Other Committee Activities Joint Expert Groups and Presentations -2022
In this guide
In this guideAssurance of Joint Expert Group opinions
1.177 The Joint Expert Groups (JEGs) were established by the FSA to assess applications for the authorisations of regulated products that were previously authorised by the European Food Safety Authority (EFSA). The three JEGS were FCM JEG which covers food contact materials, AFFAJEG which has responsibility for animal feed and feed additives, and AEJEG which has responsibility for food additives, enzymes and other regulated products. The COT provides support, challenge and assurance to the work of the three JEGS as set out below. In 2022, AFFA JEG was superseded by the reconstitution of the Advisory Committee on Animal Feeding stuffs (ACAF).
Draft Opinion on the extension of use of polyglycerol polyricinoleate
1.178 The COT considered a Risk Assessment prepared by the Joint Expert Group on Additives, Enzymes and other Regulated Products (AEJEG) regarding a n Application for the extension of use of PGPR in edible ices and emulsified sauces (RP217).
1.179 This item was reserved as it covers a draft AE JEG opinion on an application for the extension of use of the additive polyglycerol polyricinoleate, this is treated as draft policy.
1.180 A statement will be published in 2023.
Draft Opinion on the safety of the extension of use of mono- and di- glycerides (E471) for use as a surface treatment of fresh fruits and vegetables
1.181 The COT considered a Risk Assessment prepared by the AEJEG regarding an Application for the extension of use of mono- and di- glycerides for use as a surface treatment of fresh fruits and vegetables.
1.182 This item is currently reserved as it covers a draft AE JEG opinion on an application for the extension of use of the additive E471, this is treated as draft policy.
1.183 A statement will be published in 2023.
Evaluation of renewals of Smoke Flavourings authorisations
1.184 Smoke flavourings are covered by Retained EU Regulation 2065/2003 and therefore need to be authorised before they can be placed on the market in Great Britain (GB). Smoke flavouring primary product authorisations are applicant specific and are valid for 10 years. The current authorisations end in January 2024.
1.185 The FSA has received 8 applications requesting a renewal of the authorisation of smoke flavourings in June 2022, which will be evaluated by the AEJEG. The COT have been kept updated on the progress of these applications.
1.186 The AEJEG meetings for the evaluation of these Applications will commence in the first quarter of 2023.
1.187 This item was discussed as reserved business.
Presentations
UK legislation on Food Contact Materials – an overview – Presentation by FSA FCM policy team
1.188 In light of COT discussions on Biologically-Based Food Contact Materials such as bamboo composites and chitosan as well as anticipated items on ocean bound plastic (OBP) and the COT’s remit to review the output of the Joint Expert Group on Food Contact Materials (FCM JEG), it was considered that an overview of the regulations covering food contact materials would be beneficial.
1.189 FSA policy colleagues provided a brief overview of the overarching food contact material legislation. This included a summary of the enforcing regulations for the UK, which are the Materials and Articles in Contact with Food Regulations 2012 (as amended). The regulations for Great Britain (England, Wales and Scotland) enforce retained Regulation 1935/2004 (“the framework regulation”) and 10/2011 (“the plastics regulation”), with the current EU Regulations continuing to be applicable in Northern Ireland under the terms of the Northern Ireland Protocol.
1.190 All bio-based food contact materials need to meet the requirements under the retained framework regulation, including good manufacturing practice requirements. Depending on their composition some bio-based food contact materials are additionally required to adhere to the requirements under the retained plastics regulation. Finally, bio-based food contact materials are not regulated products in themselves but applications for such substances may need to be made should the business operator wish to use it in a material that falls under the scope of the plastics regulation.
1.191 Advanced materials may be categorised as active and intelligent materials and therefore would need to meet the additional requirements under retained Regulation 450/2009 (“the active & intelligent materials regulation”). Business operators have a responsibility to ensure that they are aware of the individual components of a material or article and are adhering to the requirements of the relevant regulations. Should the individual components of a material or article not fall under the scope of the additional measures, the default is that it must meet the catch-all framework and good manufacturing requirements. However, the plastics regulation does include multi-component plastic containing materials.
1.192 The EU would be implementing (and since have in October 2022) a new recycled plastics regulation and also have new proposals for legislation on FCMs in general, with a consultation running until the end of January 2023. The FSA will consider appropriate options regarding the updating of retained legislation following the EU proposals. This will provide clarity to operators if they are placing material on the UK or EU markets or both. Environmental considerations are also being taken into account as are other legitimate factors. Ultimately however, the legislation has to ensure that products placed on the market are safe for consumers with no adverse health implications.
