Annex A to TOX/2025/28
Introduction and Background
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Introduction
1. The Food Standards Agency (FSA) is considering the current advice and monitoring programme for marine biotoxins and whether there is a need to update or change existing legislative standards. The main purpose of this work is to identify any emerging marine biotoxins in UK waters, including increased occurrence due to rising temperatures as a result of climate change. The views of the Committee on the Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) were sought on whether the identified emerging marine biotoxins could pose a risk to human health.
2. A scoping paper and a summary paper were presented to the COT in 2023 and 2024 respectively (TOX/2023/59; TOX/2024/25). These provided an overview of emerging marine biotoxins with summaries of any available toxicological information, occurrence data with an emphasis on UK waters, estimated adult exposures to the marine biotoxins and any additional relevant information. The Committee decided that it was not possible to conclude on the risks of the emerging biotoxins due to a lack of information, most notably toxicologic studies, without which deriving health-based guidance values (HBGVs) was not feasible. Instead a numerical risk ranking was proposed by the Committee and discussed in March 2025 (TOX/2025/15) to assist in prioritisation of the biotoxins. Risk rankings for each group of biotoxin were generated by assigning a numerical score to each biotoxin for the following categories: toxicity, occurrence, human case reports, and monitoring.
3. The following statement provides the risk ranking and advice of the COT on whether the identified emerging marine biotoxins would pose a risk to health.
4. Please note, pinnatoxin (PnTX) (TOX/2023/37) and pectenotoxin (PTX) (TOX/2023/58) have been discussed separately and have not been included in this statement.
Background
5. Marine biotoxins are natural toxic metabolites produced by marine phytoplankton and can bioconcentrate in shellfish, and along the food chain. If concentrations of these toxins in shellfish are sufficiently high, then consumption of these shellfish can result in human illness.
6. Marine biotoxins have previously been categorised based on clinical signs but are increasingly being categorised by chemical structure. The structural toxin groups that are generally considered to be of relevance to shellfish harvested in European waters are:
- Domoic acid group (DA),
- Saxitoxin group (STX),
- Okadaic acid group (OA),
- Pectenotoxin group (PTX),
- Azaspiracid group (AZA),
- Yessotoxin group (YTX),
- Cyclic imine group (CI).
7. Marine biotoxins can also be categorised according to their water solubility which determines the extraction protocol required for analysis. The DA and STX groups are hydrophilic, while the OA, PTX, AZA, YTX and CI groups are lipophilic. The DA group is associated with amnesic shellfish poisoning (ASP), the STX group with paralytic shellfish poisoning (PSP) and the OA group with diarrhetic shellfish poisoning (DSP).
8. In the United Kingdom (UK) and European Union (EU), there are currently three major biotoxin groups that are regulated in shellfish, and which are subject to statutory testing to protect human health. The biotoxins specified within the Assimilated EU Regulation (EC) No. 853/2004 (E&W, and Scotland) and EU Regulation (EC) No. 853/2004 (NI) are PSP toxins (STX and relevant analogues), the lipophilic toxin group (OA, AZA, PTX and YTX) and ASP toxin (DA).
9. In the UK the Agri-Food and Biosciences Institute (AFBI) is the Great Britain (GB) National Reference Laboratory (NRL) for marine biotoxins. The Centre for Environment, Fisheries and Aquaculture Science (Cefas) are designated as the official laboratory (OL) for marine biotoxins in England, Wales and Scotland. Northern Ireland’s NRL for marine biotoxins is Wageningen Food Safety Research (WFSR) and the designated OL AFBI who undertake analysis and reporting of shellfish official controls (OCs). A shift from biologically based assays (such as the mouse bioassay (MBA)) for marine biotoxin testing to validated chemical methods has been implemented in the UK and EU due to their increased specificity and ethical concerns over animal use, although biological methods may still be used in limited or exceptional cases.
Emerging marine biotoxins
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
10. Emerging marine biotoxins were identified by evaluating assessments by other authorities including EFSA, Cefas, Food Safety Authority Ireland (FSAI), French Agency for Food, Environmental and Occupational Health and Safety (ANSES) and the French Research Institute for Exploitation of the Sea (IFREMER). A literature search was also conducted to identify any potential emerging marine biotoxins since the publication of these reports. The marine biotoxin groups identified were brevetoxin (BTX), palytoxin (PITX), tetrodotoxin (TTX), novel azaspiracid (AZA) and DA analogues, PTX, cyanobacterial toxins and toxins within the CI family including spirolide (SPX), gymnodimine (GYM), pteriatoxin (PtTX) and PnTX. PTX (TOX/2023/58) and PnTX (TOX/2023/37) have been discussed separately and have not been included in this statement.
11. For the majority of the biotoxins identified limited information or data was available on their toxicology, any human case reports or their occurrence in UK and/or EU waters.
12. Animal toxicological data and where available human case reports have identified five of the emerging biotoxins as neurotoxins, i.e. BTX, TTX, PITX, SPX and GYM. Data from the literature suggested the BTX, TTX and PITX groups interfere with the sodium/potassium voltage gated ion channels resulting in the depolarisation of membranes in excitable and non-excitable cells and contraction of muscle cells (EFSA, 2009b; 2010b; 2017). Hence symptoms of acute exposure to BTX, TTX and PITX in humans overlap with an array of neuromuscular and cardiorespiratory effects. Regarding the remaining CI neurotoxin groups, SPX and GYM, the evidence points to both inhibiting the muscarinic and nicotinic acetylcholine receptors in the central and peripheral nervous system and the neuromuscular junction (EFSA, 2010a). No human case reports could be identified for SPX and GYM exposure. Animal data however characterised acute toxicity of CIs by the rapid onset of systemic neurotoxicity and death. No long-term studies on CIs were available.
13. Of the cyanotoxins, MCs are the most investigated group. Current literature suggested MCs are actively transported into cells by specific organic anion transport proteins (OATPs) and due to the high number of OATPs in the liver, MCs are primarily hepatotoxic; however, distribution to other organs and tissues also occurs. MCs bind to certain protein phosphatases that are involved in a range of regulatory pathways, e.g., those responsible for cytoskeletal structures, cell replication, stress response and DNA repair (Testai et al., 2016; WHO, 2020; 2022). In humans, acute illness following consumption of drinking water contaminated with cyanobacteria typically causes gastroenteritis (Percival and Williams, 2023). Limited toxicological data was available for other cyanotoxins, i.e., anatoxins (ATX), cylindrospermopsin (CYN) and β-methylamino-L-alanine (BMAA). BMAA and ATX demonstrated neurotoxic effects whilst CYN demonstrated cytotoxicity (WHO, 2020). The mechanisms of ATX, BMAA and CYN toxicity are not well understood, and one limitation is a lack of available standards/purified toxins; therefore, only poorly characterised extracts have been used in experimental studies to date.
