The 7 Steps to Integration & Acceptance - NAMS Roadmap (2023)
In this guide
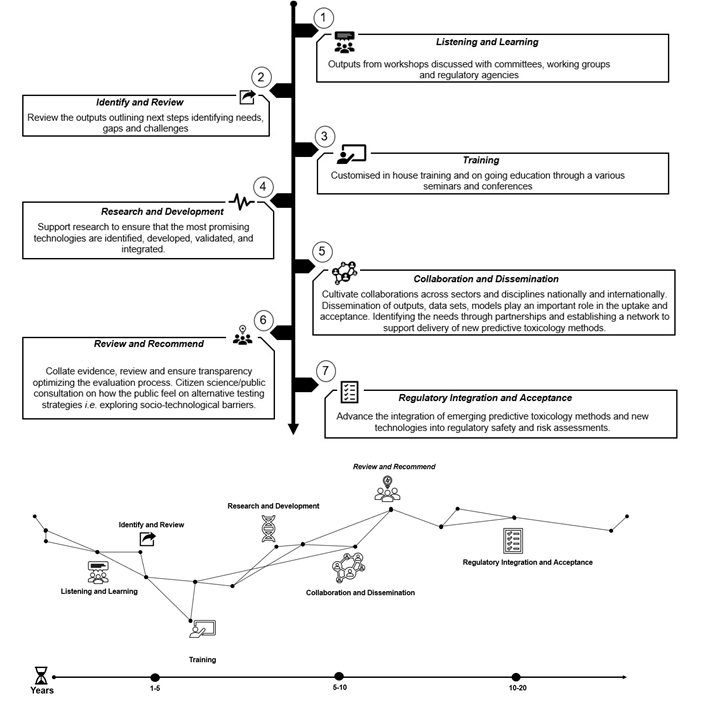
In this guideThe UK Roadmap comprises of 7 steps to integration and acceptance: (1) Listening and Learning (2) Identify and Review (3) Training (4) Research and Development (5) Collaboration and Dissemination (6) Review and Recommend (7) Regulatory Acceptance and Integration.
Figure 6. The UK Roadmap (NB The process is not linear and the steps in the roadmap diagram are not intended to represent a comprehensive set of activities with a precise timescale but should be taken as an illustration of the broad landscape vision).
Overall objectives of the roadmap are to:
- identify latest available NAMs for optimal risk assessment.
- Learn from other regulatory agencies and beyond.
- Validate through case studies.
- Build confidence in NAMs in the regulatory setting.
- Develop skills and training.
- Implement and integrate NAMs in the regulatory setting.
Listening and Learning
Outputs from workshops organised by the FSA and the COT will be discussed with relevant and appropriate committees, working groups and regulatory agencies for opportunity to comment and provide input.
The FSA will review what other regulatory agencies and industry have done and what still needs to be done.
Identify and Review
Review the outputs outlining next steps, identifying needs, knowledge and data gaps Identify opportunities and challenges.
Identify available NAMs and what the pros and cons for each are and where their strengths lie.
Formulate the problem space.
Throughout the process continue to review internal and external research and development and how that may impact on the roadmap.
Customised in-house training and ongoing education through various seminars and conferences.
Skill development: building a resilient organization.
From the perspective of FSA and COT we can then suggest that other regulators / cross government would need similar training.
Research and Development
Support and initiate research to ensure that the most promising technologies are identified, developed, validated, and integrated.
Assess the list of NAMs and other NAMs roadmaps.
Funding and Recruitment
Recruitment of Computational Fellow. Recruitment of Tox AI PhD student.
Collaboration and Dissemination
Cultivate collaborations across sectors and disciplines nationally and internationally.
Dissemination of outputs, data sets and models will play an important role in the uptake and acceptance of NAMs. Identifying the acceptance needs through partnerships and establishing a network to support delivery of new predictive toxicology methods.
Network with regulatory agencies and academics in this workspace.
Set up a hub in the FSA and Cross-Government, industry, and academia to disseminate models etc.
