Third Draft Statement on the Safety of Ginger Supplement Use in Pregnancy - Annex A
Background
In this guide
In this guideSecond Draft Statement on the Safety of Ginger Supplement Use in Pregnancy
Background
1. In 2019, the Scientific Advisory Committee on Nutrition (SACN) agreed to conduct a risk assessment on nutrition and maternal health focusing on maternal outcomes during pregnancy, childbirth and up to 24 months after delivery; this would include the effects of chemical contaminants and excess nutrients in the diet.
2. This subject was initially discussed during the COT’s horizon scanning item at their January 2020 meeting with a scoping paper being presented to the COT in July 2020. This included background information on a provisional list of chemicals proposed by SACN.
3. Following a discussion at the September 2020 meeting, the COT agreed that papers on a number of compounds should be prioritised. The following paper provides the advice of the COT on whether exposure to ginger would pose a risk to maternal health.
4. As part of the current programme of work on the maternal diet, the Committee considered the use of dietary supplements during pregnancy. A scoping paper (TOX/2020/51) was presented, reviewing the commonly used dietary supplements during pregnancy. These were supplements that were not officially recommended by the relevant authorities, but which have been promoted by anecdotal evidence and unofficial sources as having various purported benefits. The review was confined to herbal dietary supplements which would be regulated under food law, and which would not be considered to be traditional herbal medicines which are the responsibility of the Medicines and Healthcare Products Regulatory Agency (MHRA).
5. Paper TOX/2020/51 provided a detailed summary of the supplements most recommended during pregnancy (ginger, chamomile, raspberry leaf, echinacea, peppermint oil and leaves, dandelion, and evening primrose oil), focusing where available, on studies relevant to pregnancy and maternal outcomes. The main areas of investigation were general toxicity to the mother, effects on the development of the foetus or embryo, and possible interactions with medicines. The COT agreed that ginger required further investigation, noting that both human and animal in vitro and in vivo data were available.
6. In May 2021, the Committee considered the potential effects of ginger and ginger supplements during pregnancy and lactation. Paper TOX/2021/26 (Available on the COT website) reviewed the available data on toxicity to the mother, effects on the development of the foetus or embryo, and possible interactions with drugs as well as data on potential exposure.
7. Overall, it was concluded that there were limited data. The human data presented were not strongly indicative of any toxicological concern but there were some indications of possible adverse effects and a lot of uncertainties. Ginger did not appear to be systemically toxic but did appear to have reprotoxic effects at high supplemental doses. The Committee suggested looking at the animal data in closer detail to determine the point of departure (No Observed Adverse Effect Level - NOAEL), followed by calculating the potential exposure to supplements to determine whether there was cause for concern.
8. Paper TOX/2021/51 provided further information with respect to animal studies, contaminants and exposure to ginger supplements, primarily centred on the effect of ginger on prostaglandins, reproductive and developmental toxicity and the possible contaminants present in ginger.
9. Members noted that although the different ginger extracts were not comparable, from animal studies they did appear to exhibit some biological activity in the early stages of pregnancy. It was reiterated that there was no indication of general systemic toxicity from the use of ginger.
10. The COT noted that intake of ginger in foodstuffs should also be considered as ginger was consumed not only as a supplement but also as part of the diet in foods such as ginger biscuits, tea and ginger beer. Therefore, aggregate exposures would need to be considered when addressing the safety of ginger supplement use during pregnancy.
Information on ginger
In this guide
In this guide11. Ginger (Zingiber officinale) is a flowering tropical plant originating in Southeast Asia and grown in warm climates including China, India, Africa and the Caribbean. Ginger is commonly consumed in fresh root, dried root powder and capsule (encapsulated dried powder) forms, as a liquid extract, preserved in syrup or sugar and as a tea.
Uses
12. The rhizome (underground stem) of the ginger plant is commonly used as a spice and flavouring in many countries around the world and is increasingly growing in popularity as a natural remedy due to its purported immunomodulatory properties and also to alleviate motion sickness and post-operative nausea and vomiting. Ginger has been recommended as a nonpharmacological treatment for mild to moderate nausea and vomiting in pregnancy (NHS 2021; NICE 2021) and has also been used as a dietary supplement and a traditional remedy in many cultures. Ginger is included in the official pharmacopoeias of some western countries.
Constituents
13. Over 100 compounds have been identified in ginger extracts, most being terpenoids - mainly sesquiterpenoids (α-zingiberene, β-sesquiphellandrene, β-bisabolene, α-farnesene, ar-curcumene zingiberol) and smaller amounts of monoterpenoids (camphene, β-phellandrene, cineole, geraniol, curcumene, citral, terpineol, borneol) (EMA, 2012).
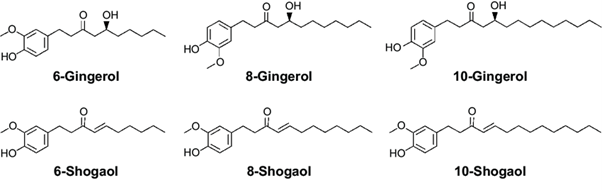
14. The ginger rhizome contains two main classes of constituents: the essential oils responsible for the aroma, and the main pungent principles, gingerols and shogaols. Organic acids are also present in smaller amounts. Depending on the area of cultivation, gingerols make up 4-7.5% of the pungent principles, the main one being 6-gingerol. Gingerols of other chain lengths are also present in smaller amounts.
Reviews by other regulatory agencies
15. Ginger is included in the official pharmacopoeias of several western countries. Ginger is classified as ‘Generally Recognised as Safe’ (GRAS) by the United States Food and Drug Administration (FDA) however, few specific studies have been carried out to evaluate the safety of ginger use during pregnancy and lactation. A report by the National Institute for Health and Care Excellence (NICE) cites a number of short duration trials which have been conducted in pregnant women (NICE, 2021).
16. In 2008, the Danish company Ferrosan A/S withdrew their product GraviFrisk – a product containing 6 g of dried ground ginger - from market, due to concerns surrounding the lack of safety data with respect to the use of supplements containing highly concentrated ginger extracts by pregnant women (Dietz et al., 2016).
17. In their 2012 report on ginger root in powdered form, the European Medicines Agency (EMA) concluded “The ginger extract dosages to provoke acute toxicity are high and much higher than usually administered dosages (factor of 10-15 for an adult). There is some evidence that ginger root may cause rodent testicular weight to increase by repeated high dosages of ginger root extract (2000 mg/kg). Ginger root has mutagenic as well as antimutagenic properties in microbial test systems. Developmental toxicity studies in rats are difficult to interpret, however, it is probably not a cause for concern. In general, toxicity studies of ginger are considered inadequate at least regarding genotoxicity, carcinogenicity and, partially, reproductive and developmental toxicity.”
18. The Norwegian Food Safety Authority have issued a warning regarding the use of ginger supplements and ginger-containing shots during pregnancy. This was based on a risk assessment carried out by the Danish Technical University and the Danish Veterinary and Food Administration (DTU, 2018). The assessment, based on animal studies, including one in which rats were treated with a fresh grated ginger preparation with ginger at concentrations of 20-50 g/L in water, found that even in the 20 g/L treatment group – the equivalent of 1,784 mg/kg bw increased the incidence of abortion in rats. The Norwegian Food Safety Authority concluded that while a woman of 70 kg would consume less ginger (124 mg to 329 mg) there remains cause for concern and fetal risk cannot be excluded.
19. Recently, the Finnish Food Authority issued a recommendation against the use of products containing ginger concentrate or extract, ginger tea and food supplements containing ginger by pregnant and breastfeeding women, infants and toddlers, schoolchildren, elderly and individuals with weakened immunity (Finnish Food Authority, 2019). It was noted that the concentrates contained substances which may be harmful and safe consumption levels were unknown.
20. The Expert Panel for Cosmetic Ingredient Safety (Panel) assessed the safety of ginger-derived ingredients for use in cosmetics and determined that they are safe in cosmetics in the present practices of use when formulated to be non-sensitising (Belsito, 2021). The report describes a short-term toxicity study where seventy participants were given an oral dose of either steamed ginger extract (200 mg in capsule form; n = 36), or a placebo (n = 34), daily. All clinical test results were normal, and all participant completed the study. No extract-related adverse effects were observed.
Health-based guidance values and Red ginger
In this guide
In this guideHealth-based guidance values
21. There are currently no health-based guidance values (HBGV) with respect to ginger or its main components. Exposure to ginger was considered based on information found on supplement and tincture composition and background diet, but the variability of available supplements meant exposure also varied, which made comparison difficult.
22. The NHS and NICE support the use of ginger tea and biscuits as a non-pharmacological intervention for nausea and vomiting in pregnancy (NHS, 2021; NICE, 2021). Generally, amounts of 1 g ginger per day are probably safe (NHS, 2022). Anecdotally, 1-1.5 g per day has also been advised during pregnancy (Healthline, 2020; Mother and baby, 2022). It is advised that supplements should be used only under the advice and supervision of a medical professional.
Red ginger
23. In traditional medicine, red ginger is used for treating headaches, indigestion, nausea, vomiting, and cancer. In addition, it is widely used to treat autoimmune diseases (psoriasis), hypertension, hypercholesteremia, hyperuricemia and bacterial infections. (Zhang et al., 2022).
24. The consumption of red ginger in the diet is not common due to the difference in taste when compared with common ginger. There is limited evidence to suggest that red ginger is commonly purchased or consumed in the UK.
25. Health claims relating to red ginger reference the benefits of consuming red ginger for emesis and pain during and following pregnancy however studies in this area are primarily from hospital obstetrics settings in Asia (largely Indonesia) where red ginger is grown and readily available.
26. There is limited toxicological data available on red ginger and studies looking at the medicinal potential of red ginger do not assess or comment on effects outside of those of interest. There are some examples of comparisons of red vs common ginger on toxicological literature. In these studies, red ginger has an enhanced effect when compared to common ginger.
27. Red ginger is discussed in Annex D.
Toxicology Overview
In this guide
In this guide28. It was noted that some studies reported effects on male testes and, though not relevant for females, they were nevertheless regarded as indicating a potential reprotoxic effect from ginger. Studies suggest that ginger affected the viability of pregnancy; however, with no strong conclusive human data, the COT concluded that more work was required, especially as these studies suggested a link between first trimester loss and ginger use. Further, the possible fetotoxicity based on evidence from animal data, genotoxicity and possible drug interactions should be further investigated.