14. Human intoxications and deaths have been reported for TTX, PITX and MCs; however, only intoxications were reported for BTX, and no human cases have been reported for ATX, BMAA, CYN or any CIs to date. It must be noted that for some cases of human intoxication, the involvement of PITX remains unconfirmed as it was unclear whether the incident could solely be attributed to PITX due to incomplete or missing toxin identification/quantification data (Cefas, 2014). Furthermore, the fatalities from MC exposure occurred not due to consumption of contaminated food or water but after mistreated water was used in renal dialysis (WHO, 2020).
15. No toxicological data, occurrence data or reports of human intoxications were available for PtTX, novel AZA and DA analogues.
16. Little information was available on whether cooking may break down or alter the concentrations of these marine biotoxins. Data was only available for MC and TTX, regarding the former, the data was inconsistent with reports of increases, decreases and no changes after cooking. For TTX the limited information available showed TTX was heat stable and did not decompose during cooking (Islam et al., 2011; Bane et al., 2014; Turner et al., 2015; FAO/WHO, 2016). Literature has shown that cooking can reduce the concentrations of STX and DA through boiling or steaming due to partial leaching into the cooking liquid (EFSA, 2009a; 2009c). However, there is no other information on how cooking effects BTX, PITX, SPX and GYM or other cyanotoxins.
17. Occurrence data for the emerging biotoxins was limited as they are not regulated or included in current routine monitoring programmes. The only recent EU monitoring program was conducted by the French Research Institute for Exploitation of the Sea (IFREMER) over a five-year period (2018-2022) (Amzil et al., 2023). The results from the monitoring programme showed that unregulated lipophilic toxins, i.e. PTXs, PnTX, GYMs, BTXs and MCs, could be identified and quantified in various species of shellfish every year. This program was the first to find MC, GYM and BTX groups in shellfish. Members of the PITXs were not detected in shellfish, but were detected in other seafood organisms, e.g., sea urchins, fish, gastropods and crustaceans.
18. Limited occurrence data on the emerging marine biotoxins was also available in academic publications. Of note was a recent report of a cyanobacterial bloom in Lough Neagh in Northern Ireland (DAERA., 2024). Species known to produce MCs such as Microcystis aeruginosa were identified and ten MC-group toxins were measured in the water with congeners MC-LR and MC-RR present at high concentrations in some algal mats (1,137–18,493 μg/L) (Reid et al., 2024). Vareli et al. (2012) also reported levels ranging from 45-142 µg MC-LR/kg fresh weight in saltwater mussels from Greece. ATX and CYN have only been detected in fish but only outside Europe while BMAA were reported in shellfish from France, Sweden and Greece (Testai et al., 2016; Amzil et al., 2023). SPXs have been identified in shellfish in Norway, Spain, Italy (EFSA., 2010a) and specifically 13-desmethyl spirolide C and 20-methyl spirolide G have been reported in shellfish from Great Britain (Alexander et al, 2024). PITX has been reported in mussels and sea urchins from other European countries, including Greece, Italy and Spain (EFSA, 2009b). TTXs and their analogues unlike the other emerging biotoxins have been reported frequently in gastropods and bivalves from European waters, such as France, Spain, Italy, Greece, the Netherlands, Ireland and the UK (EFSA, 2017; Gerssen et al., 2018; Bacciocchi et al., 2019; Blanco et al., 2019; Bordin et al., 2021; Dhanji-Rapkova et al., 2020; Hort et al., 2020).
19. Due to the limited toxicological information, no HBGVs have been established for BTX and CIs; however, the EU Community Reference Laboratory for marine biotoxins (CRLMB)/EU Regulatory Reference Laboratory (EURL) has proposed a guidance level of 400 µg sum of SPXs/kg shellfish meat (CRLMB, 2005; Pigozzi et al., 2008). Acute reference doses have been derived for PITX and TTXs of 0.2 µg/kg bw (sum of PITX and ostreocin-D) and 0.25 µg/kg bw respectively (EFSA, 2009b; 2017). The World Health Organisation (WHO) proposed a provisional tolerable daily intake (TDI) for MC of 0.04 µg/kg bw (WHO., 2020; 2022); however, the database for other cyanotoxins on repeated, long-term oral exposures was limited and not sufficient to derive a TDI without high levels of uncertainty. No chronic HBGVs or guidance levels have been set either in the EU or other countries for the emerging marine biotoxins discussed here except SPX and MC.
Unranked emerging marine biotoxins
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
20. Of the emerging biotoxins identified, no monitoring data, human case reports, occurrence or toxicological data was available for novel AZA analogues, DA analogues and PtTX. An analogue approach was investigated for ranking these groups (TOX/2025/15); however, the approach was deemed unsuitable by the Committee (see paragraphs 31 and 32).
21. For more in depth discussions on the information identified from the literature and evaluation of the data for the emerging marine biotoxins please see the previous discussion papers (TOX/2023/59; TOX/2024/25).
Risk ranking method
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
22. In the absence of data to establish HBGVs and conduct a risk assessment, a numerical risk ranking method was deemed appropriate to provide a consideration of risk that policymakers can use to inform decisions on whether legislative standards should be updated or changed.
