Risk of emerging marine biotoxins in British shellfish – Pectenotoxin group
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Background
1. The Food Standards Agency (FSA) is considering the current advice and monitoring programme for marine biotoxins and whether there is a need to update or change existing legislative standards in the UK.
2. The main purpose of this work is to identify any emerging marine biotoxin threats in UK waters. The views of the Committee on the Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) are sought on whether any of these marine biotoxins would pose a risk to human health. A scoping paper on the risk to human health from consumption of bivalve molluscs (shellfish) harvested from UK waters associated with marine biotoxins is also being presented at the December 2023 meeting (TOX/2023/59).
3. Given the recent availability of additional analytical standards for pinnatoxin (PnTX), the work also includes considerations of the toxicological database for PnTX and whether there is a public health risk present that justifies including PnTX in the current FSA England and Wales biotoxin monitoring programme. A discussion paper on PnTX was presented to the COT at the July 2023 meeting (TOX/2023/37).
4. The current paper is a discussion papers which provides information and data on the risks to human health associated with consumption of shellfish from UK waters, in relation to the class of marine biotoxin known as Pectenotoxins (PTXs). PTXs are a regulated biotoxin group in the UK and included in the group of lipophilic toxins which are monitoring routinely in UK shellfish. Maximum permitted levels are stipulated in chapter 7 of Annex 3 in the UK’s retained (EC) regulation 853/2004 (EC, 2004). However, recent amendments to the European Union (EU) legislation regarding the status of pectenotoxins (EC, 2021a, 2021b) means they are set to be removed from the list of monitored biotoxin in EU shellfish. The relevant paragraph (15) of the amendment to regulation 2004/853 in April 2021 states (EC, 2021b):
“(15) Live bivalve molluscs placed on the market may not contain marine biotoxins that exceed the limits set out in point 2 of Chapter V of Section VII of Annex III to Regulation (EC) No 853/2004. The EFSA has concluded in its Opinion on Marine biotoxins in shellfish – Pectenotoxin group (8) that there are no reports of adverse effects in humans associated with Pectenotoxins (PTX) group toxins. In addition, PTX in shellfish are always accompanied by toxins from the Okadaic acid group. It is therefore appropriate to delete the reference to PTX from point 2(c) of Chapter V of Section VII of Annex III to Regulation (EC) No 853/2004”
“(15) Live bivalve molluscs placed on the market may not contain marine biotoxins that exceed the limits set out in point 2 of Chapter V of Section VII of Annex III to Regulation (EC) No 853/2004. The EFSA has concluded in its Opinion on Marine biotoxins in shellfish – Pectenotoxin group (8) that there are no reports of adverse effects in humans associated with Pectenotoxins (PTX) group toxins. In addition, PTX in shellfish are always accompanied by toxins from the Okadaic acid group. It is therefore appropriate to delete the reference to PTX from point 2(c) of Chapter V of Section VII of Annex III to Regulation (EC) No 853/2004”
5. Similarly, an excerpt from Regulation 2021/1709 which amends Regulation 2019/627 by removing the same provisions on pectenotoxin provided the:
“(9) Live bivalve molluscs placed on the market are not to contain marine biotoxins that exceed the limits established in Annex III, Section VII, Chapter V (2) of Regulation (EC) No 853/2004 of the European Parliament and of the Council (8). Regarding Pectenotoxins (PTX), the European Food Safety Authority (EFSA) has concluded that there are no reports on adverse effects in humans associated with Pectenotoxins (PTX) group toxins (9). As PTX have been removed from the health standards for live bivalve molluscs in Commission Delegated Regulation (EU) 2021/1374 (10), it is therefore appropriate to remove them as well from the provisions of Implementing Regulation (EU) 2019/627.”
6. The changes to the Regulations are based on the conclusions and recommendation from the European Food Safety Authority’s (EFSA) 2009 assessment of PTXs. This paper, therefore, briefly summarises the main points and recommendations, as well as relevant literature covered in EFSA’s 2009 assessment, and any publications produced since with direct relevance to the potential risk of PTX to UK consumers. Details of the literature search can be found in Annex A.
Introduction
7. In retained UK and EU law, there are currently three major biotoxin groups that are regulated in shellfish, and which are subject to statutory testing to protect human health. The biotoxins are: 1) domoic acid, responsible for Amnesic Shellfish Poisoning (ASP), 2) saxitoxin and its derivatives, responsible for Paralytic Shellfish Poisoning (PSP), and 3) lipophilic toxins (LTs), which are comprised of sub-groups of compounds linked, not by their toxicology, but rather by their similar extractability in organic solvents (Dhanji-Rapkova et al., 2018).
8. Okadaic acid (OA) and its related analogues, dinophysistoxins (DTXs) were the first LTs linked to Diarrhoetic Shellfish Poisoning (DSP). Historically, PTXs have been included in the DSP toxin group due to their high acute toxicity in the mouse bioassay (MBA) in which mice exposed to whole mollusc flesh extract, via intra-peritoneal (i.p.) injection, have their survival monitored over a 24 hour period (Yasumoto et al., 1985). Due to the co-occurrence of PTXs alongside OA and DTXs, PTX1 and PTX2 have been included in the OA-group for the purpose of total regulatory limits (EC, 2004).
9. However, toxicology studies indicated that PTXs may be less toxic via the oral route than OA and DTXs, have a different mode of action (MOA) and do not induce diarrhoea (Dhanji-Rapkova et al., 2018). For these reasons, EFSA recommended that the toxicity of PTXs should not be expressed as OA equivalents (EFSA, 2009).
Other Evaluations of Pectenotoxins
EU/SANCO working group on DSP and AZP (2001)
10. The European Commission (EC) Working Group on Toxicology of DSP and azaspiracid (AZP) previously identified an oral LOAEL of 250 μg/kg bw for PTX-group toxins, based on reports of liver and intestinal injury and fluid accumulation in the small intestine (Ishige et al., 1988). A combined safety factor of 1000 for extrapolating from a LOAEL to NOAEL (10), and for inter- and intra-species extrapolation (10 x 10) was applied to derive an ARfD of 0.25 μg/kg bw (EU/SANCO, 2001). Further details were not available; however, access to the original report could not be obtained at the time.
Joint FAO/IOC/WHO ad hoc Expert Consultation on Biotoxins in Bivalve Molluscs (2004)
11. In 2003 the Codex Committee on Fish and Fishery Products (CCFFP) asked for scientific evidence to enable the establishment of maximum levels (MLs) in shellfish for shellfish toxins including the DSP and PTX-group toxins. In response, the Food and Agriculture Organization (FAO)/ Intergovernmental Oceanographic Commission (IOC)’s/World Health Organisation’s (WHO)/ joint ad hoc expert consultation on biotoxins in bivalve molluscs found there was no evidence for acute adverse or chronic health effects for PTXs in humans and that PTX-group analogues did not induce diarrhoea. Hence, they recommended PTXs should be regulated separately from OA. Overall, the database was insufficient to establish an acute or chronic health based guidance value (HBGV) for PTX-group toxins (FAO/IOC/WHO, 2004).