29. Discussion paper TOX/2021/26 reviewed the available studies on cytotoxicity, mutagenicity, acute, reproductive and developmental toxicity, lactation and possible drug interactions as well as data on potential exposure in pregnancy, covering both animal and human studies. The results from these reports were varied due to the differences in the forms and extracts tested and as a result, some findings were contradictory.
30. Paper TOX/2021/51 provided further information with respect to animal studies, contaminants and exposure to ginger supplements, primarily centred on the effect of ginger on prostaglandins, reproductive and developmental toxicity and the possible contaminants present in ginger. The Committee noted that the papers reviewed covered ginger in a range of forms including fresh, aqueous, dried and alcohol extracts.
31. The toxicological data in this report have been divided into two sections: the first includes studies in which ginger was administered similarly to traditional culinary uses; and the second includes studies using ginger extracts and other concentrated forms.
32. Dry ginger powder was administered in some of the studies. Where the dose is ≤4 g/day (equivalent to approximately 2 teaspoons) it has been included in traditional culinary uses, and if it is >4 g/day the study is included in the extracts section.
Toxicology of ginger root used traditionally
Reproductive and developmental toxicity
Animal studies
33. Reproductive and developmental toxicity has been investigated in rat studies. In a study by Wilkinson (2000), three groups of pregnant Sprague-Dawley rats were administered either a control (unspecified), or 20 g/L or 50 g/L ginger tea - prepared by the infusion of grated ginger in water then filtered and administered via the drinking water - during days 6 to 15 of gestation. No further details were provided regarding specific compounds of interest. While no maternal toxicity was observed, embryonic loss in the treated groups was found to be double that of the controls. Exposed foetuses were found to be significantly heavier than controls and showed no gross structural malformations. The authors conclude that the results of this study suggest that in utero exposure to ginger tea results in early embryonic loss and increased growth in surviving foetuses.
Human studies - exposures in pregnancy
34. In a double-blind randomised crossover trial, 27 pregnant women were administered capsules containing either 250 mg ginger in powdered root form or 250 mg lactose as a placebo, four times per day, for four days followed by a wash out period of 2 days prior to a further 4 days administration of ginger or placebo alternative to the treatment during phase 1. (Fischer-Rasmussen et al., 1990). Two subjects did not carry to term: One subject from the ginger group had a spontaneous abortion, one elected. Of the remaining 25 subjects, no adverse effects were observed.
35. Of the available human studies, few explicitly addressed the safety of ginger consumption during pregnancy, most being incidental to other studies. In a double-blind study by Vutyavanich et al. (2001), 32 women were given 1 g of dried ginger in capsule form for 4 days. Of those in the ginger group, one spontaneous abortion was reported compared to 3 in the placebo group. Equally, for delivery by caesarean section, there was no difference between groups. No congenital abnormalities were observed in all babies carried to term. The group concluded that there were no significant adverse effects of ginger on pregnancy outcome.
36. An observational study in humans examined 187 pregnant women who took ginger in their first trimester and compared them to 187 pregnant women exposed to nonteratogenic drugs that were not antiemetic drugs. The results suggested that the ginger group did not have an increased rate of major malformations above the baseline rate of 1%–3% (Portnoi et al., 2003). Three major malformations were reported in the ginger group, ventricular septal defect (VSD), right lung abnormality, and kidney abnormality (pelviectasis) and one child was diagnosed with idiopathic central precocious puberty at age 2 years. The mother was reported to have taken 250 mg of ginger in capsules four times a day from 11 to 20 weeks of gestation in addition to dimenhydrinate and doxylamine/vitamin B6 (Diclectin) during the first trimester of pregnancy. No significant difference between the two groups in terms of live births, spontaneous abortions, stillbirths, therapeutic abortions, birth weight, or gestational age were reported, however the comparison group had more infants weighing less than 2,500 g (12 vs 3, P<0.001) and the ginger group had 8 sets of twins (i.e. approximately 4 in 100 births), compared with an expected background rate of 1 in 80 to 1 in 100 births. There were no twins reported in the control group.
37. Ensiyeh et al, investigated the effectiveness of ginger versus B6 for treatment of nausea and vomiting in pregnancy (NVP) in women before 17 weeks’ gestation (2009). Seventy women were randomised to receive either ginger at a dose of 1 g per day or B6 at 40 mg per day for 4 days. The ginger group reported 2 spontaneous abortions, compared to one in the B6 group. Of the babies brought to term, no congenital anomalies were observed, and all babies were discharged in good health.
38. Whilst also examining the use of ginger in the treatment of nausea and vomiting in pregnancy, Smith et al. noted 3 spontaneous abortions in the group taking 1.05 g ginger compared to 9 in the group taking 75 mg B6 daily for 3 weeks (2004).
39. Chittumma (2007) compared the effectiveness of ginger and vitamin B6 for treatment of nausea and vomiting in pregnancy. One hundred and twenty-six pregnant women, with a gestational age of < 16 weeks received either 650 mg of ginger or 25 mg of vitamin B6 three times per day for 4 days. p. Ginger and vitamin B6 significantly reduced nausea and vomiting scores from 8.7 + 2.2 to 5.4 + 2.0 and 8.3 + 2.5 to 5.7 + 2.3 respectively, (p < 0.05). There were some minor side effects in both groups 25.4% and 23.8% (p = 0.795) respectively, such as sedation, heartburn, arrhythmia.
40. The COT considered the possible mode of action of the purported benefits of ginger on nausea. It was theorised that ginger might decrease prostaglandin levels, which were linked to nausea. The effects on prostaglandins are covered from paragraph 103 onwards.
41. Overall, it was concluded that there were limited data. The human data presented were not strongly indicative of any toxicological concern but there were some indications of possible effects and many uncertainties. Ginger did not appear to be systemically toxic but reprotoxic effects have been reported in animal studies. However, there is no convincing evidence for this outcome in human studies.
Lactation
42. With respect to lactation, the focus of available studies (Lamxay et al., 2011; Kaygusuz et al., 2021; Dilokthornsakul et al., 2021) has been on the effect of ginger on milk production and volume rather than safety and therefore, the effect of exposure during lactation has not been fully investigated.
Effect on P450 (CYP) Enzymes and Herb-Drug Interactions
43. Ginger was found to have a significant inhibitory effect on CYP3A4, CYP2C9, and P‐glycoprotein activities in vitro (Kimura et al., 2010; Zhang and Lim, 2008). It was this effect that was thought to be responsible for reported hepatic cytolysis in a 48-year-old woman being treated with crizotinib. The patient, who was being treated with 250 mg crizotinib twice a day, had been taking ginger as a tea (amounts unknown) concomitantly during treatment. A subsequent diagnostic evaluation showed an increased crizotinib concentration, 1.8-fold higher than that measured two months prior.
Anti-platelet aggregation activity
Human studies
44. Krüth et al. reported the possible over-anticoagulation resulting from a possible ginger-phenprocoumoun interaction (2003). A 76-year-old woman on long-term phenprocoumon therapy presented with epistaxis and an international normalized ratio (INR) of >10. Partial thromboplastin time (PTT) was also found to be prolonged (84.4 seconds; normal <35). For several weeks prior to the event, the woman had a regular ginger intake of dried ginger pieces and tea from ginger powder. Following treatment with vitamin K, the patient’s INR and PTT returned to within therapeutic range.
45. Young et al. investigated the synergistic effect of ginger and nifedipine on anti-platelet aggregation in healthy volunteers aged 25-60 years old and hypertensive individuals aged 35-60 years old (2006). In a five-part study, the two groups comprising of 10 males and 10 females were administered 75 mg of acetylsalicylic acid (ASA), 1 g of ginger, 10 mg nifedipine, 1 g dried ginger and 10 mg nifedipine in combination and 1 g dried ginger and 75 mg ASA in combination daily for one week each following a washout period (7 days following ASA administration, 10 days thereafter).
46. Platelet aggregation in the presence of collagen, ADP and epinephrine was 44.1%, 44.5% and 42.1% in normal subjects and 64.2%, 67.7% and 62.9% in hypertensive patients, respectively. Platelet aggregation induced by collagen, ADP or epinephrine was found to be higher in hypertensive patients than normal patients. Following administration of ginger alone, platelet aggregation was measured as 35.2%, 37.8%, 35.9% with collagen, ADP and epinephrine respectively. When administered ginger and nifedipine in combination, the percentage inhibition of platelet aggregation induced by collagen, ADP and epinephrine was 79.8%, 75.2%, 69.3% respectively.
47. Al Askar et al. (2020) investigated the effect of ginger on platelet aggregation using agonists adenosine diphosphonate, arachidonic acid, collagen, ristocetin and epinephrine. Forty healthy male and female participants were randomized (1:1) to consume ginger tea at an amount of 4 g powered ginger in 150 ml of boiling water once daily vs. 4 g twice daily for five consecutive days. Comparisons were with pre-treatment changes. Four grams of ginger powder administered daily resulted in reduced platelet aggregation in subjects using epinephrine only. No such effect was seen in the higher dose group. Essentially, ginger had no effect on platelet aggregation in this study. Platelet aggregation inhibition was found to be higher in women using arachidonic acid.
48. Srivastava (1989) investigated the effect of fresh ginger on blood platelet thromboxane synthesis in humans. In a study on 7 women aged between 25-65 years, where volunteers consumed ~5g of fresh ginger for 7 days, ginger was found to inhibit eicosanoid biosynthesis in vivo.
49. Lumb found that a dose of 2g of ginger in powder form daily produced no significant differences in platelet aggregation/function than the placebo. The authors concluded that previously reported effects on thromboxane synthetase activity may be dose dependent or attributed to fresh ginger (1994).
50. Bordia et al., (1997) found that 4 g powdered ginger administered daily over the course of 1.5 and 3 months had no effect on ADP and epinephrine-induced platelet aggregation in individuals with coronary artery disease (CAD). However, a single 10g dose of powdered ginger, administered to CAD patients resulted in a significant decrease in induced platelet aggregation.
Toxicology of ginger extracts
In this guide
In this guideCytotoxicity
51. The cytotoxicity of ginger extracts has been investigated with varied results. Plengsuriyakarn et al. (2012) examined cytotoxicity of ethanolic ginger extracts in a cholangiocarcinoma (CCA) cell line 6 (CL-6) model, compared to hepatocarcinoma (HepG2) and normal human renal epithelium (HRE) models, using calcein-AM release and Hoechst 33342 assays to assess cell viability and apoptotic activity. The median inhibitory concentration, (IC50) values for cytotoxicity of the crude ethanolic extract of ginger ranged from 11 – 245 μg/ml across the 3 cell lines and the two assays.