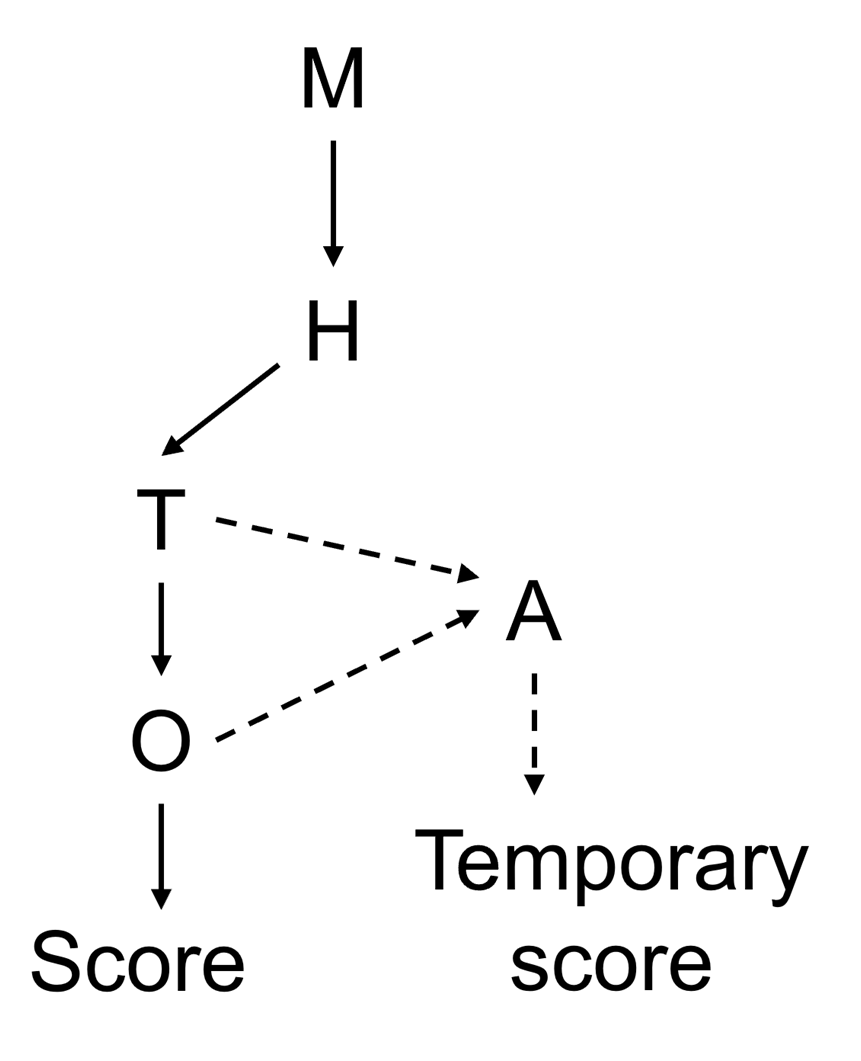
23. A decision tree was proposed to clearly depict the main considerations and to set out the amount of data available for each biotoxin, given the sometimes-limited database (Figure 1). Combining the decision tree with numerical scores for each step of the decision tree clearly depicts the underlying considerations and the weighing of the data. The decision tree considered four main categories of information: monitoring, toxicological data, i.e., human case reports and/or animal tox data, and occurrence data. Each group of emerging biotoxin is numerically scored on a scale of 1-5 for all categories generating a maximum score of 20 where higher scores represent a greater risk to public health. The considerations and weighing of evidence for each group of biotoxin were provided in tabular form, accommodated by a clear narrative explaining the underlying considerations and providing a transparent depiction of which data was driving the risk ranking.
24. An attempt at risk ranking AZA analogues, DA analogues and PtTX, groups which had insufficient data for any of the four categories, was attempted by using an analogue biotoxin (TOX/2025/15); however, the Committee concluded that using an analogue for non-hazard categories was unsuitable. The analogue approach was retained in the decision tree (Figure 1) as a potential future method for creating temporary risk rankings for other biotoxins with limited information.
Figure SEQ Figure \* ARABIC 1. Risk ranking decision tree. M = monitoring; T = toxicity; H = human case reports; O = occurrence; A = analogue. Dashed lines represent the potential path for analogues in the absence of data for T and/or O.
Monitoring
25. Monitoring considered whether the toxins were included in any recent or ongoing official marine biotoxin monitoring programmes either in the UK or EU. Toxins which were extensively monitored were considered the least risk and hence given the lowest score. Information on unofficial research monitoring programmes and monitoring in countries outside the EU may be available, resulting in higher scores. No monitoring was considered the highest risk as the prevalence of the toxin was unknown and therefore the risk was unknown. In these instances, the highest score would be applied.
Human case reports
26. Human case reports considered whether documented cases of human intoxications were available, their severity, and whether any fatalities have been reported. Higher scores were given to toxins for which both intoxications and fatalities have been reported and lower scores for toxins with reports of intoxications but no fatalities. The number of case reports was not considered as it was too variable between toxins, and the information, in general, was very limited. Toxins without information or reports of fatalities or intoxications have also been given a score; however, please note that no reports do not necessarily indicate that no intoxication (potentially even fatalities) have occurred. Underreporting has been noted as an uncertainty for marine biotoxins in general.
27. For this category there are only three scoring options as due to the limited information and uncertainties it was not considered possible to distinguish them further, but the scores have been designated 1, 3 and 5 to maintain an equal weighting of this category compared to the others.
Toxicity
28. Toxicity considered the known adverse effects of each toxin, identified from in vivo animal studies, usually mice or rat. Neurotoxic effects were ranked highest followed by gastrointestinal effects and lastly mild effects such as weakness and general unwellness. A numerical score from 1-5 has been applied, to the endpoints described above and to the consideration on the lethal dose (LD50). Whether a LD50 was considered ‘high’ or ‘low’ or rather ‘higher’ or ‘lower’, was, in this instance, determined qualitatively via the Committee’s judgement rather than quantitatively (i.e., specific LD50 ranges) due to the limited data available. The LD50s were considered to assist in differentiating toxicity profiles between biotoxins; however, the LD50s are based on a limited toxicological database and there was a high uncertainty how much weight can be assigned to them.
Occurrence
29. Occurrence considers documented cases of detection of these toxins either through official routine inspections, one off incidents and/or research efforts. Detection in UK waters was ranked highest followed by Northern EU waters, as they are most like the temperature profile in UK waters. Detection in Mediterranean EU waters would rank lower as the water profile would be different to the UK’s, however, this may change with climate change and increasing water temperatures. Detection outside the UK and EU has not been considered here and would only be considered useful, if no other data were available.
Scoring
30. Scoring was conducted as follows:
Monitoring (M):
- 1 point - extensively monitored in the UK.
- 2 points - extensive monitoring (EU/UK).
- 3 points - moderately monitored (in some countries but not across all/UK).
- 4 points - limited monitoring (in EU/UK).
- 5 points - no monitoring.
Human case reports (H):
- 5 points - documented cases of human intoxications with fatalities.
- 3 points - documented cases of human intoxications without fatalities.
- 1 point - no documented cases.
Toxicity (T):
- 5 points - causes severe neurotoxic effects with low LD50.
- 4 points - causes severe neurotoxic effects with relatively high LD50.
- 3 points - causes gastrointestinal effects with low to moderate LD50.
- 2 points - causes gastrointestinal effects with relatively high LD50.
- 1 point - causes mild other effects or high LD50 for other affects than the ones listed above.
Occurrence (O):
- 5 points - frequently detected in UK waters or no data available.
- 4 points - occasionally detected in UK waters.
- 3 points - rarely detected in UK waters.
- 2 points - detected in Northern EU waters.
- 1 point - detected only in Mediterranean EU waters.
Analogues
31. Initially an analogue approach was proposed for scoring novel AZAs and PtTXs, toxins with no to very limited information available. The approach suggested using a structurally similar analogue to fill the data gaps and generate a temporary score. However, the Committee concluded that without evidence to show that, for example, that the occurrence of one biotoxin directly relates to the occurrence of another, using analogues for all four scoring categories was associated with high uncertainty and would not result in a robust/appropriate score. The Committee suggested that in general, using suitable read-across methods could be applied in the future, especially to the hazard category, i.e., toxicity (human, animal).
