Lipid-based delivery systems
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.Background and overview
5. The oral absorption and bioavailability of certain supplement compounds, many of which are often referred to as ‘nutraceuticals’ in the wider literature, is often limited by their highly lipophilic nature. The absorption of compounds administered by the oral route is related to their relative solubility in polar (i.e., water) and nonpolar solvents (i.e., oil, fat, lipids). For absorption to be effective, compounds need to be solubilised within the aqueous polar environment of the gastrointestinal (GI) tract and to pass readily through the nonpolar phospholipid membranes of the gut wall. Highly hydrophilic or highly lipophilic compounds, therefore, are more poorly absorbed than those of intermediate solubility. Curcumin is an example of one supplement compound with low water solubility and hence poor oral bioavailability. This has led to efforts to develop alternative preparation and delivery methods.
6. The critical factors affecting oral bioavailability of supplement compounds include the proportion of the administered dose available for absorption, the proportion absorbed, and the rate and extent to which the compound is metabolised, which are referred to as bioaccessibility, absorption, and biotransformation, respectively. Formulations designed to enhance the bioavailability of lipophilic and/or poorly absorbed supplements act mechanistically through modifying these factors (McClements et al., 2015). Many of these formulations have directly followed from earlier developments in the pharmaceutical industry targeted at modifying drug pharmacokinetics and reducing toxicity profiles.
7. The most widely investigated strategy to improve oral bioavailability of lipophilic compounds is through their co-formulation in systems composed of amphipathic surfactants and/or lipophilic solvents which are then dispersed in aqueous solutions (Pouton and Porter, 2008). This approach facilitates the aqueous solubility of lipophilic molecules, leading to increased bioaccessibility and absorption when administered by the oral route.
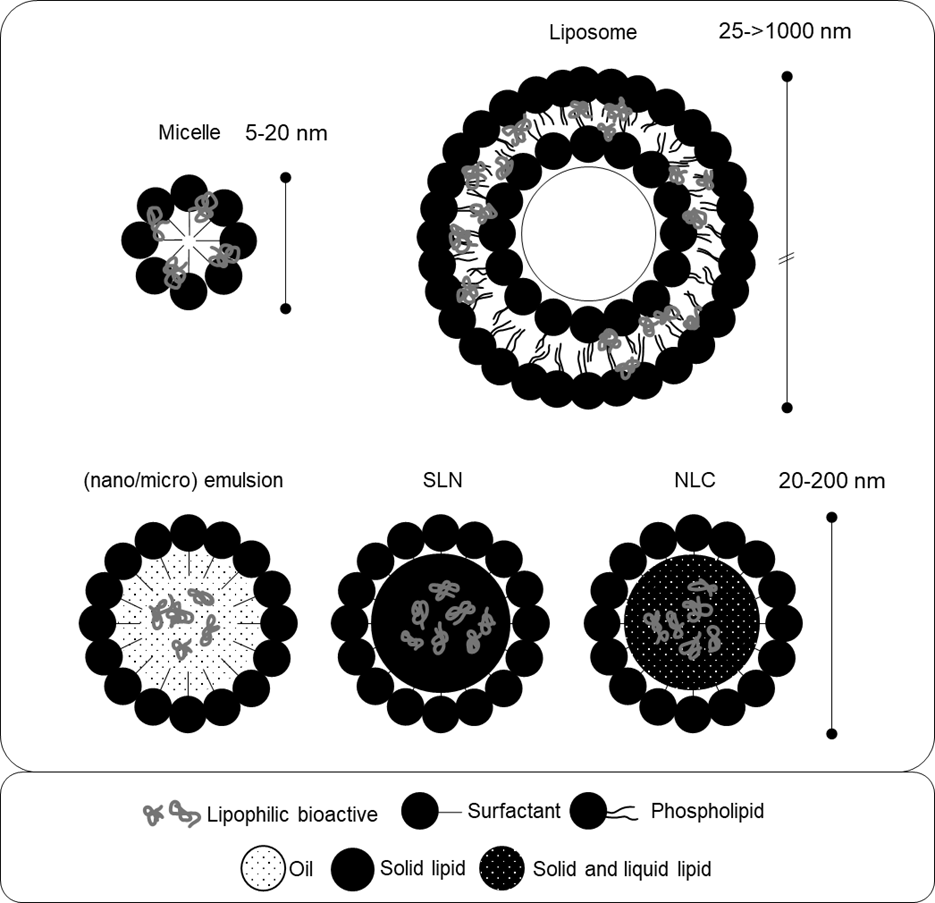
8. There are a range of different formulations, structural characteristics, and physicochemical parameters of these surfactant and/or lipid-based formulations that can be achieved by varying the concentrations and molecular identities of their constituents. As shown in figure 1, examples of some structures that have been studied for oral bioactive delivery and bioavailability enhancement include: micelles, oil-in-water micro- and nanoemulsions, self-emulsifying drug delivery systems (SEDDS), vesicles/liposomes, and lipid nanoparticles (Yao et al., 2014). Micelles, for instance, are self-assembled spherical structures of amphipathic surfactant molecules. The surfactant molecules in micelles are arranged so that the hydrophilic heads of the molecules face outwards towards the aqueous solvent forming the shell. The hydrophobic tails face inwards forming the hydrophobic core of the micelle (Haley and Frenkel, 2008).