52. Zaeoung et al. (2005) reported that the IC50 of aqueous and methanolic extracts of ginger was greater than 39.2 µg/ml against breast (MCF7) and colon (LS174T) cell lines.
53. Abudayyak et al. (2015) found the aqueous and methanolic extracts of ginger exhibited no cytotoxic activity when assessed using an MTT test (a colourimetric assay for assessing cell metabolic assay) in the rat kidney, NRK-52E cell line. The chloroform extract resulted in an IC50 value of 9.1 mg/mL.
54. However, it was noted that the inhibitory concentration (IC50) values presented in the studies reviewed were based on a small amount of data, from only a few different cell lines and therefore firm conclusions could not be drawn. Also, the purpose of most of these studies was an attempt to identify possible anti- cancer agents, rather than as an assessment on the safety of ginger as a supplement and therefore relevant endpoints would not have been assessed.
Mutagenicity
55. Nakamura and Yamamoto (1982) found that the juice of ginger rhizome possessed both mutagenic and anti-mutagenic properties, and that 6-gingerol in particular was a powerful mutagen. The group also demonstrated that 6-shogaol was much less mutagenic (strain Hs30 of Escherichia coli) than 6-gingerol (Nakamura & Yamamoto 1983). In a Salmonella typhimurium reverse mutation (Ames) assay, the urine of rats fed diets containing 0.5, 1 and 5% powdered ginger for 1 month and exposed to benzo(a)pyrene was found to display a significant reduction in mutagenicity as indicated by a reduced number of TA98 and TA100 revertants at all ginger concentrations (Nirmala et al. 2007) when tested in an Ames assay.
56. In another Ames assay, an ethanolic extract of ginger (Soudamini et al. 1995) and an essential oil from ginger (Sivaswami et al. 1991) demonstrated mutagenic activity in S. typhimurium strains TA100 and TA1535 at concentrations of 25-50 mg/plate and 5-10 mg/plate, respectively. Similarly, an ethanolic ginger extract at concentrations between 10 and 200 μg/plate, and gingerol and shogaol were mutagenic in strains TA100 and TA1538 with metabolic activation by rat liver S9 mix, while zingerone did not display mutagenic effects (Nagabhushan et al. 1987).
57. Abudayyak et al. (2015) found an aqueous ginger extract exhibited mutagenic activity when assessed using the Ames assay on S. typhimurium TA98 (in the presence of S9 mix) strain over a concentration range of 0.78–25 ug/mL however, no activity was exhibited on TA100 strain. No activity was observed with the chloroform and methanolic extracts.
58. Based on the available data, ginger showed some mutagenicity in TA100, TA1535, and TA98 strains, but this is low compared with established mutagens. An aqueous extract of ginger was not shown to be mutagenic in vivo (Nirmala, Prasanna Krishna and Polasa, 2007).
Acute toxicity
59. An acute toxicity study (Malik and Sharma, 2011) in male Wistar rats showed no signs of toxicity or mortality. The animals were administered doses of 250, 500 and 1000 mg/kg lyophilised ginger powder by gastric gavage. The authors stated that the three dose levels used in the study corresponded to 5, 10 and 20% of the NOAEL of the powder (5000 mg/kg).
Short term repeat dose studies
60. Rong et al. (2009) evaluated the safety of powdered Japanese ginger (mainly containing 6-gingerol galanolactone and 6-shogaol) by conducting a 35-day toxicity study in rats. Both male and female rats were treated with 500, 1000 and 2000 mg/kg bw/day by gavage. The results demonstrated that oral administration of up to 2000 mg/kg to male and female rats did not result in any increase in mortality, or changes to behaviour, growth, the general condition of the animals (including: changes in skin, fur, eyes, and mucous membranes, occurrence of secretions, excretions and autonomic activity), food and water consumption. At the highest dose tested (2000 mg/kg), ginger led to slightly reduced absolute and relative weights of testes (by 14.4% and 11.5%, respectively). No effects were apparent in the females.
61. The effect of oral and intraperitoneal administration of aqueous extracts of ginger root over 28 days in female rats at two dose levels (50 mg/kg and 500 mg/kg) was examined for haematological, serum and systemic toxicity (Alnaqeeb et al. 2003). Neither oral nor intraperitoneal administration resulted in mortality. Orally administered aqueous ginger extract resulted in increased levels of serum aspartate aminotransferase (AST) and decreased levels of alanine aminotransferase (ALT).
62. Jeena et al. (2011) conducted a sub chronic toxicity study of the essential oil of ginger in Wistar rats following oral administration at concentrations of 100, 250, and 500 mg/kg per day once daily for 13 consecutive weeks to assess the oral safety of ginger oil. No mortality was observed. No unusual changes in behaviour or locomotor activity were observed during the period of the study, nor were any abnormal changes observed in the relative organ weights of liver, kidney, spleen, lungs, brain, and stomach with respect to body weight in ginger oil-treated animals when compared to vehicle control animals.
63. An increase in serum sodium levels was observed in male rats treated with 500 mg/kg per day but in the absence of changes in sodium levels in females, this change was not considered significant. A slight increase in total bilirubin was observed in female rats treated with ginger oil along with a decrease in AST and ALT levels however, there were no significant changes in hepatic function parameters such as alkaline phosphatase, total protein, albumin, and globulin content.
Reproductive and developmental toxicity
In vitro studies
64. Mohammed et al investigated the effects of herbal extracts, including ginger and 6-gingerol, on chick embryonic heart micromass and mouse D3 embryonic stem cell systems (ESD3) (2016). The team observed that a different study had concluded that the use of 6-gingerol l remedies in the first trimester of pregnancy may affect foetal development (Park, 2012). However, 6-gingerol-treated primary embryonic chick cardiomyocytes showed no significant changes in contractile and cellular activity or changes in total protein content in comparison to the control. At concentrations of 0.75–6 µM, 6-gingerol treated primary embryonic chick cardiomyocytes exhibited no significant changes in contractile activity, cellular activity or changes in total protein content in comparison to the control. At concentrations of 12.5–50 µM, inhibition in contractile activity was observed at 48h. All high 6-gingerol concentrations, 12.5–100 µM, tested in micromass, significantly altered both the cellular activity and protein content in a dose-dependent manner.
65. The same concentrations of 6-gingerol were used to treat the ESD3, which showed a significant decrease in cardiomyocyte differentiation for all tested concentrations above 0.75 µM. The cellular activity and protein content of stem cell-derived cardiomyocytes also exhibited a significant decrease with increased 6-gingerol concentration exposure.
Animal studies
66. To date, the number of studies on the safety of the use of ginger supplements during pregnancy is limited. The ginger component 6-gingerol, was highlighted to affect some essential embryonic developmental processes, such as the disruption of angiogenesis. Kim et al, demonstrated the ability of 6-gingerol to inhibit proliferation and tube formation of primary cultured human endothelial cells in rat aorta by down regulation of cyclidin D and the ability to inhibit tumour growth in mice through its anti-angiogenic activity (Kim et al., 2005).
67. The teratogenicity of EV.EXT 33, a patented Zingiber officinale extract (comprising 6-gingerol, 8-gingerol, 10-gingerol, 6-shogaol, and 8-shogaol, which made up 1.9 w/w of the extract) was investigated in Wistar rats, (Weidner & Sigwart, 2001). The extracts were administered orally by gastric intubation at concentrations of 100, 333 and 1000 mg/kg, to three groups of pregnant rats from days 6 to 15 of gestation. Their bodyweight, food and water were monitored during the treatment period. The study concluded that treatment with EV.EXT 33 during the period of organogenesis resulted in neither maternal nor developmental toxicity at daily doses of up to 1000 mg/kg bw.
68. Shalaby and Hamowieh, (2010) investigated fertility, serum testosterone and acute toxicity of ginger in rats. One hundred and twenty male Sprague Dawley rats, separated into groups of 10, were orally administered either water or methanolic extracts (prepared using 100 g dry ginger roots soaked in 500 ml water or 500 ml methyl alcohol 90%) in graded doses ranging from 5 to 17.5 g/kg bw (gavage doses were not specified). Following dosing, the number of dead mice in each group after 48 hours of observation were recorded. The oral lethal doses (LD50) of the methanolic and water extracts were calculated to be 10.3 and 11.8 g/kg bw respectively. No signs of toxicity were observed at does up to 5 g/kg bw. Both extracts increased fertility index, sexual organ weight, and sperm motility and count after 65 consecutive days (see below).
69. To investigate the effect of ginger extracts on serum testosterone levels, male rats had their fertility reduced by inducing diabetes, a condition shown to reduce male fertility. The aim was to see whether ginger, with its antioxidant and androgenic effects, would restore fertility. Rats rendered diabetic by subcutaneous injection of 120 mg/kg bw alloxan for 3 days, were administered methanolic extracts of ginger for 65 days at doses of 100 and 200 mg/kg bw/d. Testosterone levels increased to 4.08 ± 0.10 and 7.13 ± 0.14 ng/dL (both significant at P < 0.001) compared to the diabetic control group which had levels of 3.30 ± 0.03 ng/dL. Serum testosterone levels also increased in rats given water extracts (150 and 300 mg/kg bw) to 4.06 ± 0.03 and 5.04 ± 0.08 ng/dL (both significant at P < 0.001 when compared to the diabetic control group) respectively.
70. The study also investigated fertility based on the fertility index (for each male this was calculated as the percentage of the number of females that become pregnant in relation to the number of mated females) and spermatogenesis. Rats were orally administered methanolic extracts at doses of 100 and 200 mg/kg bw for 65 consecutive days and water extracts at doses of 150 and 300 mg/kg bw and compared to a diabetic control group.
71. Histopathological examination of the testes of diabetic rats showed mild to moderate degenerative changes of spermatogenic cells, diffuse oedema and incomplete arrest of spermatogenesis. The testes of rats orally administered 300 mg/kg bw of water extract of ginger root showed mild degeneration of spermatogenic cells and slight oedema of interstitial cells. The testes of rats receiving orally 200 mg/kg bw of methanolic extract of ginger root showed nearly normal seminiferous tubules, showing fewer signs of degradation, suggesting a LOAEL of 200 mg/kg bw/day for the methanolic extract. The study concluded that the results suggest the intake of ginger root extract as a drink may be useful for diabetic patients suffering from sexual impotency.
