Information on ginger
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.11. Ginger (Zingiber officinale) is a flowering tropical plant originating in Southeast Asia and grown in warm climates including China, India, Africa and the Caribbean. Ginger is commonly consumed in fresh root, dried root powder and capsule (encapsulated dried powder) forms, as a liquid extract, preserved in syrup or sugar and as a tea.
Uses
12. The rhizome (underground stem) of the ginger plant is commonly used as a spice and flavouring in many countries around the world and is increasingly growing in popularity as a natural remedy due to its purported immunomodulatory properties and also to alleviate motion sickness and post-operative nausea and vomiting. Ginger has been recommended as a nonpharmacological treatment for mild to moderate nausea and vomiting in pregnancy (NHS 2021; NICE 2021) and has also been used as a dietary supplement and a traditional remedy in many cultures. Ginger is included in the official pharmacopoeias of some western countries.
Constituents
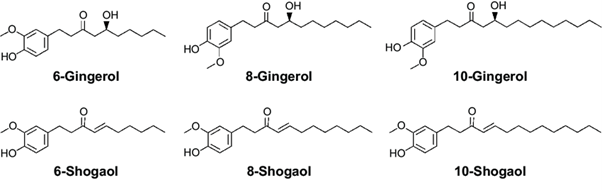
13. Over 100 compounds have been identified in ginger extracts, most being terpenoids - mainly sesquiterpenoids (α-zingiberene, β-sesquiphellandrene, β-bisabolene, α-farnesene, ar-curcumene zingiberol) and smaller amounts of monoterpenoids (camphene, β-phellandrene, cineole, geraniol, curcumene, citral, terpineol, borneol) (EMA, 2012).
14. The ginger rhizome contains two main classes of constituents: the essential oils responsible for the aroma, and the main pungent principles, gingerols and shogaols. Organic acids are also present in smaller amounts. Depending on the area of cultivation, gingerols make up 4-7.5% of the pungent principles, the main one being 6-gingerol. Gingerols of other chain lengths are also present in smaller amounts.
Reviews by other regulatory agencies
15. Ginger is included in the official pharmacopoeias of several western countries. Ginger is classified as ‘Generally Recognised as Safe’ (GRAS) by the United States Food and Drug Administration (FDA) however, few specific studies have been carried out to evaluate the safety of ginger use during pregnancy and lactation. A report by the National Institute for Health and Care Excellence (NICE) cites a number of short duration trials which have been conducted in pregnant women (NICE, 2021).
16. In 2008, the Danish company Ferrosan A/S withdrew their product GraviFrisk – a product containing 6 g of dried ground ginger - from market, due to concerns surrounding the lack of safety data with respect to the use of supplements containing highly concentrated ginger extracts by pregnant women (Dietz et al., 2016).
17. In their 2012 report on ginger root in powdered form, the European Medicines Agency (EMA) concluded “The ginger extract dosages to provoke acute toxicity are high and much higher than usually administered dosages (factor of 10-15 for an adult). There is some evidence that ginger root may cause rodent testicular weight to increase by repeated high dosages of ginger root extract (2000 mg/kg). Ginger root has mutagenic as well as antimutagenic properties in microbial test systems. Developmental toxicity studies in rats are difficult to interpret, however, it is probably not a cause for concern. In general, toxicity studies of ginger are considered inadequate at least regarding genotoxicity, carcinogenicity and, partially, reproductive and developmental toxicity.”
18. The Norwegian Food Safety Authority have issued a warning regarding the use of ginger supplements and ginger-containing shots during pregnancy. This was based on a risk assessment carried out by the Danish Technical University and the Danish Veterinary and Food Administration (DTU, 2018). The assessment, based on animal studies, including one in which rats were treated with a fresh grated ginger preparation with ginger at concentrations of 20-50 g/L in water, found that even in the 20 g/L treatment group – the equivalent of 1,784 mg/kg bw increased the incidence of abortion in rats. The Norwegian Food Safety Authority concluded that while a woman of 70 kg would consume less ginger (124 mg to 329 mg) there remains cause for concern and fetal risk cannot be excluded.
19. Recently, the Finnish Food Authority issued a recommendation against the use of products containing ginger concentrate or extract, ginger tea and food supplements containing ginger by pregnant and breastfeeding women, infants and toddlers, schoolchildren, elderly and individuals with weakened immunity (Finnish Food Authority, 2019). It was noted that the concentrates contained substances which may be harmful and safe consumption levels were unknown.
20. The Expert Panel for Cosmetic Ingredient Safety (Panel) assessed the safety of ginger-derived ingredients for use in cosmetics and determined that they are safe in cosmetics in the present practices of use when formulated to be non-sensitising (Belsito, 2021). The report describes a short-term toxicity study where seventy participants were given an oral dose of either steamed ginger extract (200 mg in capsule form; n = 36), or a placebo (n = 34), daily. All clinical test results were normal, and all participant completed the study. No extract-related adverse effects were observed.