9. The bioactive molecules formulated in these systems (i.e., the supplements or ‘nutraceuticals’ such as curcumin) are often encapsulated within the lipidic structures and are referred to as the ‘payload’ or ‘cargo molecules.’ The term ‘cargo molecules’ is used in the current paper to indicate the bioactive molecules formulated in these systems. A variety of these surfactant and/or lipid-based systems can be manufactured from food-grade ingredients and are described in paragraphs 14 - 30. Table 1 provides examples of food-grade ingredients used to produce lipid-based carrier systems, with an emphasis on commonly used lipophilic solvents and surface-active substances.
Table 1. Examples of food-grade ingredients used in the formulation of lipid-based delivery systems for supplements, adapted from Yao et al. (2014).
|
Structural component |
Example ingredients |
|
Lipophilic substances |
Triacylglycerol oils (e.g., canola, corn, fish, medium-chain triglycerides, palm, peanut, soybean, sunflower oils), Essential oils (e.g., carvacrol, lemon grass, oregano, thyme, thymol oils), Flavour oils (e.g., lemon, lime, orange, peppermint oils), Indigestible oils (e.g., waxes, hydrocarbon, paraffin, mineral oils). |
|
Surface-active substances |
Small-molecule surfactants (e.g., monoglycerides, diglycerides, polysorbates, sorbitan esters, sugar esters), Phospholipids (e.g., lecithin and lysolecithin), Proteins (e.g., casein, gelatine, soy, and whey), Polysaccharides (e.g., gum arabic and modified starch), Solid particles (e.g., silica or titanium). |
10. The structural characteristics of these systems can be engineered to formulate particles existing at the nanometre scale and are often discussed within the area of ‘nanotechnology’ (Jones et al., 2019) or described as ‘engineered lipid nanoparticles’ (Yao et al., 2014). This small size (see Table 2) and structures of these systems may significantly modulate the pharmacokinetics of associated bioactive cargo molecules through their physicochemical protection (Jones et al., 2019) and engagement of transcellular absorption pathways. These formulations also provide large surface area to volume ratios thus increasing their solubility and providing increased access for biological constituents, thereby modifying the bioavailability of the associated bioactive materials (Tamjidi et al., 2013).
11. In these lipid-based systems, lipophilic compounds preferentially dissolve in the hydrophobic compartment of surfactants composing micellar or vesicular structures and/or within the oil compartment of emulsions and lipid nanoparticle systems, thereby increasing their solubility in aqueous solution. The pathways by which lipid-based formulations alter the pharmacokinetics and bioavailability of associated cargo molecules may occur through physical, chemical, and/or biological mechanisms (Yao et al., 2014). These mechanisms include providing physical protection of cargo molecules, increasing their solubility and bioaccessibility, increasing intestinal permeability and absorption, altering the physiology of intestinal absorptive epithelial cells (enterocytes), promoting lymphatic transport, and facilitating the direct uptake of nanoparticles in the gastrointestinal (GI) tract (see paragraphs 31 – 48).
12. Fundamental to the efficacy of lipid-based delivery systems is their ability to maintain cargo molecules within a soluble and hence bioaccessible form within the GI tract. During their transit through the GI tract, lipid-based formulations are disassembled (partially digested) and reassembled into various kinds of micellar structures - mixed micelles - which facilitates their absorption into enterocytes (Yao et al., 2014). The precise structure and physicochemical properties of the formulation will dictate its fate. Delivery systems which are not digested or only partially digested may bypass first-pass metabolism through intracellular lymphatic trafficking via enterocytes, M-cells, and/or paracellular transport, thereby delivering their cargo molecules to the systemic circulation (Trevaskis et al., 2008).
13. Several common lipid-based formulation delivery systems currently under development and/or available on the market for dietary supplements are reviewed in this discussion paper. The following paragraphs give a general overview of their structures and physicochemical properties. This is followed by an overview of their modes of action and influence on the absorption and pharmacokinetics of associated bioactive cargo molecules. Several of these mechanisms are shared and overlap between the various formulations, and the pharmacokinetic parameters of a specific formulation will depend on its precise physicochemical properties and on that of the associated cargo. This is explored in detail with respect to vitamin C, curcuminoids, and CBD, in later sections of the current paper.