72. The above study has been included for completeness and as any general mechanisms may be more widely relevant: This is consistent with the findings of Hosseini et al (2015)
73. Hosseini et al. (2015, abstract only) investigated the effect of ethanolic ginger extract on serum testosterone, LH and FSH as well an effect on spermatogenic cell lines in male mature offspring rats. In this study, 72 female rats, sorted into 9 groups were orally administered an alcoholic extract of ginger at doses of 50, 100 and 200 mg/kg bw, during their neonatal and perinatal periods and saline was used as a control. Following puberty, LH, FSH, numbers of Sertoli cells, spermatogonia, spermatocytes and spermatids were counted in 8 male rat offspring from each group. Ginger was found to significantly increase testosterone levels and the number of spermatogenic cells and at doses of 100 and 200 mg/kg bw, alcoholic extract of ginger significantly reduced the FSH and LH levels compared to control groups. The authors concluded that “the oral consumption of Ginger during pregnancy and lactation dose-dependently increase the level of testosterone and the number of spermatogenic cells.”
74. Dissabandara & Chandrasekara (2007) also examined the effect of powdered ginger extract administered prenatally on the postnatal development of rats. A period of administration of the dry powdered extract orally at doses of 500 mg/kg/day or 1000 mg/kg/day (control not specified) during days 5 to 15 of gestation resulted in a lower intake of food and water and lower weight gain in dams in the ginger treated group, with some embryonic loss. Growth and physical maturation of the offspring were unaffected. It was concluded that maternal administration of ginger during mid pregnancy resulted in reduced maternal weight gain and increased embryonic loss without affecting the surviving offspring.
75. ElMazoudy and Attia (2018) investigated the effect of powdered dried ginger root on the oestrus cycle and implantation in female mice. ICR mice, were orally dosed at 250, 500, 1000, or 2000 mg/kg bw/d aqueous ginger extract. These were investigated in four different experiments: (i) treatment for 90 days and throughout mating and gestation; (ii) 35-days of treatment evaluating the effects on the oestrous cycle; (iii) treatment for 20 days and throughout mating to evaluate pre-implantation loss (antifertility); and (iv) treatment for 20 days and throughout gestation to evaluate post-implantation loss (abortifacient). In the 90-day study, the dams were terminated on gestation day 20. In the mothers one mortality was recorded in the 1000 mg/kg bw/d group on gestation day 18 and two in the 2000 mg/kg bw/d group at gestational day 16. There was a significant reduction in body weight changes in these two dose groups compared to the control group; however, food consumption was comparable.
76. In the study investigating the oestrus cycle, a significant reduction in the numbers of oestrus cycles was observed at the highest dose, with the length of the oestrus cycle in this group being significantly prolonged (10.05 ± 0.8) days compared with (4.99 ± 0.5) days recurrent and successive oestrous cycles in control mice. At the highest dose level, the length of the oestrous cycle was prolonged with a significant decrease in the duration of diestrous-metestrus (luteal) phase and prolonged proestrus-estrus (ovulatory) phase. In the study investigating pre-implantation loss, a significant decrease in the number of corpora lutea was observed at the highest dose. Implantation failure was also increased by 36% compared to the control group and pre-implantation loss at this dose group was also 16.6% higher than the control group. The authors considered that this may reflect a dose-dependent antifertility (anti-implantation) effect.
77. Regarding fertility and developmental outcomes, the female copulation index was significantly reduced at 2000 and 1000 mg/kg bw/d, whereas the female pregnancy index was significantly decreased only at the highest dose. The number of implantation sites and live fetuses in the 2000 mg/kg bw/d group was lower than the other treated and control groups. An increase in fetal resorption and post implantation loss was also seen in the highest dose group. There was no evidence of fetal malformations however growth retardation, reduced pup weight and delay in the crown-rump length was observed in this dose group as well. Finally, changes in ovarian histopathology were observed at 2000 mg/kg bw/d, following 90 days of treatment. Ovarian follicle atresia was observed. The atretic follicles contained cell debris and there was haemorrhage in the antral cavity.
78. Additionally, degenerated primordial follicles with pyknotic nuclei forming polycystic ovaries were noted. Deteriorated follicles were observed as a detaching of layers of granulosa cells from the basal membrane by dilation of zona pellucida and with evidence of apoptosis; non-visibility of the follicular nuclei was also evident in damaged ova. The authors considered the above observations as evidence that ginger possesses anti-ovulation properties. Overall, the authors concluded that ginger impairs the normal growth of the corpus luteum because of progesterone insufficiency during early pregnancy and that the results suggested that ginger can disrupt the oestrous cycle and blastocyst implantation without teratogenesis. They considered the lowest NOAEL to be 250 mg/kg bw.
79. When evaluating the effect of the aqueous extract of Ginger rhizomes on the sexual parameters of rats. Peneme et al. (2023) initially determined the acute toxicity of the aqueous extract of ginger rhizomes in accordance with OECD guideline no. 423. Rats were given 5000 mg/kg aqueous ginger extract by gavage. No change in the general condition or behaviour of the mice compared with the control batch was observed. No animal mortality was observed after 48 hours or 14 days of observation. This experiment was followed by administering aqueous ginger extract at doses of 300 and 600 mg/kg, 17β-oestradiol at a dose of 1 mg/kg or distilled water, orally to rats for 14 days. A non-significant increase and decrease in body weight was observed at doses of 300 and 600 mg/kg respectively. The authors state that rats treated with 17 β-oestradiol also showed a reduction in body weight, as with the ginger extract at 600 mg/kg, and they considered this to confirm an oestrogenic effect. The eosinophil indices for the 600 mg/kg group were similar to the 17β-oestradiol indicating disruption of the oestrus cycle. A significant increase in oestradiol levels was observed in the rats treated at 300 mg/kg, and a non-significant decrease at 600 mg/kg compared with the control batch. The rat batch treated with the reference molecule 17 β-oestradiol at 1 mg/kg also showed a drop in oestradiol levels.
80. The Committee considered the animal studies to be inconclusive.
81. ElMazoudy and Attia (2018) noted reductions in bodyweight and deaths in mice dosed up to 2000 mg/kg bw/day ginger extract and Alnaqeeb et al., (2003), observed increases in serum aspartate aminotransferase (AST) in female rats dosed up to 500 mg/kg ginger extract.
82. However, the Committee noted that the database was limited, and the extraction and concentration of ginger varied between the studies. On the basis of the available information, more data would be needed in order to allow for a robust investigation of the effects described above. Therefore, at present, the Committee were unable to determine a point of departure, to reach a conclusion.
Human studies - exposures in pregnancy
83. Willetts et al. examined the effect of ginger on pregnancy induced nausea (2003). 120 women less than 20 weeks pregnant, were given 125 mg ginger extract (EV.EXT35; equivalent to 1.5 g of dried ginger) or a placebo four times per day for 4 days. However, there is some lack of clarity in the description of this study as it is stated in the discussion "Women in the treatment arm of this trial took ginger for 8 days and those in the placebo arm took ginger for 4 days." It is not clear whether this refers to the trial described. Three spontaneous abortions were observed in the group receiving ginger, although one of these had not started taking ginger. One spontaneous abortion was observed in the placebo group.
84. In an observational study conducted by Laekman et al. (2021) 51 pregnant women could freely use ginger tablets with a maximum of 2 tablets of 50 mg EXT.GR10 a day in case of gastrointestinal discomfort during pregnancy. EXT.GR10 is a 10-times concentrated ethanolic extract of ginger root. No strict minimum number of tablets was set, and 44 out of 51 patients (86.3%) took the ginger tablets. The 44 patients took 544 tablets or a mean of 12.4 tablets per patient, with a minimum of 1 and a maximum of 55 tablets. Stillbirth, prematurity, hypertension, and gestational diabetes were reported. There were no serious complications at birth. Four cases of dysplasia of the hip and two minor malformations were recorded in the offspring. Outcomes were compared to the rate in a Flemish population delivering during the same period. Hypertension, low birth weight and premature delivery were 15.9%, 13.6% and 20.5 % respectively in the ginger cohort compared to the representative population where the rates were 5.4%, 5.6% and 5.8% respectively. The author states that there was no relationship between the events affecting the mother and child and the number of EXT.GR10 tablets taken.
Effect on P450 (CYP) Enzymes and Herb-Drug Interactions
85. CYPs are a family of enzymes responsible for the biotransformation of several drugs. Induction or inhibition of CYP enzymes is a major determinant of the occurrence of drug-drug interactions.
In silico
86. Qiu et al. (2015) estimated the molecular interactions between 12 main active components (6-gingerol, 8-gingerol, 10-gingerol, 6-shogaol, 8-shogaol, 10-shogaol, ar-curcumene, β-bisabolene, β-sesquiphelandrene, 6-gingerdione, (-)-zingiberene, and methyl-6-isogingerol) and human P450 (CYP) 1A2, 2C9, 2C19, 2D6, and 3A4 and attempted to predict the absorption, distribution, metabolism, excretion, and toxicity (ADMET) of the 12 ginger components using computational methods and literature searches. This study suggests that ginger components may regulate the activity and expression of various human CYPs, resulting in alterations in drug clearance and response with a high risk of inhibition of CYP2C9 and CYP3A4.
In vitro studies
87. Ginger extracts and the major components thereof - 6-gingerol (6G), 8-gingerol (8G), 10-gingerol (10G) and 6-shogaol (6S) - were investigated in in vitro models and shown to have an inhibitory effect on CYP enzymes CYP3A4, CYP2C9 (Kimura et al., 2010), CYP2C19 (Kim et al., 2012), and CYP1A2 and CYP2C8 with IC50 values as low 1µM, (e.g., 6-shogaol on CYP1A2; Mukkavilli et al., 2014).
Animal studies
88. Several reports have been published on the pharmacological properties of ginger, with varying results. Studies have examined the herb-drug interaction in animal models, (Okonta et al., 2008; Egashira et al., 2012) although some studies have questionable results.
89. A study into the effect of ginger on the pharmacokinetics of metronidazole was reported by Okonta et al., using rabbits (2008). In a two-phase study, five healthy local strain rabbits (3 females, two males) were each given 3 mg/kg oral metronidazole. Following a 2-week washout period, the rabbits were given 1 ml/kg of ginger extract orally daily for 3 days and immediately given 3 mg/kg metronidazole per oral on the third day. Ginger significantly increased the absorption and plasma half-life and significantly decreased the elimination rate constant and clearance of metronidazole.
90. Egashira et al., reported the interaction between ginger juice and tacrolimus in rats (2012). Tacrolimus (0.6 mg/kg) was administered intraduodenally in male Sprague-Dawley rats 1 h following oral administration of 10 mL/kg 50% ginger juice or water. CYP3A enzymes metabolize tacrolimus in the intestine as well as in the liver and the author states that ginger has been reported to change the activity of CYP3A4. Pre-treatment with ginger juice was found to significantly increase tacrolimus blood concentrations compared to those in animals pre-treated with water or orange juice.
