA. (2012) dose conversion factor of 0.09 for subchronic studies used. Funded by the National Nature Science Foundation of China and Scientific Research Fund of Liaoning Provincial Education Department. |
Table 18 Developmental toxicity studies for PFCAs - PFOA
*Derived by contractor; ** calculated according to EFSA. (2012): NR – not reported; NA – not applicable; # - no. of animals studied per endpoint differs to the no. of animals treated.
|
Substance / CAS no. / purity / reference |
Strain & species / sex / no. of animals |
Dose (mg/kg bw/day) / vehicle / route of admin / duration / GL study / GLP status |
PFAS concentration (µg/ml) |
Observed effects at LOAEL (controls vs treated groups). Recovery (controls vs treated groups). |
Published NOAEL / LOAEL (mg/kg bw/day) |
Study author conclusions |
Comments |
|
PFOA (ammonia salt) CAS No. 3825–26-1 ≥98%. Conley et al. (2022). |
Sprague-Dawley rats Female (pregnant) 5/dose.
|
0, 10, 30, 62.5, 125 or 250. 0.5 % Tween-80®. Gavage GD8-PND2 Non-GL study GLP not stated.
|
At 10 mg/kg bw/day in dams on PND2 (mean ± SE). Serum: 31.8 ± 1.1. At 10 mg/kg bw/day in pups (mean ± SE). Serum: NR. |
Maternal effects on PND2 (mean ± SE): ↓ TT4 (29.10 ± 0.96 ng/mL vs 16.18 ± 0.90 ng/mL). ↓ TT3 (0.56 ± 0.04 ng/mL vs 0.44 ± 0.02 ng/mL). ↓ FT4 (42.18 ± 7.71 pg/mL vs 25.88 ± 1.99 pg/mL). ↓ FT3 (0.82 ± 0.11 pg/mL vs 0.47 ± 0.13 pg/mL). Offspring effects on PND2 (mean ± SE): ↓ birth weight (7.05 ± 0.32 g vs 6.20 ± 0.15 g) ↓ TT3 (0.12 ± 0.01 ng/mL vs 0.09 ± 0.01 ng/mL). ↓ TT4 (5.87 ± 0.49 ng/mL vs 3.69 ± 0.35 ng/mL). ↓ rT3 on PND2 (0.07 ± 0.1 ng/mL vs 0.04 ± 0.004 ng/mL). Recovery not assessed. |
Maternal: NA / 10*. F1 pups: NA / 10*. |
Decreases in serum THs were potentially involved in the observed reductions in pup body weights and growth, which requires additional investigation, Pup birth weight was reduced in all dose groups, however after adjusting with ANCOVA for birthtime and litter size, significantly reduced only at ≥62.5 mg/kg bw/day (17% and 26% reductions at 62.5 and 125 mg/kg bw/day respectively). |
K1 TSH not measured. Decrease in TT4 and TT3 in dams show a dose response. No dose response in dams for FT4 and FT3. Decrease in TT3, TT4 and rT3 in pups shows no dose response, THs measured in 2-5 animals /dose in offspring. Funded by the US EPA Office of Research and Development and Office of Water. |
Annex B
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Per- and polyfluoroalkyl substances: evaluation of thyroid effects
Literature search
Search terms
- Search terms presented in the EFSA opinion were replicated for the searches.
Scopus (search terms in all fields)
(TITLE-ABS-KEY (perfluoro* OR pfos OR pfoa OR pfas OR ‘fluorotelomer alcohol’ AND TITLE-ABS-KEY (toxicity OR toxi* OR acute OR subacute OR subchronic OR chronic OR mutagen* OR carcino* OR reprotox* OR nephrotox* OR neurotox* OR hepatotox* OR immune OR immuno* OR ‘developmental tox*’ OR thyroid OR endocri* OR endocrine OR estrogen OR oestrogen OR fertility OR tumour OR tumor OR gestat* OR lactat* OR ‘DNA damage’ OR mortality OR adverse OR ‘adverse effect’ OR ‘blood lipid*’ OR ‘serum lipid*’ OR PPAR OR ‘ex vivo’ OR ‘in vitro’ OR ‘in vivo’ OR exvivo OR invitro OR invivo OR cell* OR tissue* OR rodent* OR mouse OR animal* OR rat* OR mice OR rabbit* OR dog* OR monkey* OR ‘experimental animal*’ OR ‘lab* animal* OR ‘lab* animal*’ AND ( EXCLUDE ( SUBJAREA , "CHEM" ) OR EXCLUDE ( SUBJAREA , "MATE" ) OR EXCLUDE ( SUBJAREA , "CENG" ) OR EXCLUDE ( SUBJAREA , "ENGI" ) OR EXCLUDE ( SUBJAREA , "PHYS" ) OR EXCLUDE ( SUBJAREA , "COMP" ) OR EXCLUDE ( SUBJAREA , "EART" ) OR EXCLUDE ( SUBJAREA , "ENER" ) OR EXCLUDE ( SUBJAREA , "SOCI" ) OR EXCLUDE ( SUBJAREA , "ECON" ) OR EXCLUDE ( SUBJAREA , "BUSI" ) OR EXCLUDE ( SUBJAREA , "ARTS" ) ) AND ( LIMIT-TO ( DOCTYPE , "ar" ) OR LIMIT-TO ( DOCTYPE , "re" ) ) AND ( LIMIT-TO ( LANGUAGE , "English" ) OR EXCLUDE ( LANGUAGE , "Russian" ) OR EXCLUDE ( LANGUAGE , "Spanish" ) ) AND ( EXCLUDE ( LANGUAGE , "Italian" ) ) AND ( EXCLUDE ( SUBJAREA , "MATH" )): 5449
PubMed (search terms in title and abstract)