Emulsions
14. Emulsions are colloidal mixtures of hydrophobic and hydrophilic liquids, surfactants and co-surfactants, in which one phase (the dispersed phase) is dispersed as droplets within the other (the continuous phase). These droplets are stabilised by an interfacial film of surfactants which prevents phase separation and contributes to thermodynamic and kinetic stability (Singh et al., 2017). Emulsions can either be oil-in-water or water-in-oil, in which the dispersed phase is oil or water, respectively, with the corresponding substance composing the continuous phase. Oil-in-water emulsions are used to encapsulate lipophilic target compounds, whereas water-in-oil emulsions encapsulate hydrophilic active compounds. The structural characteristics of the dispersed phase can be varied, giving rise to emulsions containing spheroid/micelle/reversed micelle, cylindrical, and plane-like droplets.
15. Emulsions are categorised by their droplet size and thermodynamic stability. Conventional emulsions have droplet sizes >200 nm (Choi and McClements, 2020), whilst micro and nanoemulsions have droplet sizes <200 nm. Despite their prefixes, microemulsions tend to have smaller average droplet sizes than nanoemulsions, and the key distinction between these two systems is thermodynamic stability (McClements, 2012). Microemulsions are thermodynamically stable, and nanoemulsions are thermodynamically unstable systems. The size range defined for nanoemulsions differs between publications, with upper limits set at 100 nm, 200 nm, and 500 mm (Mason et al., 2006; McClements, 2012; Choi and McClements, 2020). McClements (2012) states “there is no distinct change in the physicochemical or thermodynamic properties of an oil-in-water emulsion when one reduces the droplet size from the micrometre range to the nanometre range.” There may, however, be differences in bioavailability, as discussed below.
16. Emulsification has been used to promote the solubilisation of poorly soluble lipophilic compounds. This process takes advantage of the hydrophobic core of emulsion droplets to allow dispersion of the target compound(s) (i.e., cargo molecules) in an aqueous solution (Yao et al., 2014). In this way, emulsions enhance the physicochemical stability of target compounds, increase their solubility, and promote intestinal permeability, thereby facilitating biological uptake (Ting et al. 2014). Therefore, emulsions have been used as delivery systems for poorly soluble bioactive drugs and supplement compounds.
17. SEDDS are isotropic mixtures (meaning their physical properties remain the same when tested in different directions) of oils (lipids), surfactants, and/or co-surfactants that are incorporated into capsules. Upon physical agitation within aqueous media (i.e., in the conditions of the GIT), these systems self-emulsify. SEDDS may be designed to form mixtures that have the physical properties of micro- or nanoemulsions upon solubilisation (self-microemulsifying and self-nanoemulsifying drugs delivery systems, respectively SMEDDS and SNEDDS). (Gursoy and Benita, 2004; Tanya and Hari, 2017).
Micelles
18. Micelles are similar in composition to emulsions in that they are colloidal dispersions of spherical surfactant droplets dispersed within a continuous phase. When dispersed above a specific concentration in aqueous media – known as the critical micelle concentration – amphiphilic surfactants such as phospholipids spontaneously self-assemble into spherical structures. Micellar structures can be altered or ‘tuned’ and “combinations of surfactants with different molecular characteristics are often used to improve the formation, stability, or performance of micelles.” (Yao et al., 2014).
19. Micelles are generally considered to be primarily two-phase (rather than three-phase) systems composed of surfactant molecules and water with only low to no quantities of lipophilic solvent (Siano, 1982). As such, and as opposed to emulsions, micelles do not enclose a core of oil-based solvent. Although various size ranges have been reported for micelles, depending on the curvature and tail length of the surfactant, they are nano-sized structures existing at up to approximately 100 nm (Huang et al., 2010) and often smaller (e.g., 15-30 nm) (Ali et al., 2019). Micelles co-formulated with cargo molecules, such as supplements or drugs, have been referred to as ‘swollen micelles’ (Yao et al., 2014).
20. Micelles can also be formed through the spontaneous self-assembly of block copolymers, molecules composed of covalently linked hydrophilic and hydrophobic segments, to form polymeric micelles. These are of significant interest as drug delivery vehicles (Kulthe et al., 2012).
Liposomes
21. Liposomes are spherical vesicular structures composed of one or more phospholipid bilayers (Liu et al., 2020). They range in size from small vesicles (30 - 100 nm), to large (100 – 300 nm) and giant vesicles (1 – 100 µm), enclose an aqueous core, and may have complex internal structures including concentric bilayers (Sharma and Shamra, 1997; Liu et al., 2020). Single membrane liposomes are termed unilamellar vesicles, liposomes with one or more bilayers are termed multilamellar vesicles, whilst liposomes enclosing smaller liposomes are termed multivesicular liposomes (Akbarzadeh et al., 2013). Cholesterol and other modifiers such as polysaccharides (hyaluronic acid, mannans, dextrans, etc.) (Turánek et al., 2019) and polymers (such as polyethylene glycol) (Shen et al., 2018) may be incorporated into liposomes to increase their stability and modulate their biological activity.