91. The possible herb-drug interaction of ginger crude extract (GCE) on glibenclamide and insulin was investigated by Al Omari et al., along with its hypoglycaemic and antihyperglycemic effects in normoglycemic- and streptozotocin-induced (STZ) diabetic rats (2012). Ginger crude extract was administered to normoglycemic male rats as a single dose (1 day) and as a daily dose for 1 week. STZ diabetic rats were treated with the same GCE concentrations (25, 50 and 100 mg/kg bw) together with glibenclamide (5 mg/kg bw) or insulin (1.2 IU/kg bw).
92. Single administration of ginger crude extract resulted in a significant decrease in blood glucose level (BGL) in normoglycemic rats after 1 and 2 hours (50 mg/kg bw). In STZ- diabetic rats ginger crude extract (25 and 50 mg/kg bw) decreased non-fasting BGL (N-FBGL) significantly at 1.5, 2.5, 3.5 and 4.5 hours. Glibenclamide (5 mg/kg bw) in combination with ginger crude extract at doses 25 or 50 mg/kg bw resulted in a significant reduction in the N-FBGL by 26.3% and 25.1% respectively after 4.5 hours, compared to glibenclamide alone which exhibited a 7.9% reduction.
Human studies
93. Human data showed possible interactions with medicines, including antibiotics, immunosuppressants, and anticoagulant medications. Although, in some cases, multiple concomitant medications were being used therefore, the effects observed cannot necessarily be directly attributed to ginger supplementation (Rubin et al., 2019).
94. Conversely, whilst investigating the effects of ginger on the pharmacokinetics or pharmacodynamics of warfarin and the effect of ginger on clotting status, Jiang et al., (2005), found that neither the pharmacokinetics nor pharmacodynamics of warfarin were affected in healthy males who were treated with a single 25 mg dose of warfarin, following 7 days of pretreatment with ginger tablets (3 tablets, 3 times per day, each capsule containing extract equivalent to 0.4 g of ginger rhizome powder). Furthermore, ginger had no effect on international normalized ratio (INR) or ex vivo platelet aggregation in response to arachidonic acid.
Anti-platelet aggregation activity
In vitro studies
95. Srivastava (1986) reported an effect of ginger extracts on in vitro platelet aggregation. Ginger extracts in water, n-hexane, chloroform, and ethyl acetate were shown to inhibit platelet aggregation using arachidonic acid (AA), epinephrine, adenosine diphosphate (ADP), and collagen as agonists.
Animal studies
96. A study by ElMazoudy and Attia (2018) linked follicular failure to haemorrhagic effects in a study investigating the effect of aqueous ginger extract on the oestrus cycle and implantation, in female mice. The authors concluded that ginger impairs the normal growth of the corpus luteum and that the results suggested that ginger can disrupt the oestrous cycle and blastocyst implantation without teratogenesis. They considered the lowest NOAEL to be 250 mg/kg bw. The COT noted that this might be worth further investigation. However, it was also noted that other factors could be contributing to the results observed and the study results were inconclusive.
97. The effect of an aqueous ginger extract on platelet thromboxane-B2 (TXB2) and prostaglandin-E2 (PGE2) production was studied by Thomson et al. (2002). Adult female Sprague-Dawley rats were administered an aqueous extract of raw ginger at either 50 mg/kg or 500 mg/kg daily, by either oral gavage or intraperitoneally (IP) for a period of 4 weeks. A dose of 50 mg/kg ginger administered orally, or IP did not result in any significant reduction in serum TXB2 levels when compared to saline-treated control groups but doses at 500 mg/kg significantly reduced TXB2 levels in serum.
98. A non-significant reduction in the level of TXB2 was observed when ginger was injected IP. However, levels were not significantly different from the TXB2 levels in control rats that had received saline. 50 mg/kg of ginger administered orally resulted in serum PGE2 levels being significantly reduced and 500 mg/kg was found to be more effective in reducing PGE2 synthesis. PGE2 levels were reported to be significantly lower than the saline control in rats given 500 mg/kg ginger extract both orally and IP.
Human studies
99. Rubin et al. (2019) reported the possible effect of ginger supplementation on the (INR) in a woman taking warfarin. The 70-year-old female, who had been taking clonazepam 1 mg, metoprolol succinate 25 mg, paroxetine 10 mg, phenytoin 30 mg, rosuvastatin 20 mg, warfarin 7.5 mg daily, and warfarin 10 mg once day per week, presented with an INR of 8, an increase from 2.7, one month after taking a “Ginger Rescue,” a daily oral, chewable, 48 mg ginger supplement that had no other herbal or active ingredients. A week following cessation of the ginger supplement, the INR declined to 2.6.
100. Ginger, in powder form (5 g per day), was demonstrated to significantly (P<0.001) decrease ADP- and epinephrine-induced platelet aggregation in healthy male subjects who were fed 100 g of butter daily for seven days (Verma et al., 1993). Conversely, Bordia et al., (1997) found that 4 g powdered ginger administered daily over the course of 1.5 and 3 months had no effect on ADP and epinephrine-induced platelet aggregation in individuals with coronary artery disease (CAD). However, a single 10g dose of powdered ginger, administered to CAD patients resulted in a significant decrease in induced platelet aggregation.
Effects on blood pressure
Animal studies
101. Ghayur and Gilani (2005) reported that a crude extract of ginger administered intravenously, induced a dose-dependent (0.3–3 mg/kg) decrease in arterial blood pressure of anesthetized Sprague-Dawley rats with an EC50 value of 0.9 ± 0.1 mg/kg (mean ± SEM). In guinea pig paired atria, the crude extract exhibited cardio-depressant activity on the rate and force of spontaneous contractions with EC50 values of 0.57 ± 0.03 and 0.88 ± 0.07 mg/ml (mean ± SEM) for force and rate of contraction, respectively. In rabbit thoracic aorta preparation, when tested on the resting baseline, the ginger extract was devoid of any effect up to the dose of 10 mg/mL. The extract was then tested on high-K+ (80 mM) and phenylephrine (1 μM)-induced contractions. The extract relaxed the phenylephrine-induced vascular contraction at a dose 10 times higher than that required against K+ (80 mM)-induced contraction with an EC50 of 0.92 ± 0.04 mg/ml, compared with an EC50 of 0.11 ± 0.01 mg/ml against K+-induced contraction.
102. Ca2+ channel-blocking (CCB) activity was confirmed when the crude extract shifted the Ca2+ dose–response curves to the right, the shift being similar to that obtained with verapamil. It also inhibited the phenylephrine (1 mM) control peaks in normal-Ca2+ and Ca2+-free solution, indicating that it acts at both the membrane-bound and the intracellular Ca2+ channels. When tested in endothelium-intact rat aorta, it again relaxed the K+-induced contraction (EC50 value of 0.091 ± 0.002 mg/ml) at a dose 14 times less than that required for relaxing the PE-induced contraction (EC50 value of 1.26 ± 0.08 mg/ml). The vasodilator effect of the crude extract was endothelium-independent because it was not blocked by Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME) (0.1 mM) or atropine (1 mM) and also was reproduced in endothelium-denuded preparations at the same dose range. These data indicate that the blood pressure-lowering effect of ginger is mediated through blockade of voltage-dependent calcium channels.
Effect on Prostaglandins
In vitro
103. Ginger extracts, along with many gingerols and shogaols have been shown to suppress prostaglandin synthesis in vitro, through inhibition of cyclooxygenase (Jolad et al. 2005; Pan et al. 2008; Dugasani et al. 2010).
104. Lantz et al. (2007) investigated the anti-inflammatory effect of ginger extracts and the principal components thereof (6-, 8- 10-gingerols and 6-, 8-, 10-shogaols) in an in vitro model, U937 cells, differentiated and exposed to lipopolysaccharide (LPS) from Escherichia coli (1 µg/ml). Extracts containing predominantly gingerols were found not to be cytotoxic, while shogaols were found to be cytotoxic at concentrations above 20 µg/ml. Crude extracts of ginger inhibited LPS-induced PGE2 (IC50 < 0.1 µg/ml) production but were much less effective at inhibiting TNF-α (IC50> 30 µg/ml). Extracts containing either predominantly gingerols or shogaols were highly active at inhibiting LPS-induced PGE2 production (IC50 < 0.1 µg/ml). Extracts containing predominantly gingerols inhibited LPS-induced COX-2 expression while shogaol containing extracts had no effect on COX-2 expression.
105. Jolad et al. also demonstrated the inhibitory effect of gingerols on LPS-induced PGE2 production in HL-60 cells stimulated with 1 µg/ml of LPS (2004). None of the compounds tested were shown to be cytotoxic.
Animal studies
106. The Committee noted the potential effect of ginger on the prostaglandin pathway, in particular Cyclooxygenase-1 (COX1) and Cyclooxygenase-2 (COX2) inhibition and how this may affect early pregnancy. One study examining the effects of ginger extracts on prostaglandin E2 (PGE2) production in vitro (Lantz et al. 2007) demonstrated that crude organic extracts (dichloromethane-methanol, 1:1 v/v) of ginger were capable of inhibiting PGE2 production and that the compounds may act at several sites. The most potent effect on lipopolysaccharide (LPS) induced prostaglandin production was noted at less than 0.1 µg/ml. It was noted that the half maximal inhibitory concentration (IC50) values for a range of components were given, and it was demonstrated that the components mainly acted on COX-2. The COT concluded further studies would be needed to determine the role of decreased prostaglandin levels in the early termination of pregnancy.
107. The composition of ginger extracts also appears to vary according to whether ginger is fresh or dried. Suekawa et al., (1986, abstract only) demonstrated that (6)-shogaol, a principal component mainly found in dried ginger, inhibited carrageenan-induced swelling of rat hind paw, AA-induced platelet aggregation in rabbit and prostaglandin PGI2 release in rat aorta, suggesting a potential inhibitory action on cyclooxygenases (COX) in both platelets and aorta tissue.