1.193 Following the presentation, the Committee discussed the practical implications of regulations for testing that needs to be carried out for a new bio-based material. It was noted that a number of steps would need to be adhered to by the business operator given that the material is unlikely to have been previously used in a food contact material scenario). If the material is expected to be used, for example, in multiple material types, different food contact applications or are multifunctional, business operators will need to ensure that they are carrying out appropriate due diligence. They are responsible for ensuring that final products have undergone the appropriate testing and are safe in expected use.
1.194 The Committee acknowledged that while not directly the topic of the discussion, in some situations, i.e. for non-food applications, the food contact regulations may not be entirely applicable for the product produced and therefore, a cross-cutting approach might be needed (examples such as medical devices and biocidal products).
1.195 There continues to be close collaboration between FCM policy at the FSA and other Government Departments, allowing the business operator to be signposted to the relevant Department.
PhD Student and Postdoctoral Fellow presentations
1.196 The FSA and COT have been considering New Approach Methodologies (NAMs) in order to understand the best scientific methodologies available for use in the risk assessment of chemicals, and to consider how these can be incorporated and accepted in a regulatory context.
1.197 In 2021, the FSA started funding a computational toxicology postdoctoral Fellow at the University of Birmingham and a PhD Student at King’s College London as part of their Interdisciplinary Doctoral Program (LIDo-TOX AI). The Fellow and PhD student have been working alongside other Government Departments to understand how NAMs will improve indicative levels of safety in chemical risk assessment.
1.198 In addition, these new partnerships have helped with networking, research collaboration, training opportunities and other activities in this area. The Fellowship and studentship also compliment the work set out in the COT FSA UK Roadmap towards (see paragraph 1.169) using NAMs in chemical risk assessment.
1.199 The Postdoctoral Fellow and the PhD student prepared a yearly review and gave presentations to the Committee on their progress to date.
1.200 The Postdoctoral fellow presented two case studies. The first of these focused on the plasticiser di-2-ethylhexyl terephthalate (DEHTP). The main objective was to derive a health-based guidance value. Concentration-response data obtained from ToxCast studies, via the Chemicals Dashboard (US Environmental Protection Agency (EPA)), were used. The second case study had, as chemical of choice, a perfluorinated substance, perfluorooctanoic acid (PFOA). The main objective was to integrate an in silico workflow with transcriptomics data to derive a health-based guidance value for PFOA that could be compared with that previously recommended by EFSA. Transcriptomics data published by Health Canada were used as a data source from in vitro exposures of Human Liver Microtissues (a commercial preparation of spheroids comprising primary hepatocytes and Kupffer cells) to PFOA.
1.201 The PhD student presented on the hybrid Quantitative Structure Activity Relationship (QSAR) model of mutagenicity developed by the Kings College team, which is, on average, 78% accurate at predicting mutagenicity. The hybrid model consists of two constituent QSAR models which individually are approximately 70% accurate on average. The first QSAR model used molecular fingerprint- based similarity index calculations, whereas the second QSAR model used molecular fragmentation, to identify pro-mutagenic characteristics. Principal component analysis (PCA) was successfully used to identify the key determinants of the predictions.
1.202 The COT Members were impressed with the progress to date and gave feedback to the fellow and PhD student.
Opportunities and outlook for UK food and Chemicals regulation post EU exit Workshop
1.203 The COT, UKHSA and FSA organised a workshop in July 2022 held in Liverpool on “Opportunities and outlook for UK food and Chemicals regulation post EU exit”.
1.204 The workshop was to build on the successful: Royal Society of Chemistry (RSC) Workshop of 2019 : Drivers and scope for a UK chemicals framework.
1.205 The 2019 RSC workshop examined where chemicals regulation might be in the post EU exit landscape in the UK and the opportunities that might be realised from that.
1.206 From the 2019 workshop a number of actions were suggested.
1.207 After three intervening years (2022), several global events have impacted the economy and regulation in the UK including the post EU exit environment. In light of these events, the COT considered it would be timely to have a second workshop to review what has been achieved and what still needs to be done to realise the full potential of EU exit.
1.208 The purpose of the workshop was to review the food and chemical regulatory landscape; its transfer to the UK; future UK development (REACH) and divergence (drivers and supporting science); identify challenges and opportunities to consider where new structures and investment are required to realise and address these.
1.209 A workshop report will follow in 2023.
Working Groups
SETE
Report of the Synthesis and Integration of Epidemiological and Toxicological Evidence Subgroup (SETE) of the Committee on Toxicity and the Committee on Carcinogenicity
1.210 The UK Committees on Toxicity (COT) and on Carcinogenicity (COC) regularly review epidemiological and toxicological evidence in their risk assessments. There is, therefore, a need for guidance on the approaches used by the Committees to integrate these evidence streams, both for scientific consistency and to ensure public transparency. To that end, the Committees established the Synthesising and Integration of Epidemiological and Toxicological Evidence Subgroup (SETE) to review and document current practice and provide applicable guidance.