COT (2006)
12. COT found no clear evidence of PTX intoxication in humas. One DSP incident report in the UK with contaminated mussels confirmed the presence of PTX-group toxins, however, samples taken also contained OA and OA esters, as well as DTX1 and DTX1 esters. The Committee therefore concluded that there was uncertainty whether or how PTX-group toxins may have contributed to the effects observed.
13. While noting the conflicting results for PTX toxicity, the COT thought it pertinent not to dismiss any studies reporting adverse effects after oral administration and derived an acute reference dose (ARfD) of 0.25 μg/kg bw based on a lowest observed adverse effect levels (LOAEL) of 250 μg/kg bw and an overall uncertainty (UF) factor of 1000 to account for inter- and intraspecies variation and extrapolation from a LOAEL to a no observed adverse effect level (NOAEL). The COT recommended that the ARfD should be reviewed when further data become available.
14. Based on the ARfD of 0.25 μg/kg bw, the COT estimated a PTX concentration of 6 μg/100 g shellfish meat would be the maximum concentration without an appreciable health risk, assuming and adult bodyweight of 60 kg. Members noted at the time that this concentration was lower than the current regulatory limits, and that the MBA was not sufficiently sensitive to detect the presence of PTXs at this level.
Codex Alimentarius (2015)
15. In the Codex standard for live and raw bivalve molluscs (Codex Alimentarius, 2015), the ML for the OA group of biotoxins in mollusc flesh was 0.16 mg of OA equivalents/kg. The OA group listed OA, DTX1 and DTX2, but the PTX-group was not included.
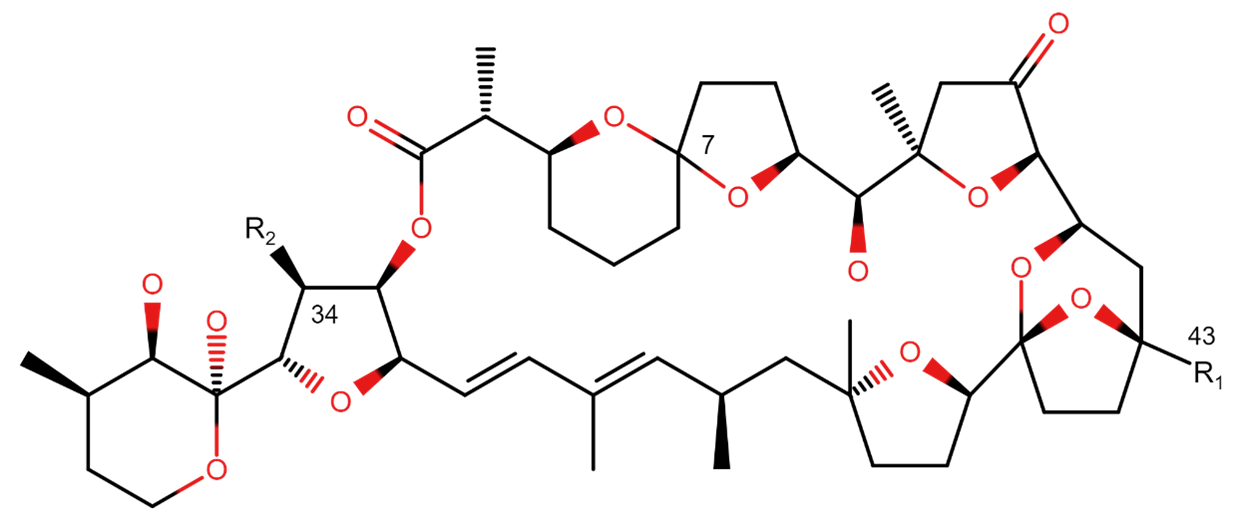
FAO/WHO (2016)
16. In 2016, the FAO/WHO concluded that there were no reports of human intoxication by PTXs. PTX2, the most common PTX-analogue, was not toxic in rodents when given orally, even at high doses (5 mg/kg), however several PTX analogues were considered to be lethal when administered by i.p. injection: PTX1-4, PTX6, and PTX11. All other analogues showed no lethality, even at high doses (>5 mg/kg). The report also noted that initial studies reported a diarrhetic effect of PTXs, but this was later proven to be wrong.
17. The FAO/WHO stated the mechanism of action of PTX was known, with PTX binding to actin and causing cytoskeleton disorganization due to F-actin depolymerization. PTXs have also been shown to be apoptotic in vitro. No information was provided on regulatory limits for the PTX-group, whether in conjunction with the OA group or separately (FAO/WHO, 2016).
NZFS (2020)
18. In 2020, New Zealand Food Safety (NZFS) published an assessment on the food safety risk presented by PTX in New Zealand shellfish using data collected from 2009-2019. As part of the risk assessment, both PTX and DSP groups were reviewed, as they are currently regulated together in New Zealand.
19. NZFS agreed with EFSA that group limits for the OA-group toxins and PTX-group toxins were inappropriate from an analytical and toxicological point of view and concluded that from a scientific standpoint “it is a straightforward decision to remove the PTX-group from the DSP group of toxins”.
20. While it was the agreed view that there was no evidence to that PTX-group toxins have caused any human illness, NZFS noted there were significant data gaps in the literature. Limited data were available on the long-term, carcinogenic, or genotoxic effects of PTX and data from reproductive toxicity studies were also needed.
21. NZFS considered it important to establish whether the PTX-group analogues induced diarrhoea as it underpins the validity of whether the PTX-group should be included in the DSP class of toxins. However, they noted that this was complicated by the fact that Dinophysis spp. produced not only PTX-group analogues but also OA and its derivatives which were well known for their diarrhoetic effect. Therefore, it was likely that algal and shellfish extracts would contain both groups of toxins. This was further compounded by the fact that PTX and DSP group toxins were hard to separate chromatographically, such that pure samples of PTX-group analogues were hard to achieve.
22. NZFS considered the ARfD proposed by EFSA overly conservative and based on data likely to be inaccurate. They considered that studies from Ishige (1988) and Ito (2006), the basis of EFSA’s ARfD, used PTX2 of unknown purity and observed diarrhoea which was accepted to not be a symptom of PTX-intoxication. Toxic effects observed in the study by Ishige (1988), involved fluid accumulation in the intestine and damage to intestinal villi. In NZFS’s opinion, these were changes that were commonly seen with OA derivatives. Therefore, NZFS concluded these data should be discounted due to the likelihood of contamination with OA-group toxins and more recent studies which employed fully authenticated material and showing considerably lower toxicity should be considered.