32. As there was no to very limited data available for PtTX and novel AZAs in all categories the Committee considered it not appropriate to apply analogues here and did not include them in their final risk ranking.
Risk ranking results
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
33. The risk rankings, following the decision tree in Figure 1, for the emerging marine biotoxins are presented in Tables 1-6. A narrative has been supplied alongside the risk ranking to clearly depict the underlying considerations as to the numerical scores applied to each biotoxin.
Table 1. Tetrodotoxin (TTX).
|
Category |
No. |
Score |
Narrative |
|
Monitoring |
4 |
Limited monitoring |
TTX is not routinely monitored; however, the French Research Institute for Exploitation of the Sea (IFREMER) conducted a five-year monitoring program of unregulated marine biotoxins between 2018 and 2022 which included TTX. |
|
Human case reports |
5 |
Documented cases of human intoxications and fatalities |
Documented cases of human intoxications and fatalities. Death, caused by respiratory failure and cardiac collapse. |
|
Toxicity |
5 |
Causes severe neurotoxic effects with low LD50 |
TTX is neurotoxic (LD50 oral administration 232 µg/kg bw and intragastric administration 532 µg/kg bw in mice). |
|
Occurrence |
5 |
Frequently detected in UK waters |
Detected at 0.0003 to 0.541 mg/kg in gastropods and bivalves in France, Spain, Italy, Greece, Netherlands, Ireland and UK. |
|
Total: |
19 |
Summary: |
For TTX all categories score high, and no specific category is driving the total score. |
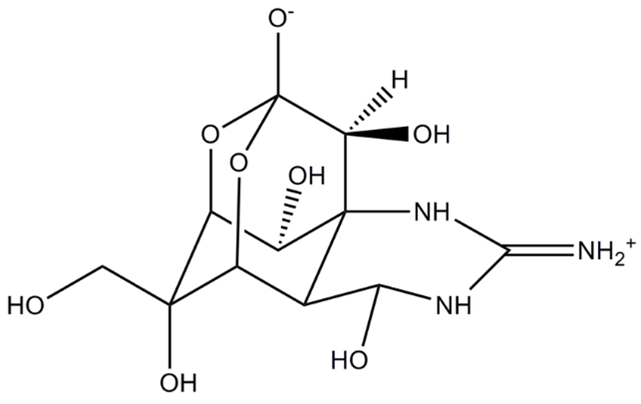
Figure 1. Chemical structure of TTX (Lago et al., 2015).
Table 2. Palytoxin (PITX).
|
Category |
No. |
Score |
Narrative |
|
Monitoring |
5 |
No monitoring |
There is currently no monitoring of PITX in the UK or EU. |
|
Human case reports |
5 |
Documented cases of human intoxications and fatalities |
Documented cases of human intoxications and fatalities. Symptoms include myalgia and weakness, possibly accompanied by fever, nausea and vomiting, and rhabdomyolysis, characterised by injury to skeletal muscle, muscle breakdown and leakage of myocytes into plasma. Renal failure and disseminated intravascular coagulation. Skin, eye and respiratory irritation. Death. |
|
Toxicity |
5 |
Causes severe neurotoxic effects with low LD50 |
PITX is neurotoxic (LD50 oral administration 510-767 µg/kg bw in mice and 40 µg/kg bw in rat). |
|
Occurrence |
2 |
Detected in Northern EU waters |
Detected at 300-625 µg/kg in shellfish meat. Detected in France, Greece, Italy and Spain. |
|
Total: |
17 |
Summary: |
For PITX all categories except occurrence scored high. Only the detection of PITX in northern EU, rather than the UK, prevents the maximum score. |
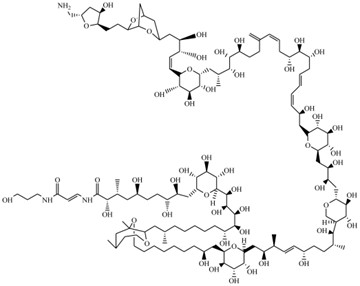
Figure 2. Chemical structure of PITX (Ramos and Vasconcelos., 2010).
Table 3. Brevetoxin (BTX).
|
Category |
No. |
Score |
Narrative |
|
Monitoring |
4 |
Limited monitoring |
BTX is not routinely monitored; however, the IFREMER conducted a five-year monitoring program of unregulated marine biotoxins between 2018 and 2022 which included BTX. |
|
Human case reports |
3 |
Documented cases of human intoxications |
A few hundred intoxications reported (ANSES, 2021). Symptoms include nausea, vomiting, diarrhoea, paraesthesia, cramps, bronchoconstriction, paralysis, seizure and coma. No human fatalities or persistent symptoms reported. |
|
Toxicity |
4 |
Causes severe neurotoxic effects with relatively high LD50 |
BTX is neurotoxic (LD50 oral administration 520-6600 µg/kg bw in mice). BTX was ranked one lower than other neurotoxins (TTX, SPX, and PITX) as the LD50 range for BTX is several folds higher than other neurotoxins. |
|
Occurrence |
2 |
Detected in Northern EU waters |
Recent report of BTX-2 and BTX-3 detected in muscles in France (82 to 345 µg/kg). |
|
Total: |
13 |
Summary: |
For BTX no maximum scores for categories were given. The severe neurotoxic effects and lack of monitoring are driving the score. However, occurrence data on BTX in northern EU was available, as well as reports of human intoxications but no deaths, overall, lowering the total score. |
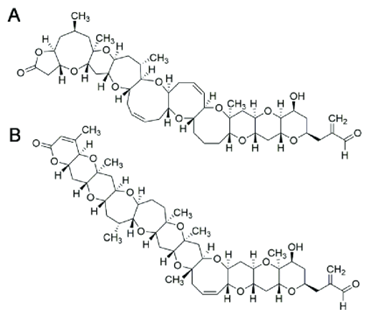
Figure 3. Chemical structure of BTX1 (A) and BTX2 (B) (Vilariño et al., 2018).