1.211 SETE recognised that issues on which advice from the Committees is sought varies considerably and hence the guidance proposed should be sufficiently flexible to address this.
1.212 Scoping and problem formulation were identified as the crucial first step in the process. This ensures the right questions are asked, helps make the most efficient use of resources and identifies the most appropriate approaches to use in the assessment. An established system or guidance to assess the separate/different evidence streams should be followed where feasible. For both epidemiological and toxicological evidence, a prescriptive checklist or scoring approach is not recommended. However, identifying the strengths and weaknesses of studies is important. The decision-making process should be robust, transparent, evidence-based, defensible and documented, but equally importantly, it should be easy to use. Collaboration and ongoing dialogue between epidemiologists, exposure experts and toxicologists are strongly advised. Information on mode of action (MOA) can be invaluable for evidence integration as it underpins weight of evidence considerations by providing the mechanistic link between empirical observation and biological plausibility.
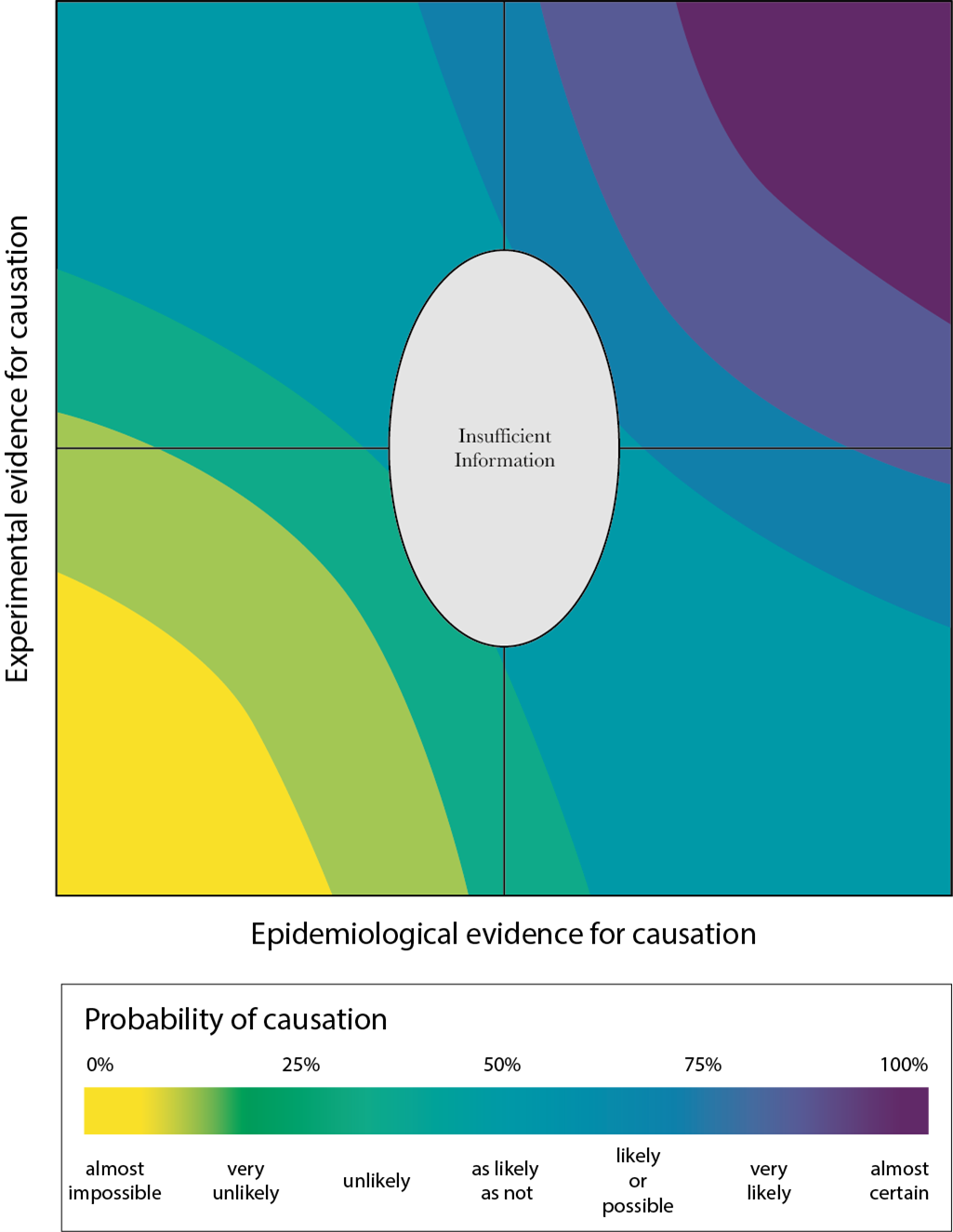
1.213 All lines of evidence should be considered, with no pre-existing hierarchy. One way to clearly depict the influence of the different lines of evidence on causality s via visual representation (Figure 1).
