The proposal: How does the FSA plan to integrate NAMs in the regulatory space? - NAMS Roadmap (2023)
In this guide
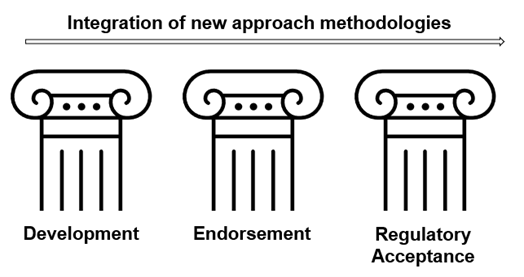
In this guideThe pillars of the NAMs integration approach in the regulatory space will be: Development, Endorsement and Regulatory Acceptance.
Pillar I: Development Formulating the problem space
It is well known that designing and structuring your problem space (Goel and Pirolli,
1992) will be fundamental in its outputs (Newell et al., 1993) and success (Goel and Pirolli, 1989). The science of design consists of the use of efficient, available computational techniques (modelling) for finding the best course of action to respond to real situations, or reasonable approximations of real situations (Simon, 1981). Problem formulation is a systematic approach that identifies all factors critical to a risk assessment (Solomon et al., 2016) and will be key to the chemical risk assessment process.
Figure 3. Landscape to integrate NAMs in the regulatory space.
The FSA plans to use problem exploration techniques which includes interactive sessions at FSA-COT-led workshops, to discuss the use of NAMs in risk assessment and define the questions that need to be answered in order to use NAMs in regulatory chemical risk assessment.
|
Exploration |
| Identification |
| Allocation |
| Adoption |
| Integration |
Figure 4. Formulating the problem space for project outputs.
Pillar II: Endorsement
Breaking Down the Silos: Drivers and Obstacles
The Malloy et al. (2017) paper recommends supporting trans-sector and transdisciplinary efforts to integrate predictive toxicology “Use existing efforts to bring together regulators, industry, civil society, and academics to agree on testing protocols for nanotechnologies as a model that then could be adopted in other fields”.
Despite advances, the scope and pace of adoption of NAMs, including toxicogenomics tools and data sets in chemical risk assessment, have generally, not met the ambitious expectations of their advocates (Pain et al., 2020).
Regulatory uptake of NAMs has been hesitant and despite notable improvements and new applications, some of the major obstacles remain, such as unconnected silos, interpretation complexities and acceptance of validation by regulators.
Pillar III: Regulatory Acceptance
Incorporation, Adopters of change, Data to Deployment
The incorporation and implementation of these new predictive capabilities by regulatory agencies brings both challenges and opportunities.
Moving from research to risk assessment to regulatory setting and beyond, the validation and acceptance of these new emerging technologies must be ensured. The FSA plan to learn from other regulatory agencies and other conceptual frameworks (e.g. IPBES Conceptual Framework (Diaz et al., 2015) i.e. to structure the syntheses that will inform policy, and to improve comparability across various assessments carried out at different spatial scales, on different themes, and in different regions) in different settings.
NAMs are gaining traction as a systematic approach (Sturla et al., 2014) to support informed decisions on chemical risk assessment. Adapting how we assess risk will be, and has always been, a challenge, but big changes have, and can, occur through innovation that facilitates and accelerates adaptation (Rogers, 2003). In Attwell’s 1992 paper, it was concluded that a sensible way forward, was to treat a knowledge barrier approach to technology diffusion, as a distinct theory in its own right. The diffusion of technology is reconceptualized in terms of organizational learning, skill development, and knowledge barriers. As knowledge barriers are lowered, diffusion speeds up, and a transition is observed from an early pattern in which the new technology is typically obtained as a service to a later pattern of in-house provision of the technology.
The “rate of adoption is the relative speed with which an innovation is adopted by members of a social system” Rogers (2003). Therefore, throughout the process the FSA will strive to be transparent and engage with the public. Techniques for adoption and integration could include the spherical cow approach in order for maximum understanding for all stakeholders, including the consumer. Data visualisation exploration techniques could be applied (e.g., Mondrian and Manet (Unwin et al., 1996)) to make use of multidimension data. Incorporating data sets such as Open FoodTox into the latest predictive models will increase predictive capacity for chemicals with little or no tox data.