Cyanotoxins
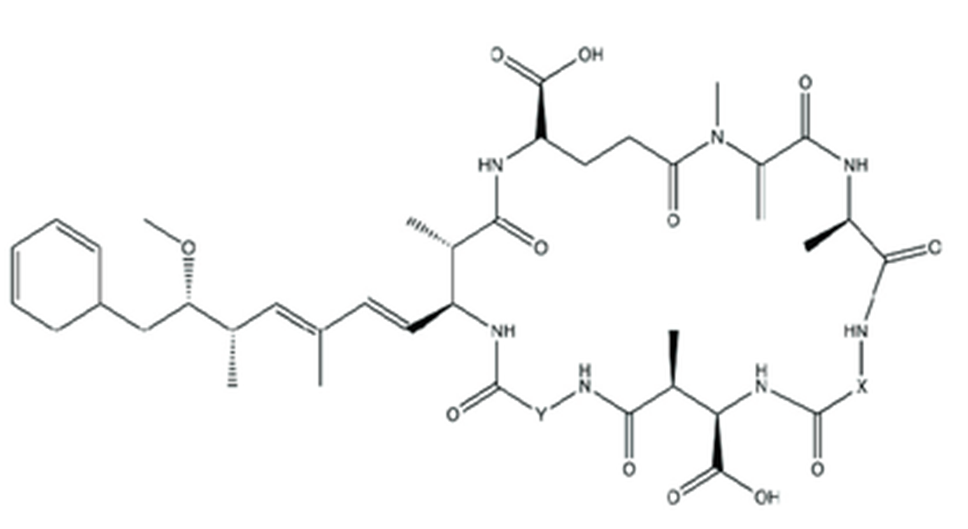
2. Cyanotoxins are a diverse group which span a variety of chemical structures and are all produced by different species and genera of cyanobacteria. MCs are the only class of cyanotoxins with information available for all categories of the risk ranking process (Figure 4). An attempt was made to risk rank other classes of cyanotoxins but due to insufficient data this was not possible.
Figure 4. General structure of MCs where X and Y are variable amino acids at positions 2 and 4 respectively (adapted from Lad et al., 2022).
Table 4. Microcystin (MC).
|
Category |
No. |
Score |
Narrative |
|
Monitoring |
4 |
Limited monitoring |
MC is not routinely monitored; however, the IFREMER conducted a five-year monitoring program of unregulated marine biotoxins between 2018 and 2022 which included MC. |
|
Human case reports |
5 |
Documented cases of human intoxications and fatalities |
Fatalities due to MC exposure have been reported. Symptoms include gastroenteritis, intrahepatic haemorrhage and death. |
|
Toxicity |
3 |
Causes gastro-intestinal effects with low to moderate LD50 |
MC most commonly causes gastroenteritis but also hepatoxic (MC-LR LD50 oral administration 5-10.9 mg/kg bw in mice and in rats > 5 mg/kg bw). |
|
Occurrence |
3 |
Rarely detected in UK waters |
Detected in Northern Ireland (Lough Neagh) and France. Reported at 45-142 µg MC-LR/kg fresh weight in saltwater mussels from Greece. |
|
Total: |
15 |
Summary: |
Reported fatalities from MC exposure, the lack of monitoring and detection in the UK albeit rare all drive the score. Only its lesser toxicity lowers the score. |
Cyclic imines (CIs)
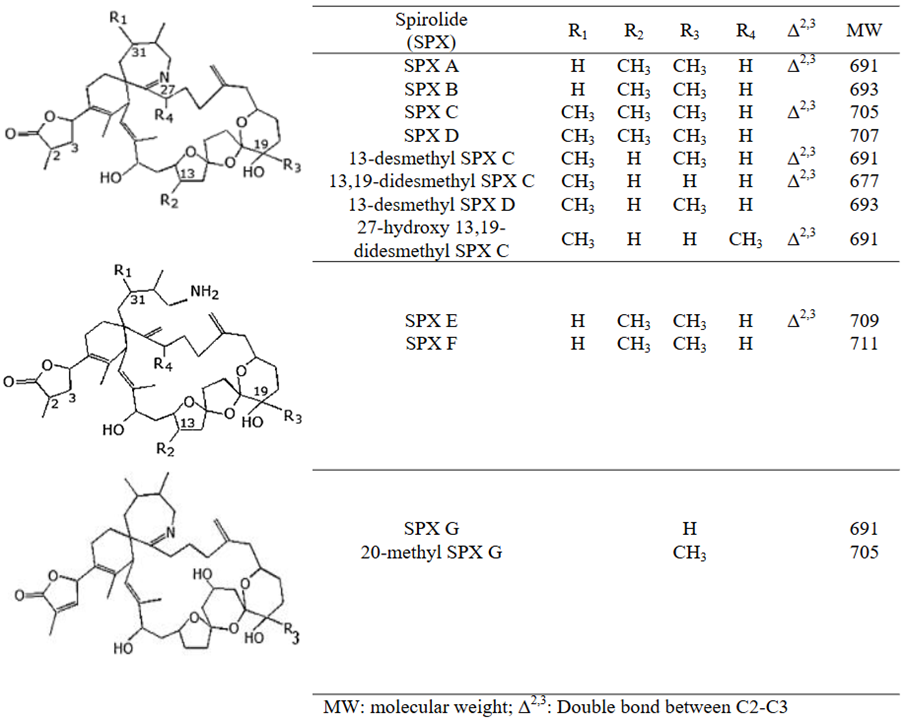
Table 5. Spirolides (SPX).
|
Category |
No. |
Score |
Narrative |
|
Monitoring |
4 |
Limited monitoring |
SPX is not routinely monitored; however, the IFREMER conducted a five-year monitoring program of unregulated marine biotoxins between 2018 and 2022 which included SPX. |
|
Human case reports |
1 |
No documented cases |
No documented cases of human intoxications for SPX |
|
Toxicity |
5 |
Causes severe neurotoxic effects with low LD50 |
SPX is neurotoxic (LD50 oral administration 53-1000 µg/kg bw in mice). |
|
Occurrence |
3 |
Rarely detected in UK waters |
Found in shellfish in France, Norway, Spain and Italy. A recent report (Alexander et al., 2024) found SPX-1 and 20-Me-SPX G in bivalve molluscs across the UK. |
|
Total: |
13 |
Summary: |
For SPX the absence of human case reports lowers the score, but the major drivers are the lack of monitoring and its severe neurotoxic effects. |
Figure 5. Chemical structure of SPXs (EFSA.,2010a).
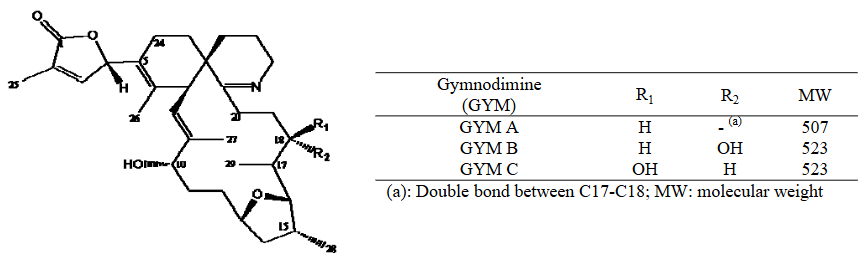
Table 6. Gymnodimine (GYM).
|
Category |
No. |
Score |
Narrative |
|
Monitoring |
4 |
Limited monitoring |
GYM is not routinely monitored; however, the IFREMER conducted a five-year monitoring program of unregulated marine biotoxins between 2018 and 2022 which included GYM. |
|
Human case reports |
1 |
No documented cases |
No documented cases of human intoxications for GYM . |
|
Toxicity |
4 |
Causes severe neurotoxic effects with relatively high LD50 |
GYM is neurotoxic (GYM-A LD50 oral administration 755-4057 µg/kg bw in mice). GYM is ranked one lower than other neurotoxins (TTX, SPX, and PITX) as the LD50 range for GYM-A is several folds higher than the other neurotoxins. |
|
Occurrence |
2 |
Detected in Northern EU waters |
Recent reports of GYM-A detected in shellfish from France in IFREMER. |
|
Total: |
11 |
Summary: |
For GYM the main drivers of the score are the lack of monitoring and its severe neurotoxic effects. The absence of human case reports and its detection in the EU but absence in the UK lowers the overall score. |
Figure 6. Chemical structures of GYMs (EFSA., 2010a).