Set up a Cross Whitehall NAMs working group so we can exchange information in this area across government departments.
[The FSA have created and lead the Cross Whitehall group on NAMs New Approaches to Chemical Risk Assessment in the Regulatory Space (NACRARS)]
Citizen science and public engagement.
Review and Recommend
Collate evidence, review, and ensure transparency. Optimizing the evaluation process.
Maintain open transparency throughout.
Public consultation on how the public feel on alternative strategies and approaches. Present work throughout to working groups and scientific advisory committees.
Regulatory Integration and Acceptance
Advance the integration of emerging predictive toxicology methods and new technologies into regulatory safety and risk assessments.
Data integrity and data capability.
Integrate the best possible methodologies in risk assessment to predict risk more efficiently, rapidly and accurately.
Virtual laboratories acceptance.
Integration of NAMs in a regulatory setting.
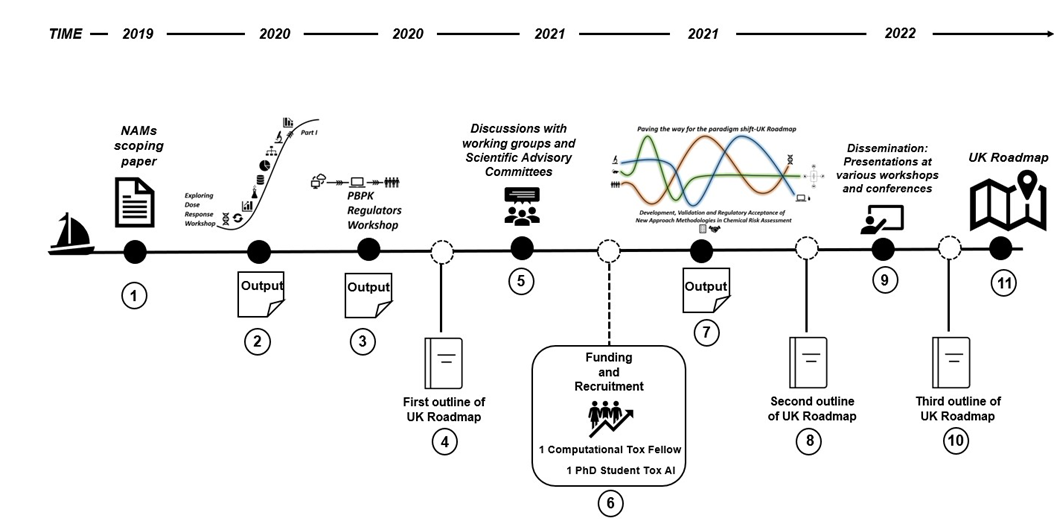
Stepping and (Mile)Stones - How the FSA and COT got here
- NAMs scoping paper taken to COT and reviewed.
- Output of the Exploring Dose Response Workshop (March 2020)
- Output of the PBPK Regulators Workshop (December 2020)
- Output of the discussions with working groups and scientific advisory committies (regular reviews)
- First outline of the UK Roadmap (2021)
- Recruitment of a PhD student and computational tox fellow.
- Output of the Paving the way for the paradigm shift-UK Roadmap Development, Validation and Regulatory Acceptance of New Approach Methodologies workshop (October 2021)
- Second outline of the UK Roadmap (2022)
- Dissemination presentations at varoius workshops and conferences
- Third outline of UK Roadmap (2023)
- Finalisation of the UK Roadmap.
Finding Balance
We are now at a pivotal point of finding the balance between in vitro/in vivo/in chemico/in silico data (Figure 7). Traditional methods were the best available methods at the time and these included the use of animals and it is important to recognise that these have served us well. However, we have now entered a new era that requires us to adopt new technologies to ensure that we continue to use the best methodologies available to make our risk assessment process as reliable and relevant as possible. This will require an integrated workflow approach (Figure 2).
Figure 7. Finding the balance from Mice to Mouse.