23. Analysis of the available monitoring data from 2009-2019 found that the main PTX analogue observed in shellfish, PTX2, was detected in 1.3% of New Zealand shellfish samples analysed. The maximum concentration found during that period was 0.079 mg/kg but there was no evidence that PTX resulted in any human illness during this period.
24. NZFS concluded the that overall risk of exposure to DSP observed in New Zealand was small. Whilst they thought it sensible to remove the PTX-group toxins from the DSP group, they did not recommend changing the regulatory limits for the OA-group toxins (NZFS, 2020).
FDA (2022)
25. The American Food and Drug Administration’s (FDA) 2022 guidance on Fish and Fishery Products Hazards and Controls noted that PTX was detected in phytoplankton and/or molluscan shellfish from Australia, Italy, Japan, New Zealand, Norway, Portugal, Spain, and the U.S. but did not state any specific expectations regarding controls for PTX in its Hazard Analysis Critical Control Point (HACCP) plans (FDA, 2022).
26. The FDA and Environmental Protection Agency (EPA) both list safety levels for DSP as ≥0.16 mg/kg total OA equivalents (i.e., combined free OA, DTX1 and DTX1, and their acyl-esters), the PTX-group was not included (FDA, 2022).
Summary of EFSA (2009) Opinion: Marine biotoxins in shellfish – Pectenotoxin group
27. In their 2009 report, EFSA assessed the current EU limits regarding human health and methods of analysis for the PTX biotoxins group.
28. As PTX-group toxins are exclusively produced by Dinophysis spp., which also produce OA-group toxins, both toxin groups co-occur in shellfish. On this basis they appear to have originally been grouped in EU regulation (EFSA, 2009).
Chemical characterisation
29. PTXs are macrolides containing multiple polyether ring units (Figure 1) isolated from species of shellfish and from dinoflagellates of the genus Dinophysis. To date, 15 PTX-group toxins have been isolated and characterised. PTX2, the main analogue produced by Dinophysis algae, is the precursor for other PTX-group analogues that are produced through metabolic biotransformation in the gut of bivalves.
Figure 1. Chemical structures of pectenotoxin
30. Oxidation of the C-43 methyl group in PTX2 in the digestive gland of the shellfish can give rise to the alcohol (PTX1), aldehyde (PTX3) and carboxylic acid (PTX6) forms of the toxin. PTX4 and PTX7, spiroketal isomers of PTX1 and PTX6 respectively, have also been isolated from mussels and scallops. PTX8 and PTX9 are isomers of PTX4 and PTX7, which are obtained after acid treatment of the latter. These are not know to be naturally occurring in algae or shellfish, but artificial toxins generated during isolation or acid treatment (Sasaki et al., 1998). PTX11, also isolated from samples of Dinophysis, is the 34S-hydroxy analogue of PTX2, and is more resistant to enzymatic hydrolysis than PTX2 when exposed to mussel hepatopancreas (Suzuki et al., 2006).
31. In most bivalve species, PTX2 is metabolized to PTX2 seco acid (PTX2 SA), in which the lactone ring of PTX2 is hydrolysed to the seco acid form. Epimerization of PTX2 SA yields the thermodynamically more stable 7-epi-PTX2 SA.
Reports of Human intoxication by PTX
32. EFSA found no reports of human illness that were causally associated with PTX-group toxins consumption. They noted that as PTX-group toxins always co-occur with OA toxins, it was difficult to assess whether PTX-group toxins may contribute to human cases of DSP.
33. In 2001, Burgess and Shaw (2001) detailed a mass outbreak of food poisoning in Australia initially attributed to the presence of PTX2SA. 100 people were poisoned following consumption of pipis (Plebidonax deltoides) resulting in 56 cases of hospitalisation. Symptoms included nausea, vomiting and diarrhoea. While it had initially been suggested that PTX2 SA and 7-epi-PTX2 SA may have been responsible, the symptoms were later attributed to OA esters (EFSA, 2009). These were not detected at the time as they require a hydrolysis step during sample preparation to allow detection (Burgess, 2003).
Toxicokinetics
34. Limited data were available on the absorption, distribution, metabolism, and excretion (ADME) of PTX-group toxins and no data exist on the toxicokinetics in humans. Although not specifically designed to measure bioavailability of PTX-group toxins, EFSA described two studies which provide limited information.
35. Burgess (2003) administrated a single oral dose of 5.7 μg PTX2/animal to mice (n=3) and found 1 μg of the parent compound in the GI content and faeces after 24 hours, with only traces of PTX2 in the GI tissue. No detectable amounts were found in other internal organs and urine. In a second experiment a mix of PTX2 and PTX2 SA was administered by oral gavage. A similar pattern was observed, most of the toxins remained within the GI tract and were almost fully excreted in the faeces without being absorbed. Following i.p. administration of PTX2 and PTX2 SA, both compounds were detected in blood and internal organs as well as the GI tract. All detectable PTX was excreted in the faeces rather than in urine.
36. EFSA cited a second (unpublished) study, by Espenes et al. A single dose of PTX2 was administered by gavage at 5 mg/kg bw to mice. PTX2 was found at the highest concentration in the stomach (7 µg/g) and at the lowest amounts in the duodenum (0.27 µg/g), small intestine (0.13 µg/g) and colon (0.05 µg/g) and trace quantities were fund in the liver, kidney, heart and whole blood. EFSA concluded that these experiments suggested a low absorption of PTX-group analogues in the gut of mice following oral administration (EFSA, 2009).
Molecular mechanism of action
37. EFSA (2009) noted that while OA inhibits serine/threonine phosphoprotein phosphatases in in vitro experiments at nM concentrations, PTX1 by contrast does not inhibit these enzymes (Bialojan and Takai, 1988; Fladmark et al., 1998). This finding provided a molecular basis to distinguish PTX-group toxins from OA-group toxins.
38. Several papers indicated that PTX-group toxins induced morphological alterations in animal tissue in vitro, through effects on the cellular cytoskeleton (Table 9, EFSA, 2009). This suggested that PTX1 and PTX2 can interact and disrupt stress fibres though F-actin depolymerization (EFSA, 2009; Hori et al., 1999; Spector et al., 1999; Zhou et al., 1994).
39. EFSA considered the study by Chae et al. (2005) to provide direct evidence that agents, such as PTX2, altering the actin-based cytoskeleton triggered the intrinsic mitochondrial apoptotic pathway (Chae et al., 2005). This, in turns indicated that PTX-group toxins induced F-actin depolymerization was the causative event of subsequent cell death. EFSA therefore found it reasonable to conclude that the PTX-actin interaction and the consequent perturbation of actin cytoskeletons could be the molecular basis of cellular vacuolization in animal models and cell damage in biological systems exposed to PTX-group toxins (EFSA, 2009).
Toxicity in animals
Intraperitoneal administration of PTX-group toxins
40. The toxicological database was limited, and no data were available on the chronic effects of PTX-group toxins.
40. The toxicological database was limited, and no data were available on the chronic effects of PTX-group toxins.
41. The acute toxicity of the different PTX analogues after i.p. administration are summarised in Table 3 (Originally Adapted from Munday, 2008). Information on feeding method, fasting state, strain, and sex of mice was not available for the majority of the available data.