Discussion
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
35. The risk ranking method was applied to six emerging marine biotoxins scoring each 1-5 points according to four different categories, i.e., monitoring, human case reports, toxicity and occurrence data where a maximum possible score of 20 points could be achieved indicating the highest possible risk. An overview of the rankings has been provided in Table 7.
Table 7. Summary table of risk rankings generated for each of the six groups of emerging marine biotoxins according to four categories (maximum score of 20).
|
Toxin |
Score |
M |
T |
H |
O |
|
TTX |
19 |
4 |
5 |
5 |
5 |
|
PITX |
17 |
5 |
5 |
5 |
2 |
|
MC |
15 |
4 |
3 |
5 |
3 |
|
BTX |
13 |
4 |
4 |
3 |
2 |
|
SPX (CI) |
13 |
4 |
5 |
1 |
3 |
|
GYM (CI) |
11 |
4 |
4 |
1 |
2 |
M = Monitoring; T = Toxicity; H = Human case reports; O = Occurrence; CI = Cyclic imine.
36. The decision tree and weighing the available data provided a priority list for the six emerging marine biotoxin groups, ranking them according to their potential risk to human health in the UK.
37. TTX and PITX were ranked as high risk due to their neurotoxic endpoints observed in animal studies and their case reports of human fatalities from intoxication. Both scored high due to a lack of monitoring, however compared to TTX, PITX has not yet been detected in UK waters or shellfish thus scoring lower overall.
38. MCs rank third despite toxicological data reporting moderate adverse health effects including gastroenteritis and hepatoxicity, compared to more severe neurotoxic endpoints of the other marine biotoxins. MCs rank higher due to reports of human deaths after intoxication, i.e., compared to BTX for which only intoxications were reported and the CIs SPX and GYM which have no known human case reports. The detection of MCs in Lough Neagh Northern Ireland also attributes to the risk of MC over BTX and GYM which have both only been reported in northern EU.
39. Two toxins SPX and BTX achieved an identical score of 13 with the differences being due to their H, O and T scores. The COT agreed that in the instance of a tied score human data would be prioritised followed by toxicology/experimental animal data, and lastly occurrence data. Applying the Committees weighing of the evidence, BTX ranks higher than SPX, due to reported intoxications of BTX compared to no available human information for SPX.
40. GYM achieved the lowest score due to an absence of any published reports of intoxication in humans. In addition, GYM also achieved a low occurrence score as it has only been reported in France.
Uncertainties
41. The key challenge in risk ranking these emerging marine biotoxins is the lack of toxicological/human data and occurrence data in UK waters. Most of the toxins are not routinely monitored, in the UK or other EU countries and therefore it is unclear whether these biotoxins could already be in UK waters. This adds considerable uncertainty when considering the prioritisation of which toxins pose the greatest risk to the UK population.
42. The potential underreporting of intoxications, especially in individuals suffering from mild to moderate adverse health effects, such as nausea and vomiting, could lead to a considerate underestimation of the risk, resulting in a lower risk ranking. Given the severity of the effects, underreporting may potentially be less significant for neurotoxic endpoints, but this may especially be a problem for gastrointestinal symptoms. There is also considerable uncertainty whether reported adverse health effects were caused by one specific biotoxin, or a combination of biotoxins or other potential complications. In a lot of cases, data on the specific marine biotoxin was lacking. Reports of mild or moderate health effects were likely not monitored long term so symptoms could have worsened, or other issues could have arisen later, that were not directly thought to be associated with the biotoxin.
43. Toxicity data is limited for all emerging marine biotoxins discussed in this statement. LD50s from a limited number of animal studies were used to help distinguish risk profiles; however, the small number of studies limited the reliability of the risk estimation and added further to the overall uncertainty of the rankings.
44. Insufficient toxicological data also means HBGVs could not be derived and a reliable estimate of exposure to emerging marine biotoxins could not be conducted.
45. The approach proposed here to risk rank the emerging marine biotoxins cannot account for the possibility of exposure to multiple toxins.
46. For cyanotoxins there is a substantial lack of data for all except MCs, hence they have not been included in this risk ranking. Sufficient data were not available to apply a read across method. Hence, it is unclear whether or how they may contribute to the reported adverse effects of MC.
Conclusions
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
47. The FSA is reviewing its current advice and monitoring programme for marine biotoxins to determine whether updates to existing legislative standards are necessary. To support this, the views of the COT were sought to ascertain the potential risks posed by emerging marine biotoxins to human health. The COT recommended using a numerical risk ranking method to provide the FSA with robust evidence to help inform any decisions on revising legislative standards.
48. The risk ranking, numerical scores provided alongside a narrative, successfully managed to distinguish higher risk biotoxins, notably TTX and PITX, and lower risk biotoxins such as BTX, SPX and GYM. Data on human case reports was prioritised over other data to distinguish the scores for BTX from SPX.
49. There are a number of uncertainties underlying the risk ranking: (1) the absence of routine monitoring means it is unclear whether emerging biotoxins are already present in UK waters or shellfish; (2) potential underreporting of human intoxications, especially in cases with only mild to moderate symptoms such as gastrointestinal effects; (3) a lack of detail on human reports such as complicating factors, cooccurrence of biotoxins or persistent symptoms; (4) LD50s used to distinguish toxicity profiles are based on a limited number of studies (5) limited toxicological data prevented derivation of HBGVs thus estimated exposures cannot be compared to a standard level of known risk preventing clear conclusions on current risk to public health.
50. Despite these uncertainties the risk ranking alongside the narrative provides a current priority list of emerging biotoxin groups to assist policy in their decision making. It is important to note that the risk ranking is based on limited knowledge and that as more information becomes available the potential risk to these marine biotoxins could change.