((“perfluoro*“[Title/Abstract] OR “pfos” [Title/Abstract] OR “pfoa”[Title/Abstract] OR “pfas” [Title/Abstract] OR “fluorotelomer alcohol” [Title/Abstract] AND “toxi*”[Title/Abstract] OR “acute” [Title/Abstract] OR” subacute” [Title/Abstract] OR “subchronic” [Title/Abstract] OR “chronic” [Title/Abstract] OR “mutagen*” [Title/Abstract] OR “carcino*”[Title/Abstract] OR “reprotox*” [Title/Abstract] OR “nephrotox*” [Title/Abstract] OR “neurotox*”[Title/Abstract] OR “hepatotox* [Title/Abstract] OR ”immune” [Title/Abstract] OR “immuno*”[Title/Abstract] OR “developmental tox*”[Title/Abstract] OR” thyroid” [Title/Abstract] OR “endocri*” [Title/Abstract] OR “endocrine”[Title/Abstract] OR “estrogen”[Title/Abstract] OR “oestrogen”[Title/Abstract] OR “fertility” [Title/Abstract] OR ”tumour”[Title/Abstract] OR “tumor”[Title/Abstract] OR “gestat*” [Title/Abstract] OR “lactat*”[Title/Abstract] OR “DNA damage” [Title/Abstract] OR “mortality” [Title/Abstract] OR “adverse” [Title/Abstract] OR “adverse effect” OR “blood lipid” [Title/Abstract] OR “serum lipid*” [Title/Abstract] OR “PPAR” [Title/Abstract] OR “ex vivo”[Title/Abstract] OR “in vitro” [Title/Abstract] OR “in vivo” [Title/Abstract] OR “exvivo”[Title/Abstract] OR “invitro” [Title/Abstract] OR “invivo” [Title/Abstract] OR” cell*”[Title/Abstract] OR “tissue*”[Title/Abstract] OR “rodent*”[Title/Abstract] OR “mouse”[Title/Abstract] OR “animal*”[Title/Abstract] OR “rat*"[Title/Abstract] OR E”mice” [Title/Abstract] OR “rabbit*”[Title/Abstract] OR “dog*”[Title/Abstract] OR “monkey*”[Title/Abstract] OR “experimental animal*”[Title/Abstract] OR “lab* animal*”[Title/Abstract] OR “‘lab* animal*[Title/Abstract] AND (English[Filter])): 2569
- Data from both searches were combined and duplicates removed giving a total of 3397 papers.
- To identify papers on specific endpoints key words were used to search in all fields in Endnote. Key words included, but were not limited to,
- Thyro,
- TSH,
- T3,
- T4,
- Thyroxine,
- Triiodothryronine,
- Hypertrophy,
- Thyroid stimulating hormone.
Inclusion and exclusion criteria
- Generic inclusion criteria and exclusion criteria were applied to the data retrieved (Table 19).
Table 19. Generic inclusion and exclusion criteria used during primary screening of titles
|
Inclusion Criteria |
Exclusion criteria |
|
Articles in English language.
|
Articles in other languages |
|
Peer reviewed publications. |
Expert opinions by authoritative bodies commentaries, editorials and letters to the editor. PhD Theses. Extended abstracts, conference proceeding. |
|
Relevant reviews. |
Studies not reporting original results, including comments, letters or editorials Papers without an abstract. |
|
Papers concerning toxicological effects. |
Papers concerned only with methodology. |
|
Studies in humans, rats, mice, rabbits, dogs or monkeys. |
Studies in other species such as fish, birds, foxes, frogs, bears, chickens. Transgenic animals. In vitro studies. Human (epidemiology) studies. |
|
Any experimental animal study, all ages, male and females. |
None. |
|
Oral (feeding, gavage and drinking water studies), Inhalation studies, Dermal studies, Subcutaneous injection (s.c.), Intraperitoneal injection (i.p.), Intramuscular injection (i.m.). |
None. |
|
Any PFAS. |
Mixtures of PFAS. |
Annex C
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Table 20 PFSA chemical structures and molecular weight
CAS Number is either that given in the study or identified based on the chemical name given in the study.
|
PFAS |
CAS no. |
Structure (source: ChemSpider) |
Molecular weight (g/mol) |
|
PFBS. |
375-73-5. |
300.1. |
|
|
PFHxS (potassium salt). |
3871-99-6. |
438.21. |
|
|
PFOS. |
1763-23-1. [potassium salt 2795-39-3] |
500.13 [538.22] |
Table 21 PFCA chemical structures and molecular weight
CAS Number is either that given in the study or identified based on the chemical name given in the study.
|
PFAS |
CAS no. |
Structure (source: ChemSpider) |
Molecular weight (g/mol) |
|
PFBA (ammonium salt). |
10495-86-0. |
231.09. |
|
|
PFHxA (sodium salt). |
2923-26-4. |
336.03. |
|
|
PFHxA. |
307-24-4. |
314.05. |
|
|
PFOA .
|
335-67-1. [ammonium salt 3825-26-1] |
414.07. [431.1] |
|
|
PFNA. |
375-95-1. |
464.08. |
|
|
PFDA . |
335-76-2. |
514.08. |
|
|
PFTeDA. |
376-06-7. |
714.11. |
|
|
PFHxDA. |
67905-19-5. |
814.13. |