Effect on animals with induced diabetes
108. Luo et al. (2022) determined the effects of ginger on gestational diabetes in rats. In this study, 40 adult female rats were divided into 4 equal groups: pregnant rats, pregnant rats with diabetes, pregnant rats consuming ginger powder (100 mg/kg, by gavage), and pregnant rats with diabetes consuming ginger powder. The results of this study showed that one of the mechanisms of physiological metabolic adaptations during pregnancy is a change in the expression of mTORc1, SREBP-1c, PPAR-α, and PPAR-g genes. Disruption of their expression can lead to metabolic disorders and hyperglycaemia and even in advanced cases cause gestational diabetes. However, the results in the groups receiving ginger showed that ginger can significantly improve the metabolic status by modulating the expression of these genes. There were no reported adverse effects resulting from the administration of ginger when compared to the control.
109. Streptozotocin induced rats were utilized as a diabetic model and received 200 or 400 mg/kg/day ginger extract for eight weeks. (Raoufi et al., 2023) Ginger at both levels ameliorated the levels of glucose, testosterone, and MDA. At the higher dose group elevated the levels of insulin, 17β-oestradiol, and progesterone were seen.
Contaminants
In this guide
In this guide110. Differences in cultivation conditions and extraction methods could lead to possible sources of contamination from toxins, microbes, pesticides, heavy metals and residual solvents. Studies investigating contamination in ginger are limited, however of the few studies available, the main sources of contamination reported are heavy metals (Wagesho & Chandravanshi, 2015; Goroya et al., 2019, Kilic & Soylak, 2019; Xu et al., 2020) and mycotoxins (Ałtyn and Twarużek, 2020; Wen et al., 2014; Omotayo et al., 2019; Lippolis et al., 2017).
111. Ginger can be exposed to mycotoxin contamination during harvesting, storage and handling. Whilst information on mycotoxin contamination in ginger is limited, ginger has been demonstrated to be particularly exposed to aflatoxins and ochratoxin A (OTA). This is reflected in GB legislation where maximum levels for these toxins for spices including ginger are established in Assimilated EU Law 1881/2006. Maximum levels are 5 µg/kg for aflatoxin B1 (AFB1), 10 µg/kg for all aflatoxins (sum of AFB1, AFB2, AFG1, and AFG2) and 15 µg/kg for OTA, for ginger and its products.
112. A study evaluating the heavy metal content of ginger from turkey found that the permissible limit values in edible plants determined by FAO/WHO were exceeded for Fe, Zn, Cd, Pb and Cu (Karagözoğlu, 2023).
113. The Committee discussed the potential presence of contaminants in ginger and noted that the ginger products used in the studies reported were sourced locally in markets or herbalists (Wagesho & Chandravanshi, 2015; Goroya et al., 2019). Members queried whether there were any specific data on contaminants in ginger supplements available in the UK.
114. The Committee noted it was unknown how much ginger and particularly, highly concentrated juice extracts, would contribute to overall contaminant exposure in the UK.
Exposure
In this guide
In this guide115. TOX/2021/26 discussed exposure to ginger via the diet and in supplement form. TOX/2020/51 examined in more detail exposure to ginger in the form of highly concentrated juices (‘shots’). This statement reviews ginger from all sources described previously.
116. A number of ginger supplements (Tables 1 and 2, Annex C) are purported to support digestive and joint health, alleviate nausea, upset stomach, and travel sickness. Currently, a number of commercially available pregnancy supplements, including ‘Seven Seas Pregnancy’ and ‘Seven Seas Pregnancy Plus Follow On’, contain ginger extracts in their formulations.
117. The availability of supplements in different forms, along with a lack of information with regards to extraction processes involved and therefore composition of the extracts, meant it was not possible to consider aggregate exposures. As such, ginger exposure from the diet and from supplements were separately considered.
118. In addition to supplements, pregnant women may also consume ginger as part of their general diet to various degrees. There are anecdotal reports of women consuming ginger products (Tables 3, Annex C) such as ginger biscuits and ginger ale, to alleviate morning sickness and nausea. Some may use these in combination with juice shots or tinctures (Table 4, Annex C).
119. Table 1 shows calculated exposures from the diet, supplements and drinks (including teas and shots). Mean acute ginger exposure from the diet of women aged 16-49 years old was 0.026 g/kg bw/day, and 97.5th percentile exposure was 0.16 g/kg bw/day. The corresponding mean and 97.5th percentile chronic exposures were 0.0083 and 0.058 g/kg bw/day, respectively. The upper value of the range of exposure from drinks and supplements was more than double (%) that of those estimated from 97.5th percentile acute exposure from the diet.
Table 1: Estimated mean and 97.5th percentile acute and chronic ginger exposures from a variety of sources in women aged 16 – 49 years old.
|
Commodity |
Range of daily exposures (g/day) |
Range of daily exposures (g/kg bw/day) |
Mean acute exposure* (g/day) |
Mean acute exposure* (g/kg bw/day) |
97.5th percentile acute exposure* (g/day) |
97.5th percentile acute exposure* (g/kg bw/day) |
Mean chronic exposure* (g/day) |
Mean chronic exposure* (g/kg bw/day) |
97.5th percentile chronic exposure* (g/day) |
97.5th percentile chronic exposure* g/kg bw/day |
|
Fooda |
NA |
NA |
1.7 |
0.026 |
11 |
0.16 |
0.55 |
0.0083 |
3.4 |
0.058 |
|
Drinks (Including tea and shots) b1,b |
0.5 - 32.5 |
0.0071 - 0.46 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
|
Supplements c |
0.010 - 24 |
0.00014 - 0.34 |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
NA |
1This assumes only one serving is consumed per day.
a Data obtained from the National Diet and Nutrition surveys years 1-8 calculated from women of a childbearing age (16-49 years) (Bates et al., 2014; 2016; Roberts et al., 2018).
b Data obtained online from retailers, see Appendix 1 for further details.
c Data obtained online from retailers, see Appendix 1 for further details.
*Rounded to 2 significant figures.
120. As previously mentioned, 1 - 1.5 g per day of ginger may be advised during pregnancy (NHS, 2022, Healthline, 2020; Mother and baby, 2022). Some highly concentrated ginger shots commercially available contain up to 30 g of fresh ginger per serving, over 30 times that recommended by healthcare professionals.
121. As the NDNS does not provide data for pregnant women, there was uncertainty as to whether the data presented an accurate reflection of consumption during pregnancy. This uncertainty also extended to data presented for drinks and supplements, as the pattern of consumption during pregnancy to alleviate symptoms of sickness is unknown.
Toxicology conclusions
Reproductive and developmental toxicity
122. The COT considered a number of epidemiological studies investigating the use of ginger during pregnancy (TOX/2021/26). For the most part, few studies explicitly addressed the safety of ginger consumption during pregnancy. Most were focused on the use of ginger as a treatment for nausea (Fischer-Rasmussen et al., 1990; Smith et al., 2004; Ensiyeh et al., 2009), age-related neurological disorders or pregnancy-induced sickness and therefore focused on efficacy (Willetts et al., 2003; Stanisiere et al., 2018). However, safety was considered in a few studies. The studies considered by the Committee included observational and randomised clinical studies, lasting from 4 days to 20 weeks in duration (Vutyavanich et al., 2001; Portnoi et al., 2003). Ginger in various forms was investigated in doses ranging from 750 mg/day to the equivalent of 7 g/day.
123. The animal studies on reproductive toxicity considered in TOX/2021/26 reported a number of findings, including reduced maternal weight gain, increased foetus weight, increased serum testosterone level in F1 generation males and an increase in embryonic loss.
124. The study results in pregnant women were also varied and the overall findings inconclusive. Findings reported included abdominal discomfort, vomiting and diarrhoea. There were reports of incidences of spontaneous abortion (Portnoi et al., 2003, Ensiyeh et al., 2009), however, this effect was observed in both the treated and control groups and therefore, cannot directly be attributed to the consumption of ginger. Portnoi et al., reported 8 spontaneous abortions in the comparator group, compared to 3 occurring in the group taking ginger and Ensiyeh et al., reported 2 spontaneous abortions in the ginger group compared to 1 in the group taking vitamin B6. This study reported no congenital abnormalitiespost-partum following exposure to ginger.
125. In their 2003 review of interventions for nausea and vomiting in early pregnancy (first trimester), Mathews concluded high-quality consistent evidence is lacking to support advice regarding the safety of ginger during pregnancy (Mathews et al., 2015). However, it was noted that a review by Bryer et al. (2005) concluded that maternal consumption of ginger shows no evidence of teratogenicity in infants. More recently, Stanisiere et al. (2018) conducted a review of the safety and efficacy of ginger rhizome for decreasing nausea and vomiting in women during early pregnancy, based on systematic literature searches until the end of December 2017. The group concluded that the in vivo results do not suggest any major concerns with respect to reproductive and developmental safety of ginger root, as no associations were found between the use of ginger and malformations in humans. However, in vitro results could not be extrapolated to humans and safety could be dependent on ginger quality. The majority of the studies included in this review have already been included in this draft statement. Some recent studies have been conducted evaluating the effectiveness and safety of ginger in pregnancy, and these will be discussed in detail. Overall, most studies reported gastrointestinal effects such as abdominal discomfort, vomiting and diarrhoea. Other effects included dizziness, headaches and drowsiness with some more serious effects such as spontaneous abortion also being reported in 5 out of the 14 randomized clinical studies. The review by Jewell and Young focuses on the reported effects rather than statistical significance, therefore more details on the studies reporting more serious effects are given below.
Anti-platelet aggregation activity
126. Several reports have been published on the pharmacological properties of ginger, with varying results. The potential effect of ginger extract and components thereof on the reduction of platelet aggregation and their potential antithrombotic activity has been noted as a concern in both literature and by health professionals.
127. Ginger was reported to have antiplatelet activity (Srivastava, 1986,1989; Young et al., 2006), with some studies reporting effects in animals at doses of 500 mg/kg bw (Thomson et al., 2002). Ginger was found to inhibit platelet thromboxane and prostaglandin endoperoxcides (PGF2α, PGE2 and PGD2) in human platelets, in a dose-dependent manner (Srivas, 1984).
128. With regards to the relevance of such effects in pregnancy, literature reports note that pregnancy is associated with an increased incidence of thrombotic events; mainly related to a pro-thrombotic state, physiologically useful to reduce bleeding at delivery. These changes are more pronounced in the third trimester (Patti et al., 2014). It has also been hypothesised that antiplatelet agents might prevent or delay the development of pre-eclampsia (Duley et al., 2019). The implications and clinical significance of the anti-platelet activity of ginger exposure during different stages of pregnancy remain undetermined.
129. This further highlighted the need to differentiate exposure from the normal diet to that from supplements. Members noted that associations with haemorrhagic effects were reported following supplemental exposure to ginger, (Kruth et al., 2003; Rubin et al., 2019; Al Askar et al., 2020) though these were inconclusive.