COT
July 2025
List of Abbreviations and Technical terms
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
|
Acronym |
Definition |
|
AFBI |
Agri-Food and Biosciences Institute |
|
ANSES |
Environmental and Occupational Health and Safety |
|
ASP |
Amnesic shellfish poisoning |
|
ATX |
Anatoxin |
|
AZA |
Azaspiracid |
|
BMAA |
β-methylamino-L-alanine |
|
BTX |
Brevetoxin |
|
Cefas |
Centre for Environment, Fisheries and Aquaculture Science |
|
CI |
Cyclic imine |
|
COT |
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment |
|
CRLMB |
Community Reference Laboratory for marine biotoxins |
|
CYN |
Cylindrospermopsin |
|
DA |
Domoic acid |
|
DSP |
Diarrhetic shellfish poisoning |
|
EFSA |
European Food Safety Authority |
|
EU |
European Union |
|
EURL |
EU Regulatory Reference Laboratory |
|
FSA |
Food Standards Agency |
|
FSAI |
Food Safety Authority Ireland |
|
GB |
Great Britain |
|
GYM |
Gymnodimine |
|
HBGV |
Health-based guidance value |
|
IFREMER |
French Research Institute for Exploitation of the Sea |
|
LD50 |
Lethal dose |
|
MBA |
Mouse bioassay |
|
MC |
Microcystin |
|
NRL |
National Reference Laboratory |
|
OA |
Okadaic acid |
|
OATP |
Organic anion transport proteins |
|
OC |
Official control |
|
OL |
Official laboratory |
|
PITX |
Palytoxin |
|
PnTX |
Pinnatoxin |
|
PSP |
Paralytic shellfish poisoning |
|
PtTX |
Pteriatoxin |
|
PTX |
Pectenotoxin |
|
SPX |
Spirolide |
|
STX |
Saxitoxin |
|
TTX |
Tetrodotoxin |
|
UK |
United Kingdom |
|
WFSR |
Wageningen Food Safety Research |
|
WHO |
World Health Organisation |
|
YTX |
Yessotoxin |
References
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Alexander, R. P., O’Neill, A., Dean, K. J., Turner, A. D., & Maskrey, B. H. (2024). Detection of the Cyclic Imines Pinnatoxin G, 13-Desmethyl Spirolide C and 20-Methyl Spirolide G in Bivalve Molluscs from Great Britain. Marine Drugs, 22(12), 556. https://doi.org/10.3390/md22120556.
ANSES, (2021). Opinion on the state of knowledge on brevetoxins in shellfish, data on toxicity, occurrence and brevetoxin-producing microalgae. ANSES revised OPINION on the state of knowledge on brevetoxins in shellfish, data on toxicity, occurrence and brevetoxin-producing microalgae.
Amzil Z, Derrien A, Terrillon AT, Savar V, Bertin T, Peyrat M, Duval A, Lhaute K, Arnich N, Hort V, Nicolas M (2023). Five years monitoring the emergence of unregulated toxins in shellfish in France (EMERGTOX 2018-2022). Marine Drugs, 21, 435. https://doi.org/10.3390/md21080435.
Aune, T., Espenes, A., Aasen, J. A. B., Quilliam, M. A., Hess, P., & Larsen, S. (2012). Study of possible combined toxic effects of azaspiracid-1 and okadaic acid in mice via the oral route. Toxicon, 60(5), 895–906. https://doi.org/10.1016/j.toxicon.2012.06.007.
Bacciocchi S, Campacci D, Siracusa M, Dubbini A, Leoni F, Tavoloni T, Accoroni S, Gorbi S, Guiliani ME, Stramenga A, Piersanti A (2021). Tetrodotoxin (TTX) and Vibro alginolyticus in mussels from Central Adriatic Sea (Italy): Are they closely related? Marine Drugs, 19(6), 304. https://doi.org/10.3390/md19060304.
Bane V, Lehane M, Dikshit M, O’Riordan A, Furey A (2014). Tetrodotoxin: chemistry, toxicity, source, distribution and detection. Toxins, 6(2), 693–755. https://doi.org/10.3390/toxins6020693.
Blanco L, Lago J, Gonzalez V, Paz B, Rambla-Alegre M, Cabado AG (2019). Occurrence of tetrodotoxin in bivalves and gastropods from harvesting areas and other natural spaces in Spain. MDPI, 11(6), 331. https://doi.org/10.3390/toxins11060331.
Bordin P, Dall’Ara S, Tartaglione L, Antonelli P, Calfapietra A, Varriale F, Guiatti D, Milandri A, Dell’Aversano C, Arcangeli G, Barco L (2021). First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control, 120(4), 107510. http://dx.doi.org/10.1016/j.foodcont.2020.107510.
Cefas (2014). Final Report: Research to support the development of a monitoring programme for new or emerging marine biotoxins in shellfish in UK waters. Final_Report_-_Research.pdf.
CRLMB (Community Reference Laboratory for Marine Biotoxins), (2005). Report on toxicology working group meeting, Cesenatico, Italy, 24-25 October 2005.
DAERA (Department of Agriculture, Environment and Rural Affairs), (2024). The Lough Neagh Report, Blue Green Algae and Water Quality in Northern Ireland, July 2024. The Lough Neagh Report.
Davidson K, Baker C, Higgins C, Higman W, Swan S, Veszelovski A, Turner AD (2015). Potential threats posed by new or merging biotoxins in UK waters and examination of detection methodologies used for their control: Cyclic imines. Marine Drugs, 13(12), 7087-112. https://doi.org/10.3390/md13127057.
Dhanji-Rapkova M, Turner AD, Baker-Austin C, Huggett JF, Ritchie JM (2021). Distribution of tetrodotoxin in Pacific oysters (Crassostrea gigas). MDPI, 19(2), 84. https://doi.org/10.3390/md19020084.
EFSA (2008). Marine biotoxins in shellfish – Azaspiracid group. Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA Journal, 6(10): 723. https://doi.org/10.2903/j.efsa.2008.723.
EFSA (2009a). Scientific Opinion on marine biotoxins in shellfish – domoic acid. EFSA Journal, 7(7), 1181. https://doi.org/10.2903/j.efsa.2009.1181.
EFSA (2009b). Scientific Opinion on marine biotoxins in shellfish – Palytoxin group. EFSA Journal, 7(12): 1393. https://doi.org/10.2903/j.efsa.2009.1393.
EFSA (2009c). Scientific Opinion on marine biotoxins in shellfish – Saxitoxin group. EFSA Journal, 1019, 1-76. https://doi.org/10.2903/j.efsa.2009.1019.
EFSA (2010a). Scientific Opinion on marine biotoxins in shellfish – Cyclic imines (spirolides, gymnodimines, pinnatoxins and pteriatoxins). EFSA Journal, 8(6): 1628. https://doi.org/10.2903/j.efsa.2010.1628.
