What have the FSA/COT done so far? - NAMS Roadmap (2023)
In this guide
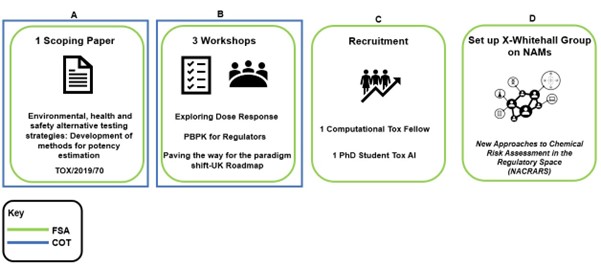
In this guideFigure 1. Diagram of what the FSA and COT have done so far in the NAMs space.
(A) Scoping paper on NAMs (B) 3 international workshops (C) recruited a computational toxicology fellow in Advanced in silico methods of assessing toxicological risk and a PhD Student on artificial intelligence tools to predict chemical risk (D) Set up a Cross Whitehall group on NAMs.
The scoping paper “Environmental, health and safety alternative testing strategies: Development of methods for potency estimation” (TOX/2019/70) was reviewed by the COT in December 2019. The COT were provided with a concise review of currently available methods, which included databases, different kinds of quantitative structure activity relationships (QSAR) methods, adverse outcome pathways (AOPs), High Throughput Screening (HTS), read-across models, molecular modelling approaches, machine learning, data mining, network analysis tools, and data analysis tools using artificial intelligence (AI).
The full workshop reports will be made available through the COT website once published (currently under reserved business). In 2021, the FSA recruited a computational toxicology postdoctoral Fellow at the University of Birmingham and a PhD Student at King’s College London as part of their Interdisciplinary Doctoral Program (LIDo-TOX AI). The fellow and PhD student have been working alongside other Government Departments to understand how NAMs will improve indicative levels of safety in chemical risk assessment. In addition, these new partnerships have helped with networking, research collaboration, training opportunities and other activities in this area. Some of the initial work was presented in 2022 to the COT (COT October 2022 Final Minutes) and we will continue to report on the projects. In 2022, the FSA created a Cross Whitehall steering group: New Approaches to Chemical Risk Assessment in the Regulatory Space (NACRARS). The role of the group is to encourage discussion and partnerships that will be instrumental in creating confidence and reliance in the use of NAMs in chemical risk assessment more widely.
In addition, this work will complement the report produced by Synthesis and Integration of Epidemiological and Toxicological Evidence Subgroup (SETE) of the COT and Committee on Carcinogenicity (COC) which was set up in 2019. Its aim was to review the approaches for synthesising and integrating epidemiological and toxicological evidence that are used by the COT and COC in chemical risk assessments and to provide a pragmatic guidance and transparent reflection of how the COT and COC review data.