Introduction - Risk Assessment of T-2 and HT-2 mycotoxins in Food
In this guide
In this guide7. T2/HT2 are type A trichothecenes and are produced by a variety of Fusarium and other fungal species. Fusarium species grow and invade crops and produce T2/HT2 under cool, moist conditions prior to harvest. T2/HT2 are found predominantly in cereal grains (between 11 – 14% of the samples tested were contaminated) and in particular oats, barley and wheat products (JECFA, 2016). The presence of T2/HT2 is dependent on weather at key growth stages e.g. flowering, and can demonstrate large annual variability.
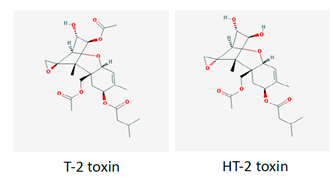
8. The chemical structures of T2/HT2 are shown below in Figure 1.
Figure 1. Chemical structures of T2/HT2.
Occurrence
9. While there are good agricultural practices deployed to manage the presence of mycotoxins in general, they have not proven effective for T2/HT2, given the large dependence on climate/weather. Similarly, reliable rapid testing is not currently available; recent assessments by industry see large variability between LC-MS/MS methods and Calibre/Charm Elisa semi-rapid methods. . Moreover, rapid analytical methods for T2/HT2 are not yet validated, making it difficult to reliably detect and mitigate these toxins at the field level. A science and evidence-based review of the UK oat supply in the context of the FSA/FSS call for data on T2/HT2 has been provided to COT Members (Croucher, 2023). This unpublished review was provided to the FSA by the British Oat & Barley Millers’ Association (BOBMA) and has been provided to Members on a confidential basis.
10. On 2th March 2013, Commission Recommendation 2013/165/EU set out indicative levels for a number of commodities, which are not legally binding but have been used by industry in the EU and UK to gather monitoring data to further understand the risk. The EU have been considering the issue of T2/HT2 for a number of years and proposed maximum levels in 2023, that are due to come into force in July 2024.
11. There are currently no maximum levels agreed at CODEX for T2/HT2 and it is unlikely that any work will be undertaken at CODEX until further geographically representative data allow for a refined exposure assessment by JECFA.
Toxicokinetics
12. The toxicokinetics of T2/HT2 have been reviewed previously by JECFA (2001) and EFSA (2017a).
13. In summary, there is very little information on the in vivo absorption of T2/HT2 in animals after oral administration. In studies in which tritiated T2 was administered directly into the small intestine of male rats, 40 to 57% of radioactivity was found in bile and blood. Only low amounts of T2 were observed in these studies, suggesting extensive hydrolysis to HT2 and other metabolites during the rapid intestinal absorption of T2 (EFSA, 2017a).
14. The presumed rapid absorption is consistent with the fact that the excretion of total radioactivity in the urine and faeces of rats was completed 48 hours after a single oral dose of tritium-labelled T2 administered by gavage (Pfeiffer et al., 1988). T2 radioactivity was rapidly distributed to the liver, kidney and other organs without accumulation in any organ in orally dosed rats and mice (EFSA, 2017a). The metabolism of T2/HT2 in humans and other species is complex and was reviewed previously by EFSA in 2011. Phase I metabolites arise from either hydrolysis of ester group, hydroxylation, or de-epoxidation. These reactions may also occur in combination. Glucuronides are the most prevalent mammalian phase II metabolites of T2/HT2 (EFSA, 2017a).
15. The major metabolic pathway of T2, regardless of the animal species, is rapid deacetylation at the fourth carbon position of T2 resulting in the formation of HT2 (Nathanail et al. 2015).
Toxicity
16. The toxicity of T2/HT2 has been reviewed previously by EFSA (2011, 2017), JECFA (2002, 2016, 2022) and the SCF (2002). All Committees agreed that these trichothecenes were haematotoxic, immunotoxic and caused reduced body weight, and emesis. These effects occurred at lower doses than other toxic effects such as dermal toxicity, developmental and reproductive toxicity, and neurotoxicity. Haematotoxicity was the critical chronic effect of T2; the underlying mode of action (MOA) being the inhibition of protein synthesis, the induction of ribotoxic stress (a response that stimulates MAP kinase signalling) and apoptosis. Mink and pigs have been identified as the most sensitive species to the toxic effects of trichothecenes.