Table 3: Acute toxicity of the different PTX analogues after of i.p. administration (Adapted from Munday, 2008).
|
Compound |
Dose |
Parameter |
Reference |
|
PTX1 |
250 |
MLD |
(Yasumoto et al., 1985) |
|
PTX2 |
219 411 192-400
|
LD50 LD50 MLD |
(Miles et al., 2004) (Yoon and Kim, 1997) (Miles et al., 2004; Yasumoto et al., 1989, 1985; Yoon and Kim, 1997) |
|
PTX3 |
350 |
MLD |
(Murata et al., 1986) |
|
PTX4 |
770 |
MLD |
(Yasumoto et al., 1989) |
|
PTX6 |
500 |
MLD |
(Yasumoto et al., 1989) |
|
PTX7 |
>5000 |
MLD |
(Sasaki et al., 1998) |
|
PTX8 |
>5000 |
MLD |
(Sasaki et al., 1998) |
|
PTX9 |
>5000 |
MLD |
(Sasaki et al., 1998) |
|
PTX11
|
244 250 |
LD50 MLD |
(Suzuki et al., 2006) |
|
PTX2 SA MLD |
>5000 |
MLD |
(Suzuki et al., 2006) |
|
7-epi-PTX2 SA |
>5000 |
MLD |
(Miles et al., 2006, 2004) |
MLD, minimum lethal dose; LD50, lethal dose (dose required to kill 50% of a tested animal population); bw, body weight.
42. LD50 values for PTX2 ranged from 219-411 μg/kg bw (Miles et al., 2004; Yoon and Kim, 1997). Death after injection of PTX2 occurred in most cases between 4 and 10 hours after dosing. Mice which received lethal doses of PTX-2 showed severe signs of general toxicity: hunched posture and lethargy, laboured respiration, ataxia and cyanosis (Miles et al., 2004). The limited data available indicated that the acute toxicity of PTX1, PTX3 and PTX11 were comparable (Murata et al., 1986; Suzuki et al., 2006; Yasumoto et al., 1985). Contrary, PTX4 and PTX6 appeared to be slightly less toxic with lethal doses of 770 and 500 µg/kg bw, respectively (Yasumoto et al., 1989). Studies on i.p. administration of PTX7, PTX8, PTX9, PTX2SA and 7-epi-PTX2SA in mice all indicated low toxicity, with no deaths observed at a dose of up to 5000 µg/kg bw (Munday, 2008).
43. Histopathological studies showed evidence of significant liver toxicity after i.p. injection of PTX-group toxins. Terao et al. (1986) reported severe liver pathology in mice 60 minutes after treatment with 1 mg/kg bw of PTX1. Toxicity was dose dependent with effects seen with doses as low as 150 µg/kg bw. Similarly, (Yoon and Kim, 1997) reported that i.p. injection of PTX2 induced a dose-dependent increase in liver enzymes. Ito et al., (2008) found i.p. injection of 500 µg/kg bw PTX6 induced hepatic haemorrhage as well as gastric injuries and kidney toxicity. By contrast, no histological changes were seen in any major organs 24 hours after injection of PTX2 SA or 7-epi-PTX2SA at doses of up to 5000 µg/kg (Miles et al., 2006).
44. In suckling mice which received i.p. doses of PTX1 of up to 1 mg/kg bw, no diarrhoea was observed (Terao et al., 1986). Similarly, no diarrhoea was observed after i.p. administration of PTX2, PTX2 SA, 7-epi-PTX2 SA or PTX11 to mature mice at doses of 5 mg/kg bw (Miles et al., 2006, 2004; Suzuki et al., 2006).
45. In the study by Yoon and Kim (1997), repeated daily i.p. administration of 20 or 100 µg/kg bw PTX2 in mice over a 1- or 2-week period did not cause death or changes in chemical biomarkers of liver or kidney toxicity. However, at repeated daily doses of 200 µg/kg bw PTX2, 50% of the animals died. In a separate study, administration of PTX2 via i.p. injection at 100 µg/kg bw for 20 days to nude mice saw no effect on body weight (Chae et al., 2005).
Oral administration of PTX-group toxins
46. EFSA concluded that oral toxicity of PTX-group toxins generally appeared to be lower than toxicity following i.p. administration.
47. In a limited study in mice (n=5) by Miles et al. (2004), a single oral administration of PTX2 or PTX2 SA at doses up to 5000 μg/kg bw caused no overt signs of toxicity, including diarrhoea. Similarly, in a report by Suzuki et al. (2006), PTX11 toxicity after oral administration was found to be low with no signs of toxicity observed in any of the five mice dosed with either PTX11, or its isomer, 7-epi-PTX2 SA at a dose rate of 5000 µg/kg, and no diarrhoea was reported.
48. Ishika et al. (1988) reported swollen intestine filled with fluid at an oral dose of 250 μg PTX2/kg bw in a single mouse tested. Diarrhoea and intestinal and liver toxicity was observed at doses of 1000 μg/kg bw and above. Ito (2006) similarly reported that in mice, at a single oral dose of 400 μg/kg bw PTX2 and above, GI tissue injury with vacuole formation was observed in epithelial cells and fluid accumulation was observed in the small intestine.
49. In a later study by Ito et al. (2008) mice and rats were given single oral doses of PTX6 by gavage. In mice, little toxic effects were observed at doses up to 5000 µg/kg bw, with only slight and transient injury of the small intestinal villi. No other mouse organs were affected. In rats, small-intestinal injury was observed at a single oral dose of 2000 µg/kg bw; no other doses were investigated. Diarrhoea was not observed in either species. However, in the same study, PTX2 caused intestinal fluid secretion in mice and slight fluid secretion in rats at single doses of 500 and 1500 µg/kg bw, respectively. In contrast PTX6 (5000 µg/kg bw) did not increase the intestinal fluid secretion in mice.
50. Hamano et al. (1986) tested the effects of individual DSTs such as DTX1 and DTX3, PTX1 and OA on suckling mice for diarrhoeagenicity. The authors reported that DTX1, DTX3 and OA induced diarrhoea, whereas PTX1 did not induce diarrhoea at doses up to 2 μg/mouse (equivalent to about 100 µg/kg bw).
51. However, EFSA noted a study by Ogino et al. (1997) which found that as little as 25 μg/kg bw of PTX2 could be lethal in mice. EFSA noted in their assessment that this study (4-5 mice per dose group) showed no dose response. The lethality observed at 25, 100, 200, 300, and 400 µg/kg bw was 25, 0, 20, 40 and 25 %, respectively.
52. There were no data available on the possible effects of PTX-group toxins following repeated oral administration (EFSA, 2009).