EFSA (2010b). Scienitfic Opinion on marine biotoxins in shellfish – Emerging biotoxins: Brevetoxin group. EFSA Journal, 8(7):1677. https://doi.org/10.2903/j.efsa.2010.1677.
EFSA (2017). Risk for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA Journal, 15(4), 4752. https://doi.org/10.2903/j.efsa.2017.4752.
FAO/WHO (2016). Technical paper on toxicity equivalency factors for marine biotoxins associated with bivalve molluscs. Rome. 1–133. Toxicity equivalence factors for marine biotoxins associated with bivalve molluscs - Technical paper.
Gerssen A, Bovee THF, Klijnstra MD, Poelman M, Portier L, Hoogenboom RLAP (2018). First report of the occurrence of tetrodotoxins in bivalve mollusks in the Netherlands. MDPI, 10(11), 450. https://doi.org/10.3390/toxins10110450.
Hort V, Arnich N, Guerin T, Lavison-Bompard G, Nicolas M (2020). First detection of tetrodotoxins in bivalves and gastropods from the French Mainland Coasts. Toxins, 12(9), 599. (anses-02965280). https://doi.org/10.3390/toxins12090599.
Islam QT, Razzak MA, Islam MA, Bari MI, Basher A, Chowdhury FR, Sayeduzzaman AB, Ahasan HA, Faiz MA, Arakawa O, Yotsu-Yamashita M, Kuch U, Mebs D (2011). Puffer fish poisoning in Bangladesh: clinical and toxicological results from large outbreaks in 2008. Transactions of the Royal Society of Tropical Medicine and Hygiene, 105(2), 74–80. https://doi.org/10.1016/j.trstmh.2010.10.002.
Ito, E., Satake, M., Ofuji, K., Kurita, N., McMahon, T., James, K., & Yasumoto, T. (2000). Multiple organ damage caused by a new toxin azaspiracid, isolated from mussels produced in Ireland. Toxicon, 38(7), 917–930. https://doi.org/10.1016/S0041-0101(99)00203-2.
Jauffrais, T., Marcaillou, C., Herrenknecht, C., Truquet, P., Séchet, V., Nicolau, E., Tillmann, U., & Hess, P. (2012). Azaspiracid accumulation, detoxification and biotransformation in blue mussels (Mytilus edulis) experimentally fed Azadinium spinosum. Toxicon, 60(4), 582–595. https://doi.org/10.1016/j.toxicon.2012.04.351.
Krock B, Tillmann U, Tebben J, Trefault N, Gu H (2019). Two novel azaspiracids from Azadinium poporum, and a comprehensive compilation of azaspiracids produced by Amphidomatacae, (Dinophyceae). Harmful Algae, 82, 1-8. https://doi.org/10.1016/j.hal.2018.12.005.
Lad, A., Breidenbach, J. D., Su, R. C., Murray, J., Kuang, R., Mascarenhas, A., Najjar, J., Patel, S., Hegde, P., Youssef, M., Breuler, J., Kleinhenz, A. L., Ault, A. P., Westrick, J. A., Modyanov, N. N., Kennedy, D. J., & Haller, S. T. (2022). As We Drink and Breathe: Adverse Health Effects of Microcystins and Other Harmful Algal Bloom Toxins in the Liver, Gut, Lungs and Beyond. Life, 12(3), Article 3. https://doi.org/10.3390/life12030418.
Lago, J., Rodríguez, L. P., Blanco, L., Vieites, J. M., & Cabado, A. G. (2015). Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses. Marine Drugs, 13(10), Article 10. https://doi.org/10.3390/md13106384.
Pigozzi, S., Bianchi, L., Boschetti, L., Cangini, M., Ceredi, A., Magnani, F., Milandri, A., Montanari, S., Pompei, M., Riccardi, E., & Rubini, S. (2008). First evidence of spirolide accumulation in northwestern Adriatic shellfish. Copenhagen. 319-322.
Ramos, V., & Vasconcelos, V. (2010). Palytoxin and analogs: Biological and ecological effects. Marine Drugs, 8(7), 2021–2037. https://doi.org/10.3390/md8072021.
Reid, N., Reyne, M. I., O’Neill, W., Greer, B., He, Q., Burdekin, O., McGrath, J. W., & Elliott, C. T. (2024). Unprecedented Harmful algal bloom in the UK and Ireland’s largest lake associated with gastrointestinal bacteria, microcystins and anabaenopeptins presenting an environmental and public health risk. Environment International, 190, 108934. https://doi.org/10.1016/j.envint.2024.108934.
Testai E, Buratti FM, Funari E, Manganelli M, Vichi S (2016). Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA supporting publication: EN-998, 309pp. https://doi.org/10.2903/sp.efsa.2016.EN-998.
Tillmann, U., Jaén, D., Fernández, L., Gottschling, M., Witt, M., Blanco, J., & Krock, B. (2017). Amphidoma languida (Amphidomatacea, Dinophyceae) with a novel azaspiracid toxin profile identified as the cause of molluscan contamination at the Atlantic coast of southern Spain. Harmful Algae, 62, 113–126. https://doi.org/10.1016/j.hal.2016.12.001.
Turner A, Powell A, Schofield A, Lees D, Baker-Austin C (2015). Detection of the pufferfish toxin tetrodotoxin in European bivalves, England, 2013 to 2014. Eurosurveillance, 20(2), 1–7. https://doi.org/10.2807/1560-7917.es2015.20.2.21009.
Vareli K, Zarli E, Zacharioudakis Vagenas G, Varelis V, Pilidis G, Briasoulis E, Sainis I (2012). Microcystin producing cyanobacterial communities in Amvrakikos Gulf (Mediterranean Sea, NW Greece) and toxin accumulation in mussels (Mytilus galloprovincialis). Harmful Algae, 15, 109-118. http://dx.doi.org/10.1016/j.hal.2011.12.005.
Vilariño, N., Louzao, M. C., Abal, P., Cagide, E., Carrera, C., Vieytes, M. R., & Botana, L. M. (2018). Human Poisoning from Marine Toxins: Unknowns for Optimal Consumer Protection. Toxins, 10(8), Article 8. https://doi.org/10.3390/toxins10080324.
WHO (2020). Cyanobacterial toxins: Microcystins. Background document for development of WHO Guidelines for drinking-water quality and Guidelines for safe recreational water environments. Microsoft Word - GDWQ.2ndEdit.Cyanobacterial.toxins.doc.
WHO (2022). Guidelines for drinking water quality. Fourth edition incorporating the first and second addenda 9789240045064-eng.pdf.
Žegura, B., Štraser, A., & Filipič, M. (2011). Genotoxicity and potential carcinogenicity of cyanobacterial toxins – a review. Mutation Research/Reviews in Mutation Research, 727(1), 16–41. https://doi.org/10.1016/j.mrrev.2011.01.002.