Conclusions of the Committee
In this guide
In this guide130. Ginger is commonly used as a spice and flavouring in many countries worldwide and is increasingly growing in popularity as a natural remedy, due to its purported immune system-boosting properties, for easing motion sickness and post-operative nausea and vomiting, and pregnancy related nausea.
131. Several ginger supplements are commercially available, ranging from dried root in capsule form to tincture form, all with varying amounts of ginger. In addition to this, concentrated ginger shots (liquid form), containing large amounts of pressed ginger, are increasingly becoming popular. The variability in the composition of these supplements adds uncertainty to the amount of ginger actually being consumed.
132. Study authors noted that some of the toxicity observed varied according to the nature of extraction solvent; organic solvent extracts exhibited more toxicity than aqueous extracts, which presumably indicates extraction of differentially toxic compounds. Hence, studies of individual extracts might not give the whole picture.
133. Overall, the Committee concluded that based on the available information it was not possible to determine a point of departure to use in the risk assessment of ginger when used as a supplement.
134. Members noted that although the different ginger extracts were not comparable, there did appear to be some biological activity in the early stages of pregnancy. It was stressed that in general there was no indication of systemic toxicity from the use of ginger in the diet as food.
135. The lack of safety and toxicological information available on ginger use in pregnancy make it difficult to fully characterise the risks in this respect. The committee noted that while there was some equivocal evidence for the possible effect of ginger on reproduction, it was not possible to characterise this based on the data available.
136. Also, consumption data was based on women of childbearing age and therefore may not be representative of the maternal diet, leading to an under/overestimation of the actual exposure.
137. There is no clear indication that ginger is detrimental to pregnant women, although a signal for some adverse effects cannot be ruled out. Generally, normal levels of consumption of ginger within a diet is not considered a health concern. The Committee noted that from the evidence presented, the potential for contamination of ginger with heavy metals and/or mycotoxins cannot be excluded.
COT Secretariat
December 2023
References
In this guide
In this guideAbudayyak, M., Özdemir Nath, E., & Özhan, G. (2015). Toxic potentials of ten herbs commonly used for aphrodisiac effect in Turkey. Turkish journal of medical sciences, 45(3), 496–506. https://doi.org/10.3906/sag-1401-153.
AlAskar, A; Shaheen, NA; Khan, AH; AlGhasham, N; Mendoza, MA; Matar, DB; Gmati, G; AlJeraisy, M; AlSuhaibani, A: (2020). Effect of daily ginger consumption on platelet aggregation. Journal of Herbal Medicine. Volume 20, 100316. https://doi.org/10.1016/j.hermed.2019.100316.
Alnaqeeb MA, Thomson M, Al-Qattan KK, Kamel F, Mustafa T, Ali M. (2003): Biochemical and histopathological toxicity of an aqueous extract of ginger. Kuwait J Sci Eng, 30: 35-48.
Al Omari, I; Afifi, F; Salhab, A. (2012). Therapeutic Effect and Possible Herb Drug Interactions of Ginger (Zingiber officinale Roscoe, Zingiberaceae) Crude Extract with Glibenclamide and Insulin. Pharmacognosy Communications. 2. 12-20. http://dx.doi.org/10.5530/pc.2012.1.4.
Ałtyn, I., & Twarużek, M. (2020). Mycotoxin Contamination Concerns of Herbs and Medicinal Plants. Toxins, 12(3), 182. https://doi.org/10.3390/toxins12030182
The Committee on Toxicity of Chemicals in Food, Consumer Products and the environment (COT) (2020). Scoping Paper on Herbal Supplements Used in Pregnancy.
Belsito, M. D., Cohen, D. E., Klaassen, C. D., Liebler, D. C., Peterson, L. A., Shank, R. C., Snyder, P. W. (2021). Safety Assessment of Zingiber officinale (Ginger)–Derived Ingredients as Used in Cosmetics. Ginger.pdf.
Bryer, E. (2005). A literature review of the effectiveness of ginger in alleviating mild-to-moderate nausea and vomiting of pregnancy. Journal of midwifery & women's health, 50 (1), e1-e3. https://doi.org/10.1016/j.jmwh.2004.08.023.
Chittumma, P., Kaewkiattikun, K., & Wiriyasiriwach, B. (2007). Comparison of the effectiveness of ginger and vitamin B6 for treatment of nausea and vomiting in early pregnancy: a randomized double-blind controlled trial. Journal-medical association of thailand, 90(1), 15. PMID: 14649969.
The Committee on Toxicity of Chemicals in Food, Consumer Products and the environment (COT) (2021). The potential effects that ginger and ginger supplements may have during pregnancy and lactation.
Dietz, B. M., Hajirahimkhan, A., Dunlap, T. L., & Bolton, J. L. (2016). Botanicals and Their Bioactive Phytochemicals for Women's Health. Pharmacological reviews, 68(4), 1026-1073.
DTU Food Institute, (2019). The safety of pregnant women when ingesting ginger shots made from the root from real ginger (Zingiber officinale Roscoe). (Available in Danish only).
Dissabandara DLO, Chandrasekara MS. (2007). Effects of prenatal ginger rhizome extract treatment on pregnancy outcome and postnatal development of Sprague Dawley rats. Ceylon J Med Sci 2007, 50: 1-7. DOI: 10.4038/cjms.v50i1.116 116-1-474-1-10-20081020.pdf.
Egashira, K; Sasaki, H; Higuchi, S; Ieiri, I (2012). Food-drug Interaction of Tacrolimus with Pomelo, Ginger, and Turmeric Juice in Rats, Drug Metabolism and Pharmacokinetics, Vol 27, 2, 242-247. https://doi.org/10.2133/dmpk.DMPK-11-RG-105.
ElMazoudy, Reda & Attia, Azza. (2018). Phytomedicine. 50. 2018, 300-308, Ginger causes subfertility and abortifacient in mice by targeting both estrous cycle and blastocyst implantation without teratogenesis.
Ensiyeh, J.; Sakineh, M.A. (2009). Comparing ginger and vitamin b6 for the treatment of nausea and vomiting in pregnancy: A randomised controlled trial. Midwifery 2009, 25, 649–653. https://doi.org/10.1016/j.midw.2007.10.013.
European Commission (EC) (2023). Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Publications Office (europa.eu).
EMA (European Medicines Agency) (2012): Assessment report on Zingiber Officinale Roscoe, rhizome; EMA/HMPC/577856/2010.
Finnish Food Authority, (2019). General Instructions on Safe Use of Foodstuffs.
Fischer-Rasmussen, W.; Kjaer, S.K.; Dahl, C.; Asping, U. (1991). Ginger treatment of hyperemesis gravidarum. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991, 38, 19–24. https://doi.org/10.1016/0028-2243(91)90202-v
Getaneh, A., Guadie, A., & Tefera, M. (2021). Levels of heavy metals in ginger (Zingiber officinale Roscoe) from selected districts of Central Gondar Zone, Ethiopia and associated health risk. Heliyon, 7(4), e06924. https://doi.org/10.1016/j.heliyon.2021.e06924.
Ghayur, M. N., & Gilani, A. H. (2005). Ginger lowers blood pressure through blockade of voltage-dependent calcium channels. Journal of cardiovascular pharmacology, 45(1), 74-80. DOI: 10.1097/00005344-200501000-00013.
Goroya K, Mitiku Z, Asresahegn Y, (2019). Determination of concentration of heavy metals in ginger using flame atomic absorption spectroscopy. Afr. J. Plant Sci. 13, 163–167. DOI: 10.5897/AJPS2019.1787.
Healthline (2020). Ginger Tea in Pregnancy: Benefits, Safety, and Directions. Ginger Tea in Pregnancy: Benefits, Safety, and Directions.
Jiang, X., Williams, K. M., Liauw, W. S., Ammit, A. J., Roufogalis, B. D., Duke, C. C., Day, R. O., & McLachlan, A. J. (2005). Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. British journal of clinical pharmacology, 59(4), 425–432. https://doi.org/10.1111/j.1365-2125.2005.02322.x.
Karagözoğlu, Y., & Kıran, T. R. (2023). Investigation of Heavy Metal Contents in Thyme (Thymus vulgaris) and Ginger (Zingiber officinale) Sold in Bingöl Herbalists. Middle Black Sea Journal of Health Science, 9(1), 88-97. https://doi.org/10.19127/mbsjohs.1203882
Kilic S, Soylak M (2020). J Food Sci Technol 57, 927–933 (2020). Determination of trace element contaminants in herbal teas using ICP-MS by different sample preparation method.
Kim, IS, Kim, SY, Yoo, HH (2012). Effects of an aqueous-ethanolic extract of ginger on cytochrome P450 enzyme-mediated drug metabolism. Die Pharmazie, 67(12), 1007–1009.
Kimura Y, Ito H, Hatano T (2010). Effects of mace and nutmeg on human cytochrome P450 3A4 and 2C9 activity. Biol Pharm Bull. 2010;33(12):1977-82. doi: 10.1248/bpb.33.1977. PMID: 21139236.
Krüth P, Brosi E, Fux R, Mörike K, Gleiter CH (2004). Ginger-Associated Overanticoagulation by Phenprocoumon. Ann Pharmacother. Feb;38(2):257-60. doi: 10.1345/aph.1D225.
Lantz, R. C., Chen, G. J., Sarihan, M., Sólyom, A. M., Jolad, S. D., & Timmermann, B. N. (2007). The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine: international journal of phytotherapy and phytopharmacology, 14(2-3), 123–128. https://doi.org/10.1016/j.phymed.2006.03.003.
Laekeman, G. M., Van Calsteren, K., Devlieger, R., Sarafanova, E., Van Limbeek, J., & Dierckxsens, Y. (2021). Ginger (Zingiber officinale) root extract during pregnancy: a clinical feasibility study. Planta Medica, 87(10/11), 907-912. DOI: 10.1007/s00228-012-1331-5.
Lippolis V, Irurhe O, Porricelli, ACR, Cortese M, Schena R, Imafidon T, Oluwadun A, Pascale M (2017). Natural co-occurrence of aflatoxins and ochratoxin A in ginger (Zingiber officinale) from Nigeria. Food Control 2017, 73, 1061–1067.
Lumb A. B. (1994). Effect of dried ginger on human platelet function. Thrombosis and haemostasis, 71(1), 110–111. PMID: 8165628.