Toxicity Equivalency Factors and Health Based Guidance Values
53. EFSA concluded that the available data on lethality in mice was not sufficient to establish robust toxicity equivalency factors (TEFs). To be prudent, however, EFSA proposed a provisional TEF value of 1 to be used for PTX1-4, PTX6 and PTX11, until better data become available. PTX7-9, PTX2 SA and 7-epi-PTX2 SA were considered less toxic and were not assigned TEFs. EFSA found no data on possible long-term effects of PTX-group toxins on humans or animals and no data on genotoxicity were identified.
54. Due to the lack of data on repeated oral administration in humans or animals EFSA were unable to establish a tolerable daily intake (TDI) for PTX-group toxins. However, due to the acute nature of PTX toxicity, EFSA set an ARfD of 0.8 µg PTX2 equivalents/kg bw. The ARfD was based on a LOAEL of 250 µg/kg bw (Ishige et al., 1988) and 300 µg/kg bw (Ito, 2006), for intestinal effects in rats. They applied an overall uncertainty factor of 300, 10 for interspecies and 10 for intraspecies variation and an additional factor of 3 for the extrapolation from a LOAEL to NOAEL.
55. Consumption of a 400 g portion of shellfish meat - identified as a “large portion size” - containing PTX-group toxins at 160 µg/kg shellfish meat (the current EU limit for lipophilic toxins) would result in an intake of 64 µg toxin (equivalent to ~1 µg/kg bw in a 60 kg adult). This intake was slightly higher than the ARfD of 0.8 µg PTX2 equivalents/kg bw. EFSA estimated, for a 60 kg adult to avoid exceeding the ARfD of 0.8 μg PTX2 eq/kg bw, a 400 g portion of shellfish should not contain more than 48 μg PTX2 equivalents. Based on current consumption and occurrence data, EFSA estimated there was a small chance (approximately 0.2 %) to exceed the ARfD of 0.8 μg PTX2 equivalents/kg bw when consuming shellfish currently available on the European market (EFSA, 2009).
EFSA’s conclusions
56. EFSA considered that the MBA, the official reference method for lipophilic biotoxins, has significant shortcomings and in their view was not considered an appropriate tool for control purposes. The method had a high variability in results, an insufficient detection capability, and limited specificity.
57. Group limits for OA-group toxins and PTX-group toxins were inappropriate from an analytical and toxicological point of view. Since PTX-group toxins do not share the same MOA or toxicological profile as OA-group toxins they should not be included in the regulatory limit for OA-group toxins and PTX-group toxins should not be expressed as OA equivalents/kg as laid out in the existing legislation. Instead, EFSA recommended separate regulatory limits for OA-group toxins and PTX-group toxins should be used (EFSA, 2009).
Relevant Publications since EFSA’s 2009 assessment
Regulatory limits for DSP toxins and PTX in other countries
58. For a number of marine toxins, including PTX, the Regulation varies among countries; some countries regulate specific toxins, while other countries did not consider there to be enough evidence to demonstrate a hazard to human health and therefore no regulations are in place (NZFS, 2020).
59. A summary of the regulatory limits for DSP toxins and whether the PTX-group forms part of the OA eq./kg limit is presented in Table 4 (Vilariño et al., 2015).
Table 4: Summary of regulatory limits for DSP toxins and whether the PTX-group forms part of the OA eq./kg limit (Adapted from Vilariño et al. (2015).
|
Toxin |
Codex |
NZ |
Aus |
Jap |
Can |
USA |
Mex |
Chi |
EU |
|
Max Limits (µg OA eq/kg) |
160 |
160 |
200 |
160 |
200 |
160 |
160 |
160 |
160 |
|
Includes PTX |
No |
Yes |
No |
No |
Yes |
No |
No |
Yes |
Yes |
NZ, New Zealand; Aus, Australia; Jap, Japan; Can, Canada; USA, United States of America; Mex, Mexico; Chi, Chile; EU, European Union.
In Vitro Studies
60. In 2012, Butler et al. examined the inhibitory effects of PTX2 and PTX2 SA on the four major actin isoforms (skeletal, cardiac, smooth muscle and non-muscle). PTX2 caused a concentration-dependent decrease in both rate and yield of skeletal muscle actin polymerisation in an in vitro fluorescent skeletal muscle actin assay (IC50 values of 44 and 177 nM; respectively), with no significant effects on depolymerization. Similar inhibitory effects were noted for all other actin isoforms. In contrast, PTX2 SA showed no effect on the polymerisation of any of the actin isoforms.
61. Reale et al. (2019) examined the toxicological effects of OA, PTX2, yessotoxin (YTX), azaspiracid-1 (AZA1), 13-desmethyl-spirolide C (SPX), and palytoxin (PlTX) on the rat enteric glial cells (EGC) cell line CRL2690 in vitro. No significant cytotoxicity was observed following treatment with PTX2 up to a concentration of 64 nM. However, PTX2 induce rapid morphological changes, causing cell shrinkage at concentrations of 4 nM and above and neurite atrophy at concentrations of 16 nM and above. At 24 hours treatment, PTX2 increased oxidative stress up to 3-fold at the highest dose tested (64 nM). The authors also reported PTX2 to cause a concentration-dependent increase of active caspase-3 in EGC cells, a marker of apoptosis, as well as a significant increase in γH2AX, a marker of DNA double strand breaks, at concentrations of 16 nM and above.
62. Based on the fact that OA is often simultaneously found in shellfish with PTX2, SPX, or YTX, a study by Alarcan et al. (2019) examined the combined effects of lipophilic biotoxins on human intestinal Caco-2 cells in vitro. OA induced cytotoxicity, DNA strand breaks and interleukin 8 release while PTX2 induced DNA strand breaks. PTX2 alone was not toxic to Caco-2 cells, whereas OA showed concentration-dependent toxicity (IC50 331 nM). The combination of OA with a second toxin resulted in reduced toxicity at low concentrations, which the authors suggested could be due to antagonistic effects. In contrast, at higher concentrations, increased toxicity was observed, which the authors suggested could indicate additive or synergistic effects. The authors concluded that combination effects of phycotoxins may occur which might have the potential to impact on risk assessment of these compounds.
63. A study by Sandvik et al. (2020) provided limited information on the potential biotransformation of PTX-group toxins by examining in vitro the metabolism of PTX2 using primary hepatocytes from Wistar rats in suspension. Results showed that purified PTX2 was rapidly metabolised, and that two major and several minor oxidized PTX2 metabolites were formed, none of which had retention times corresponding to PTX1, PTX11, or PTX13. The authors noted that rapid oxidative metabolism may help explain the low oral toxicity of PTXs observed in in vivo studies
DSP and PTX-group toxin occurrence in UK and Irish waters
64. In their most recent report on the official control (OC) monitoring programmes in England and Wales (2022), the Centre for Environment, Fisheries, and Aquaculture Science (CEFAS) reported that DSP toxins (OA-, DTX- and PTX-group) were detected in 83 samples from nine production areas in England. Eight mussel samples, originating from three production areas in South Cornwall exceeded the maximum permitted level (MPL). No data was available on the levels of PTX alone in these samples.