Figure 1: Example for the visual representation of the likelihood of a causal relationship, considering both epidemiological and toxicological data.
1.214 Decisions on whether there is sufficient information to reach a conclusion or whether a causal relationship in humans is more likely or unlikely can be reached based on where the causal relationship appears on a graph. It is important to begin with the initial estimate of causal relationship at the centre of the graph. Depending on whether the toxicological, mechanistic or epidemiological evidence assessed supports or discounts (or has no clear influence on) a conclusion of causality, placement on the graph is then moved accordingly, either in a positive or negative direction. The movement is influenced by several factors, including the strength or weakness of the evidence, any relative weighing given to epidemiological and toxicological studies and the uncertainties associated with the data. As more information is included in the process and/or becomes available, the placement of the toxicological and/or epidemiological evidence can be easily adjusted and the impact on any possible conclusion easily seen.
1.215 In contrast to other approaches, the above visualisation aims to provide a pictorial representation of the consensus views of a Committee on the influence of the different lines of evidence on causation, assessed by debate and agreement of scientific experts. In this way, it provides a more objective means of collating the views of the Committee and communicating the agreed conclusion of a Committee on the likelihood of causation.
1.216 The conclusion should be stated, with an estimate of the overall uncertainty and, where appropriate, guidance on how data gaps could be filled.
1.217 The full SETE report and guidance document (Annex 1) can be found at SETE | Committee on Toxicity (food.gov.uk).
1.218 Please note, the guidance will be trialled by the COT for 2 years and then reviewed.
Joint SACN/COT Working Group on Plant Based Drinks
1.219 The Office of Health Improvement and Disparities (OHID) (previously Public Health England) and the FSA received an increasing number of enquiries regarding the use of plant-based drinks in the diets of infants and young children aged 6 months to 5 years of age.
1.220 Current UK government advice regarding the use of plant-based drinks for infants and young children is that unsweetened calcium-fortified plant-based drinks, such as soya, oat and almond drinks, can be given to children from the age of 12 months as part of a healthy balanced diet; however, rice drinks should not be given due to the levels of arsenic in these products.
1.221 In 2021, the COT was asked to consider the potential risks posed by soya, almond and oat drinks consumed in the diets of these age groups. A COT Statement was published in 2021.
1.222 Overall, the Committee concluded that neither the safety of these drinks, nor the suitability of the current guidance, could be confirmed from a toxicological perspective. The Members agreed that, in addition to potential toxicological concerns, consideration of nutritional issues would also be required to assess whether it was necessary to issue additional advice on the consumption of plant-based drinks in children aged 6 months to 5 years of age. As a result, a joint SACN/COT Working Group was established in 2021, to consider the benefits and risks of plant-based drinks in diets across all life stages.
1.223 In 2022, a call for evidence was issued which aimed to seek evidence with regards to specific aspects of nutrition, safety and toxicity of plant-based drinks. Details on the call for information can be found at: Call for Evidence.
1.224 The Joint Working Group considered the information received in response to this call for evidence, the COT Opinions on plant-based drinks and cow’s milk as well as exposure information and utilised the Benefit-Risk Analysis for Foods (BRAFO) framework in order to compare the health risks and benefits of plant based drinks.
1.225 It is hoped that the report will be published in 2023.
Joint ACNFP/COT Working Group on Cannabidiol (CBD)
1.226 A joint Working Group has been established to review the data on cannabidiol (CBD) submitted as a part of the novel foods applications process.
1.227 The first meeting of the ACNFP/COT subcommittee was held in July and considered the toxicology datasets collated for CBD isolate applications and ultimately concluded that a list of toxicological uncertainties must be tackled before opinions on the safety of CBD isolates can be made.
1.228 The next meeting was held in September and the main topic of discussion was an in-depth review of the data on CBD isolates and synthetic CBD products. By using a refined but detailed table format, the Secretariat hopes to prompt further discussion amongst the subcommittee members. This will inform the next steps to support the review of dossiers in this group by the ACNFP.