Luo, L., Zhu, S., Akbari, A., & Tan, B. (2022). Ginger could improve gestational diabetes by targeting genes involved in nutrient metabolism, oxidative stress, inflammation, and the WNT/β-Catenin/GSK3β signaling pathway. Natural Product Communications, 17(12). https://doi.org/10.1177/1934578X221141276.
Matthews, A., Haas, D. M., O'Mathúna, D. P., & Dowswell, T. (2015). Interventions for nausea and vomiting in early pregnancy. Cochrane Database of Systematic Reviews, (9). https://doi.org/10.1002/14651858.CD007575.pub4.
Mohammed, OJ, Latif, ML Pratten, MK(2016). Evaluation of embryotoxicity for major components of herbal extracts using the chick embryonic heart micromass and mouse D3 embryonic stem cell systems. Reproductive Toxicology, Vol 59, 2016, 117-127, ISSN 0890-6238, https://doi.org/10.1016/j.reprotox.2015.12.003 .
Mother and baby (2022) Ginger in pregnancy: Safety, benefits and guidelines.
Mukkavilli, R., Gundala, S. R., Yang, C., Donthamsetty, S., Cantuaria, G., Jadhav, G. R., Vangala, S., Reid, M. D., & Aneja, R. (2014). Modulation of cytochrome P450 metabolism and transport across intestinal epithelial barrier by ginger biophenolics. PloS one, 9(9), e108386. https://doi.org/10.1371/journal.pone.0108386.
Nirmala, K., Prasanna Krishna T. and Polasa, K. (2007). In vivo Antimutagenic Potential of Ginger on Formation and Excretion of Urinary Mutagens in Rats. International Journal of Cancer Research, 3: 134-142. https://doi.org/10.3923/ijcr.2007.134.142.
NICE (2021). Antenatal care. Management of nausea and vomiting in pregnancy. NG201 Evidence review R.
NHS (2021) Vomiting and morning sickness. Vomiting and morning sickness - NHS Accessed: 10/08/2023.
NHS (2021) Women and Health. Nausea and vomiting in pregnancy.
NHS Specialist Pharmacy Service (2022). Herbal medicines: safety during pregnancy. Page not found – SPS - Specialist Pharmacy Service – The first stop for professional medicines advice.
Okonta JM, Uboh M, Obonga WO. (2008). Herb-drug interaction: a case study of effect of ginger on the pharmacokinetic of metronidazole in rabbit. Indian J Pharm Sci. 2008 Mar-Apr;70(2):230-2. doi: 10.4103/0250-474X.41462. PMID: 20046719; PMCID: PMC2792472.
Omotayo, O. P., Omotayo, A. O., Babalola, O. O., & Mwanza, M. (2019). Comparative study of aflatoxin contamination of winter and summer ginger from the North West Province of South Africa. Toxicology reports, 6, 489–495. https://doi.org/10.1016/j.toxrep.2019.05.011.
Park, S. A., Park, I. H., Cho, J. S., Moon, Y. M., Lee, S. H., Kim, T. H., ... & Lee, H. M. (2012). Effect of [6]-gingerol on myofibroblast differentiation in transforming growth factor beta 1–induced nasal polyp–derived fibroblasts. American Journal of Rhinology & Allergy, 26(2), 97-103. https://doi.org/10.2500/ajra.2012.26.3736.
Peneme B.M.L., Akassa, H., Ondélé, R., Lanzah A, B., Backala, A., Etou Ossibi, A. W., and Abena, A. A., (2023). Effects of the Aqueous Extract of the Rhizomes of Zingiber officinale (Ginger) on Sexual Parameters in Female Wistar Rats. European Journal of Medicinal Plants, 34(10), 1-11. https://doi.org/10.9734/ejmp/2023/v34i101161.
Plengsuriyakarn, T.; Viyanant, V.; Eursitthichai, V.; Tesana, S.; Chaijaroenkul, W.; Itharat, A.; Na-Bangchang, K. (2012). Cytotoxicity, Toxicity, and Anticancer Activity of Zingiber Officinale Roscoe Against Cholangiocarcinoma, Asian Pacific Organization for Cancer Prevention, 13(9), pp. 4597–4606. doi: 10.7314/apjcp.2012.13.9.4597.
Portnoi, G., Chng, L. A., Karimi-Tabesh, L., Koren, G., Tan, M. P., & Einarson, A. (2003). Prospective comparative study of the safety and effectiveness of ginger for the treatment of nausea and vomiting in pregnancy. American Journal of Obstetrics and Gynecology, 189(5), 1374–1377. https://doi.org/10.1067/s0002-9378(03)00649-5
Qiu, J. X., Zhou, Z. W., He, Z. X., Zhang, X., Zhou, S. F., & Zhu, S. (2015). Estimation of the binding modes with important human cytochrome P450 enzymes, drug interaction potential, pharmacokinetics, and hepatotoxicity of ginger components using molecular docking, computational, and pharmacokinetic modeling studies. Drug design, development and therapy, 841-866. DOI:10.2147/DDDT.S74669.
Raoufi, M. F., Farahani, T. M., Jadidi, E. S. M. S., & Gardeshi, T. M. (2023). Protective Effects of Ginger Extract on Oxidative Stress and Steroidogenesis-related Genes in The Ovary of Streptozotocin-induced Diabetic Rats. International Journal of Medical Laboratory. https://doi.org/10.18502/ijml.v10i3.13750.
Rubin D, Patel V, Dietrich E. (2019). Effects of Oral Ginger Supplementation on the INR. Case Rep Med. Jun 11; 2019:8784029. doi:10.1155/2019/8784029. PMID: 31281366; PMCID: PMC6594244.
Shalaby, M.A.; Hamowieh, A.R. (2010) Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats, Food and Chemical Toxicology, Vol 48, Issue 10, 2920-2924, https://doi.org/10.1016/j.fct.2010.07.028.
Smith, C; Crowther, C; Willson, K; Hotham, N; McMillian, V. (2004). A Randomized Controlled Trial of Ginger to Treat Nausea and Vomiting in Pregnancy. Obstetrics and Gynecology. 103. 639-45. DOI: 10.1097/01.AOG.0000118307.19798.ec.
Soudamini KK, Unnikrishnan MC, Sukumaran K, Kuttan R (1995). Mutagenicity and anti-mutagenicity of selected spices. Indian J Physiol Pharmacol, 39: 347-353.
Srivas K. C. (1984). Effects of aqueous extracts of onion, garlic and ginger on platelet aggregation and metabolism of arachidonic acid in the blood vascular system: in vitro study. Prostaglandins, leukotrienes, and medicine, 13(2), 227–235. https://doi.org/10.1016/0262-1746(84)90014-3.
Srivastava KC (1986). Isolation and effects of some ginger components of platelet aggregation and eicosanoid biosynthesis. Prostaglandins Leukot Med. Dec;25(2-3): 187-98. doi: 10.1016/0262-1746(86)90065-x. PMID: 3103137.
Srivastava KC (1989). Effect of onion and ginger consumption on platelet thromboxane production in humans. Prostaglandins Leukot Essent Fatty Acids. Mar; 35(3):183-5. doi: 10.1016/0952-3278(89)90122-1 PMID: 2710801.
Stanisiere, J., Mousset, P. Y., & Lafay, S. (2018). How Safe Is Ginger Rhizome for Decreasing Nausea and Vomiting in Women during Early Pregnancy? Foods (Basel, Switzerland), 7(4), 50. https://doi.org/10.3390/foods7040050
Thomson M, Al-Qattan KK, Al-Sawan SM, Alnaqeeb MA, Khan I, Ali M (2002). The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins Leukot Essent Fatty Acids. Dec; 67(6):475-8. DOI: 10.1054/plef.2002.0441. PMID: 12468270.
Tiran, D. (2012). Ginger to reduce nausea and vomiting during pregnancy: Evidence of effectiveness is not the same as proof of safety. Complementary Therapies in Clinical Practice 18 (2012) 22-25.
Vutyavanich T, Kraisarin T, Ruangsri R. (2001). Ginger for nausea and vomiting in pregnancy: randomized, double-masked, placebo-controlled trial. Obstet Gynecol. 2001 Apr;97(4): 577-82. DOI: 10.1016/s0029-7844(00)01228-x. PMID: 11275030.
Wagesho Y, Chandravanshi BS, (2015). Levels of essential and non-essential metals in ginger (Zingiber officinale) cultivated in Ethiopia. SpringerPlus 4, 1–13. https://doi.org/10.1186/s40064-015-0899-5.
Wen J, Kong W, Hu Y, Wang J, Yang M (2014). Multi-Mycotoxins analysis in ginger and related products by UHPLC-FLR detection and LC-MS/MS confirmation. Food Control 2014, 43, 82–87. doi: 10.1016/j.foodcont.2014.02.038.
Willetts KE, Ekangaki A, Eden JA. (2003). Effect of a ginger extract on pregnancy-induced nausea: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2003 Apr;43(2):139-44. doi: 10.1046/j.0004-8666.2003.00039.x. PMID: 14712970.
Wilkinson, J. M. (2000). Effect of ginger tea on the fetal development of Sprague-Dawley rats. Reproductive Toxicology, 14(6), 507-512. DOI: 10.1016/s0890-6238(00)00106-4
Xu J, Zhang J, Lv Y, Xu K, Lu S, Xiaohui Liu, Yang Y, (2020). Effect of soil mercury pollution on ginger (Zingiber officinale Roscoe): Growth, product quality, health risks and silicon mitigation. Ecotoxicology and Environmental Safety, Volume 195, 2020, 110472, ISSN 0147-6513. https://doi.org/10.1016/j.ecoenv.2020.110472.
Young HY, Liao JC, Chang YS, Luo YL, Lu MC, Peng WH (2006). Synergistic effect of ginger and nifedipine on human platelet aggregation: a study in hypertensive patients and normal volunteers. The American Journal of Chinese Medicine. 34(4):545-551. DOI: 10.1142/s0192415x06004089
Yu, Y., Zick, S., Li, X., Zou, P., Wright, B., & Sun, D. (2011). Examination of the pharmacokinetics of active ingredients of ginger in humans. The AAPS journal, 13(3), 417–426. https://doi.org/10.1208/s12248-011-9286-5.
Verma, S. K., Singh, J., Khamesra, R., & Bordia, A. (1993). Effect of ginger on platelet aggregation in man. The Indian journal of medical research, 98, 240–242.
Zaeoung, S.; Plubrukarn, A.; Keawpradub, N. (2005) Cytotoxic and free radical scavenging activities of zingiberaceous rhizomes. Songklanakarin J. Sci. Technol. 2005, 27, 799–812.