65. In a separate report on OC monitoring results for shellfish from Scotland, CEFAS, (2023) reported OA/DTX/PTX group toxins in 682 shellfish samples. 110 samples (all from mussels) recorded results above the MPL. These results were recorded between May and September 2022. The highest level recorded during 2022 was 2215 µg OA eq./kg, almost 14 times the regulatory limit, in a sample from Loch Laxford in July 2022. No data was available on the levels of PTX alone in these samples.
66. In March 2023, the Agri-Food and Biosciences Institute (AFBI) published their annual report for the marine biotoxin OC monitoring programme for Northern Ireland, covering testing performed from January to December 2022. 511 shellfish samples (253 oyster, 258 mussels) were tested for a variety of biotoxins including DSP (OA/DTX/PTX-group) toxins. There were no positive (levels above the regulatory limits) samples reported during 2022, with the highest levels of DSP toxins reported in mussels from the Belfast Lough (71 µg/kg). No data was available on the levels of PTX alone in these samples.
67. Dhanji-Rapkova et al. (2018) profiled the LTs monitoring results in shellfish from Great Britain collected between July 2011 and December 2016 with a focus on OA-group toxins, specifically OA, DTX1, DTX2, as well as PTX1 and PTX2. Although PTX-group toxins are regulated and reported together with OA and DTXs as total OA equivalents, this study evaluated them separately. Where present, PTX2 usually co-occurred with OA and/or DTXs. The authors reported that between July 2011 and December 2016, PTX2 was detected above the reporting limit (RL) in 34 shellfish samples (0.16%). Nine of these were Pacific oyster samples originating from a single monitoring point in Scotland. Twelve PTX2 positive mussel samples were found in seven different monitoring points in Scotland, the majority in Shetland Islands during the 2013 DSP incident, and in the Isle of Arran during 2012. Thirteen PTX2 positive mussel samples originated in south-west England where the highest PTX2 concentrations (∼ 80 μg/kg) were recorded in 2014. The authors note that the removing the PTX2 levels would not have impacted on the number of samples exceeding the MPL threshold. No PTX1 or PTX11 was detected above the RL in any OC sample subjected to analysis during the study period.
68. In 2019, researchers from the Marine Institute in Ireland published a review of DSP Toxicity in Irish waters, examine 25,595 water samples and 18,166 records of shellfish flesh from 2005 to 2017, for the presence and concentration of DSP toxins OA, DTX-1, DTX-2 (and their hydrolysed esters), as well as PTXs. Since monitoring began (the lipophilic method was modified in 2012 to include the detection and quantification of PTX1 and PTX2), quantifiable concentrations of PTX were rarely seen. In fact, no PTX-equivalent values have been observed above the RLs. The highest levels of PTX2 detected were off the Southwest coast of the country at levels between 0.01-0.13 mg/kg. The highest concentrations of PTX coincided with high levels of DTX-2, DTX-2 esters and OA esters in shellfish (Salas and Clarke, 2019).
Summary and conclusions
69. PTXs are produced by dinoflagellates, which also produce OA and its related analogues, DTXs, and bioaccumulate in filter feeding shellfish such as mussels. Hence, they are of potential risk to human health.
70. Historically, PTXs have been included in the DSP toxin group due to their high acute toxicity in the MBA and co-occurrence with OA and DTXs in shellfish. PTX is a regulated biotoxins in the UK and is included in the group of lipophilic toxins which are monitored routinely in UK shellfish. Based on the 2009 EFSA opinion on PTXs, recent amendments to EU legislation are set to remove PTX from the list of monitored biotoxin in EU shellfish.
71. In their opinion, the EFSA panel concluded that there were no confirmed human cases of intoxication from PTX-group toxins reported to date. Due to the fact PTX-group toxins were much less toxic via the oral route than OA and DTXs and have a different MOA, EFSA recommended that PTXs should not be included in the OA-group toxins and regulated separately.
Questions for the Committee
i. Do the Committee agree with EFSA’s 2009 conclusions that PTXs are less toxic via the oral route, have a different mode of action and do not induce diarrhoea?
ii. Do the Committee agree PTX-group toxins should not be included in the DSP-toxin group?
iii. Do the Committee agree that the PTX-group toxins should not be expressed as OA equivalents?
iv. Do the Committee think there is a toxicological risk from exposure to PTX?
v. Do the Committee have any other comments?
Secretariat
December 2023
Abbreviations
|
ARfD |
Acute Reference Dose |
|
AFBI |
Agri-Food & Biosciences Institute |
|
AZP |
Azaspiracid |
|
bw |
Bodyweight |
|
CCFFP |
Codex Committee on Fish and Fishery Products |
|
CEFAS |
The Centre for Environment, Fisheries and Aquaculture Science |
|
COT |
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment |
|
DSP |
Diarrhoetic Shellfish Poisoning |
|
DTX |
Dinophysistoxins |
|
EFSA |
The European Food Safety Authority |
|
EC |
European Commission |
|
EGC |
Enteric Glial Cells |
|
EU |
European Union |
|
FAO |
Food and Agriculture Organization |
|
FSA |
Food Standards Agency |
|
IOC |
Intergovernmental Oceanographic Commission |
|
i.p. |
intraperitoneal |
|
LC-MS/MS |
Liquid Chromatography with Mass Spectrometry |
|
LD50 |
Lethal Dose 50% - The dose of substance that kills 50% of the test sample |
|
LOAEL |
Lowest Observed Adverse Effect Levels |
|
MBA |
Mouse Bioassay |
|
ML |
Maximum Levels |
|
MOA |
Mode of Action |
|
MPL |
Maximum Permitted Level |
|
NOAEL |
No Observed Adverse Effect Levels |
|
OC |
Official Control |
|
PlTX |
palytoxin |
|
PTX |
Pectenotoxin |
|
PSP |
Paralytic Shellfish Poisoning |
|
RBA |
Rat Bioassay |
|
RL |
Reporting Limit |
|
SANCO |
Directorate General for Health and Consumer Affairs |
|
TDI |
Tolerable Daily Intake |
|
UF |
Uncertainty factor |
|
WHO |
World Health Organisation |
|
YTX |
Yessotoxin |
References
AFBI, 2023. Annual report for marine biotoxin analysis, official control monitoring programme for Northern Ireland.
Alarcan, J., Barbé, S., Kopp, B., Hessel-Pras, S., Braeuning, A., Lampen, A., Le Hégarat, L., Fessard, V., 2019. Combined effects of okadaic acid and pectenotoxin-2, 13-desmethylspirolide C or yessotoxin in human intestinal Caco-2 cells. Chemosphere 228, 139–148. Combined effects of okadaic acid and pectenotoxin-2, 13-desmethylspirolide C or yessotoxin in human intestinal Caco-2 cells - ScienceDirect.