As stated in the KTN Innovation 4.0 Playbook “Technological applications of AI require explicit consideration of ‘explainability’ and trust”. The FSA propose to have data integrity and data capability ambitions to fulfil this.
Data integrity and data capability
The FSA will strive to the following ambitions:
- facilitate data tools.
- enhance computational resources.
- develop and support training and skills in computer science/data.
- build confidence and trust in data usage.
- provide data integrity and data cleaning.
- demonstrate leadership in developing new methodologies towards using the best scientific available tools towards risk assessment.
Transition and Integration
Innovative technologies should be reviewed and evaluated once, prior to integration into the risk assessment/as a chemical testing method, as part of the risk assessment strategies for chemical testing for human health and the environment. Using an evidence-driven approach, to address the data gaps in the risk assessment process, will facilitate the acceptance and validity of these NAMs, as well as pave the way for alternative testing strategies with confidence.
It is hard for new technologies to be accepted because regulations, infrastructure, user practices and maintenance networks are aligned to the existing technology (Geels, 2002). In order for these new technologies to progress, users have to integrate them into their practices, organisations and routines. This involves learning/training, adjustments and ‘domestication’ (Lie and Sørensen, 1996). It is known that links between technical and social elements provide stability leading to sociotechnical change.
The FSA will strive to take into account using the Political, Economic, Sociological, Technological, Legal and Environmental (PESTLE) analysis method towards making strategic decisions. Using guiding principles from the Green Book we can start to analyse the design and use of monitoring and evaluation before, during and after implementation.
Inserting economics values such as Disability-adjusted life years (DALYs), quality- adjusted life year (QALY) Willingness-To-Pay (WTP) and even into chemical space will be key to demonstrate that applying such values would lead to better and more informed decisions. In HSEs Value of a Life Year (VOLY) report it stated that it would have major implications not only for efficiency of government spending but also for equity in population wellbeing.
This COT FSA UK roadmap will pave the way towards acceptance and integration of NAMs into safety and risk assessments from a regulatory perspective and make use of the UK government Research & Development Roadmap which will form part of the wider systems thinking approach.
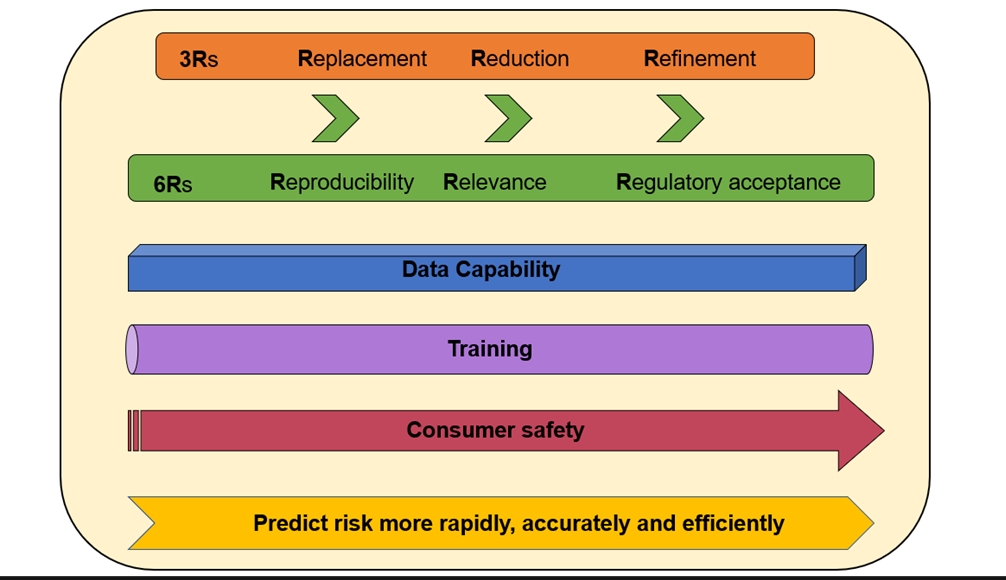
Integration of these technologies as part of the chemical risk assessment process will be fundamental in the future of human safety i.e. the consumer. This will use the best science available to predict and assess risk more rapidly, accurately, and efficiently (Figure 5).
Figure 5. Paradigm shift to towards the vision of predicting risk more rapidly, accurately and efficiently.