Bialojan, C., Takai, A., 1988. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J 256, 283–290. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics | Biochemical Journal | Portland Press
Burgess, V., 2003. Investigations into the toxicology of pectenotoxin-2-seco acid and 7-epi pectenotoxin 2-seco acid to aid in a health risk assessment for the consumption of shellfish contaminated with these shellfish toxins in Australia. ENTOX / Griffith University, Queensland.
Burgess, V., Shaw, G., 2001. Pectenotoxins — an issue for public health: A review of their comparative toxicology and metabolism. Environment International 27, 275–283. Pectenotoxins — an issue for public health: A review of their comparative toxicology and metabolism - ScienceDirect
Butler, S.C., Miles, C.O., Karim, A., Twiner, M.J., 2012. Inhibitory effects of pectenotoxins from marine algae on the polymerization of various actin isoforms. Toxicol In Vitro 26, 493–499. Inhibitory effects of pectenotoxins from marine algae on the polymerization of various actin isoforms - ScienceDirect.
CEFAS, 2023. The Shellfish official control monitoring programmes for Scotland: Summary report for 2022.
CEFAS, 2022. The Biotoxin and Phytoplankton official control monitoring programmes for England and Wales: Summary report for 2021.
Chae, H.-D., Choi, T.-S., Kim, B.-M., Jung, J.H., Bang, Y.-J., Shin, D.Y., 2005. Oocyte-based screening of cytokinesis inhibitors and identification of pectenotoxin-2 that induces Bim/Bax-mediated apoptosis in p53-deficient tumors. Oncogene 24, 4813–4819. Oocyte-based screening of cytokinesis inhibitors and identification of pectenotoxin-2 that induces Bim/Bax-mediated apoptosis in p53-deficient tumors | Oncogene (nature.com).
Codex Alimentarius, 2015. Standard for Live and Raw Bivalve Shellfish STAN 292-2008.
COT, 2006. COT statement on risk assessment of marine biotoxins of the okadaic acid, pectenotoxin, azaspiracid and yessotoxin groups in support of human health. Committee on Toxicology.
Dhanji-Rapkova, M., O’Neill, A., Maskrey, B.H., Coates, L., Teixeira Alves, M., Kelly, R.J., Hatfield, R.G., Rowland-Pilgrim, S.J., Lewis, A.M., Algoet, M., Turner, A.D., 2018. Variability and profiles of lipophilic toxins in bivalves from Great Britain during five and a half years of monitoring: Okadaic acid, dinophysis toxins and pectenotoxins. Harmful Algae 77, 66–80. Variability and profiles of lipophilic toxins in bivalves from Great Britain during five and a half years of monitoring: Okadaic acid, dinophysis toxins and pectenotoxins - ScienceDirect.
EC, 2021a. Commission Implementing Regulation (EU) 2021/1709 of 23 September 2021 Amending Implementing Regulation (EU) 2019/627 as Regards Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin; European Commission: Brussels, Belgium.
EC, 2021b. Commission Delegated Regulation (EU) 2021/1374 of 12 April 2021 Amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council on Specific Hygiene Requirements for food of Animal Origin; European Commission: Brussels, Belgium.
EC, 2004. Regulation (EC) No 853/2004 of the European Parliament and of the Council laying down specific hygiene rules for food of animal origin; European Commission: Brussels, Belgium.
EFSA, 2009. Marine biotoxins in shellfish – Pectenotoxin group. EFSA Journal 7, 1109. Marine biotoxins in shellfish – Pectenotoxin group - - 2009 - EFSA Journal - Wiley Online Library.
EU/SANCO, 2001. Report of the meeting of the working group on toxicology of DSP and AZP. Brussels.
FAO/IOC/WHO, 2004. Report of the Joint FAO/IOC/WHO ad hoc Expert Consultation on Biotoxins in Bivalve Molluscs - Technical report.
FAO/WHO, 2016. Technical paper on Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs. Rome.
FDA, 2022. Fish and Fishery Products Hazards and Controls Guidance, Department of Health and Human Services, Public Health Service, Office for Food Safety.
Fladmark, K.E., Serres, M.H., Larsen, N.L., Yasumoto, T., Aune, T., Døskeland, S.O., 1998. Sensitive detection of apoptogenic toxins in suspension cultures of rat and salmon hepatocytes. Toxicon 36, 1101–1114. Sensitive detection of apoptogenic toxins in suspension cultures of rat and salmon hepatocytes - ScienceDirect.
Hamano, Y., Kinoshita, Y., Yasumoto, T., 1986. Enteropathogenicity of Diarrhetic Shellfish Toxins in Intestinal Models. J. Food Hyg. Soc. 27.
Hori, M., Matsuura, Y., Yoshimoto, R., Ozaki, H., Yasumoto, T., Karaki, H., 1999. [Actin depolymerizing action by marine toxin, pectenotoxin-2]. Nihon Yakurigaku Zasshi 114 Suppl 1, 225P-229P. Inhibition of actin polymerization of pectenotoxin-2, a naturally bioactive substance derived from the ocean. (jst.go.jp).
Ishige, M., Satoh, N., Yasumoto, T., 1988. Pathological studies on the mice administered with the causative agent of diarrhetic shellfish poisoning (okadaic acid and Pectenotoxin-2). 38: Hokkaidoritsu Eisei Kenkyushoho 38, 15–18.
Ito, 2006. Verification of diarrhetic activities of PTX-2 and okadaic acid in vivo. Presented at the 12th International Conference on Harmful Algae.
Ito, E., Suzuki, T., Oshima, Y., Yasumoto, T., 2008. Studies of diarrhetic activity on pectenotoxin-6 in the mouse and rat. Toxicon 51, 707–716. Studies of diarrhetic activity on pectenotoxin-6 in the mouse and rat - ScienceDirect.
Miles, C.O., Wilkins, A.L., Munday, J.S., Munday, R., Hawkes, A.D., Jensen, D.J., Cooney, J.M., Beuzenberg, V., 2006. Production of 7-epi-pectenotoxin-2 seco acid and assessment of its acute toxicity to mice. J Agric Food Chem 54, 1530–1534. Production of 7-epi-Pectenotoxin-2 Seco Acid and Assessment of Its Acute Toxicity to Mice | Journal of Agricultural and Food Chemistry (acs.org).
Miles, C.O., Wilkins, A.L., Munday, R., Dines, M.H., Hawkes, A.D., Briggs, L.R., Sandvik, M., Jensen, D.J., Cooney, J.M., Holland, P.T., Quilliam, M.A., Lincoln MacKenzie, A., Beuzenberg, V., Towers, N.R., 2004. Isolation of pectenotoxin-2 from Dinophysis acuta and its conversion to pectenotoxin-2 seco acid, and preliminary assessment of their acute toxicities. Toxicon 43, 1–9. Isolation of pectenotoxin-2 from Dinophysis acuta and its conversion to pectenotoxin-2 seco acid, and preliminary assessment of their acute toxicities - ScienceDirect.
Munday, J., 2008. Toxicology of the pectenotoxins. In: Seafood and Freshwater toxins: Pharmacology, Physiology and Detection, 2nd edition. ed. CRC Press (Taylor and Francis Group), Florida.
Murata, M., Sano, M., Iwashita, T., Naoki, H., Yasumoto, T., 1986. The Structure of Pectenotoxin-3, a New Constituent of Diarrhetic Shellfish Toxins. Agricultural and Biological Chemistry 50, 2693–2695. Structure of Pectenotoxin-3, a New Constituent of Diarrhetic Shellfish Toxins | Bioscience, Biotechnology, and Biochemistry | Oxford Academic (oup.com).
NZFS, 2020. Risk Assessment of Pectenotoxins in New Zealand Bivalve Molluscan Shellfish, 2009-2019. Toxins (Basel) 12, 776. Toxins | Free Full-Text | Risk Assessment of Pectenotoxins in New Zealand Bivalve Molluscan Shellfish, 2009–2019 (mdpi.com).
Ogino, H., Kumagai, M., Yasumoto, T., 1997. Toxicologic evaluation of yessotoxin. Nat Toxins 5, 255–259. Toxicologic evaluation of Yessotoxin - Ogino - 1997 - Natural Toxins - Wiley Online Library.
Reale, O., Huguet, A., Fessard, V., 2019. Novel Insights on the Toxicity of Phycotoxins on the Gut through the Targeting of Enteric Glial Cells. Mar Drugs 17, 429. Marine Drugs | Free Full-Text | Novel Insights on the Toxicity of Phycotoxins on the Gut through the Targeting of Enteric Glial Cells (mdpi.com).
Salas, R., Clarke, D., 2019. Review of DSP Toxicity in Ireland: Long-Term Trend Impacts, Biodiversity and Toxin Profiles from a Monitoring Perspective. Toxins 11, 61.
Sandvik, M., Miles, C.O., Wilkins, A.L., Fæste, C., 2020. In vitro hepatic biotransformation of the algal toxin pectenotoxin-2. Toxicon X 6, 100031. In vitro hepatic biotransformation of the algal toxin pectenotoxin-2 - ScienceDirect.
Sasaki, K., Wright, J.L.C., Yasumoto, T., 1998. Identification and Characterization of Pectenotoxin (PTX) 4 and PTX7 as Spiroketal Stereoisomers of Two Previously Reported Pectenotoxins. J Org Chem 63, 2475–2480. Identification and Characterization of Pectenotoxin (PTX) 4 and PTX7 as Spiroketal Stereoisomers of Two Previously Reported Pectenotoxins | The Journal of Organic Chemistry (acs.org).
Spector, I., Braet, F., Shochet, N.R., Bubb, M.R., 1999. New anti-actin drugs in the study of the organization and function of the actin cytoskeleton. Microsc Res Tech 47, 18–37. Microscopy Research and Technique | Microscopy Journal | Wiley Online Library.
Suzuki, T., Walter, J.A., LeBlanc, P., MacKinnon, S., Miles, C.O., Wilkins, A.L., Munday, R., Beuzenberg, V., MacKenzie, A.L., Jensen, D.J., Cooney, J.M., Quilliam, M.A., 2006. Identification of pectenotoxin-11 as 34S-hydroxypectenotoxin-2, a new pectenotoxin analogue in the toxic dinoflagellate Dinophysis acuta from New Zealand. Chem Res Toxicol 19, 310–318. Identification of Pectenotoxin-11 as 34S-Hydroxypectenotoxin-2, a New Pectenotoxin Analogue in the Toxic Dinoflagellate Dinophysis acuta from New Zealand | Chemical Research in Toxicology (acs.org).
Terao, K., Ito, E., Yanagi, T., Yasumoto, T., 1986. Histopathological studies on experimental marine toxin poisoning. I. Ultrastructural changes in the small intestine and liver of suckling mice induced by dinophysistoxin-1 and pectenotoxin-1. Toxicon 24, 1141–1151. Histopathological studies on experimental marine toxin poisoning. I. Ultrastructural changes in the small intestine and liver of suckling mice induced by dinophysistoxin-1 and pectenotoxin-1 - ScienceDirect.
Vilariño, N., Louzao, M.C., Fraga, M., Botana, L.M., 2015. From science to policy: dynamic adaptation of legal regulations on aquatic biotoxins, in: 13. From Science to Policy: Dynamic Adaptation of Legal Regulations on Aquatic Biotoxins. De Gruyter, pp. 441–482. 13. From science to policy: dynamic adaptation of legal regulations on aquatic biotoxins (degruyter.com).
Yasumoto, T., Murata, M., Oshima, Y., Sano, M., Matsumoto, G.K., Clardy, J., 1985. Diarrhetic shellfish toxins. Tetrahedron 41, 1019–1025. Diarrhetic shellfish toxins - ScienceDirect.
Yasumoto, T., Murata, T.M., Lee, J.S., Torigoe, K., 1989. Polyether toxins produced by dinoflagellates. In: Mycotoxins and Phycotoxins. Elsevier, Amsterdam.
Yoon, M.Y., Kim, Y.C., 1997. Acute toxicity of pectenotoxin-2 and its effects on hepatic metabolising enzyme system in mice. [in Korean]. Korean Journal of Toxicology 13, 183–186.
Zhou, Z.H., Komiyama, M., Terao, K., Shimada, Y., 1994. Effects of pectenotoxin-1 on liver cells in vitro. Nat Toxins 2, 132–135. Effects of pectenotoxin‐1 on liver cells in vitro - Zhou - 1994 - Natural Toxins - Wiley Online Library.
TOX/2023/XX Annex A
Risk of emerging marine biotoxins in British shellfish – Pectenotoxin group
Literature Review
Papers were selected from PubMed, using the year filter from 2009 to 2023 to search for papers published after the EFSA 2009 opinion. An initial search using the term ‘pectenotoxin’ produced 205 results, 77 of which were filtered via the free full text filter. All 205 results were scanned for keywords and relevance regarding either new information on the toxicology of PTX or its occurrence in UK waters. Further searches included the terms ‘pectenotoxin toxicology’ producing 8 results, ‘pectenotoxin England’ producing 2 results, and ‘pectenotoxin United Kingdom’ which produced 6 results.
Google was also used to find CEFAS reports for monitoring of shellfish including the harmful algal blooms (HABS) surveillance programmes and monitoring data. The term ‘CEFAS pectenotoxin’ was used for this search. Overall, from these searches, 9 sources regarding occurrence in the UK were selected and 5 In Vitro studies identified to be relevant.