22. Conjugation of liposomal phospholipids to polyethylene glycol, for instance, has been frequently used to increase liposome circulation time within pharmaceutical research and development. Within the food and feed industry, however, liposomes with surface modifications are rarely formulated (Liu et al., 2019). The major phospholipid used in liposomes for food-based applications is phosphatidylcholine derived from soy lecithin, egg lecithin (Akbarzadeh et al., 2013), marine lecithin (Imran et al., 2015), and milk phospholipids (Thompson et al., 2007).
23. Liposomes form spontaneously after phospholipid precipitated from organic solvent evaporation are dispersed in aqueous solution, although more recent methods for their production include the use of supercritical fluids and microfluidisation (Liu et al., 2020; Ajeeshkumar et al., 2021). The resulting preparation may be further modified to refine particle size, structure, and distribution, and loaded with bioactive cargo (e.g., curcumin) through active or passive loading methods (Akbarzadeh et al., 2013).
24. Due to the amphipathic nature of the phospholipid building blocks, liposomes can serve as carrier vehicles for lipophilic and/or hydrophilic bioactive cargo molecules; whilst hydrophilic molecules may be encapsulated within the core of liposomes, lipophilic molecules are embedded in the lipid bilayer, with molecules of intermediate polarity distributing between the components dependent upon their preferential solubility in polar and nonpolar substances (referred to as the ‘LogP’, i.e., the partition coefficient as derived from the experimental solubilisation of a solute in water and octanol).
25. The liposomal encapsulation of drug molecules was initially developed by the pharmaceutical industry to enhance the pharmacokinetics of therapeutic compounds, including oral bioavailability, tissue distribution, and toxicity profile, although they have also been used in the cosmetic industry (Müller et al., 2000). A key example is the liposomal formulation of the chemotherapeutic agent doxorubicin that was approved by the FDA in 1995 (Liu et al., 2022). Liposomal encapsulation reduces the inherent cardiotoxicity associated with oral doxorubicin administration and improves the overall response rate by modulating its tissue distribution profile (Xing et al., 2015). Use of liposomes in the food and feed sector follows these earlier developments in the pharmaceutical industries. Their use in supplements is primarily tied to attempts to enhance the oral bioavailability of poorly absorbed compounds and for the physicochemical protection of target molecules that are sensitive to environmental and physiological conditions (e.g., UV, pH, temperature).
26. As they interact with the biomolecular milieu of the blood stream, liposomes and other (lipid) nanoparticles become coated with a “protein corona”. The protein corona imparts a “new biological identity” to circulating nanoparticles, thereby modulating their fate and which may have important effects on the bioavailability of associated bioactive molecules (Giulimondi et al., 2019).
Lipid nanoparticles
Solid lipid nanoparticles
27. Solid lipid nanoparticles (SLNs) also have a similar structure to nanoemulsions in that they are colloidal dispersions of (nano-sized) lipid droplets (20-200 nm) within aqueous media stabilised by a surfactant interfacial region. However, in SLNs the core of these particles is either fully or partially crystalline at body temperature, as opposed to a liquid (Yao et al., 2014). The lipidic component of SLNs can consist of triglycerides, mono-, di-, and triglyceride mixtures, waxes, and hard fats. Lipophilic bioactive molecules may be incorporated in the lipidic phase of SLNs and can either be dispersed, enriched in the core or enriched in the shell of the particle (Müller et al., 2000).
28. The solid state of the lipidic component improves control over the release of encapsulated molecules out of the particle (Weiss et al., 2008). For instance, prednisolone encapsulated in SLNs demonstrated a prolonged and sustained in vitro release for up to 7 weeks that was modulated by particle size, preparation method, and composition of the lipid matrix (Zur Mühlen and Mehnert, 1998). SLNs also provide physicochemical protection to encapsulated molecules, thereby increasing their stability under various environmental and physiological conditions (Müller et al., 2000). For instance, SLN encapsulation may protect labile bioactive molecules from degradation with the stomach thus enhancing transit of intact molecules to the small intestine for absorption. Coating of SLNs with chitosan and other modifications have been proposed that might further enhance the stability of SLNs within the GI tract and prevent burst-release of bioactive cargo molecules in the stomach (Ganesan et al., 2018).
Nanostructured lipid carriers
29. Nanostructured lipid carriers (NLCs) are a modified form of SLN in which the lipid phase is composed of both solid and liquid lipids at body temperature. Consequently, NLCs exhibit a more amorphous and less crystalline internal structure than SLNs. NLCs were developed to enhance entrapment of bioactive cargo molecules and to prevent their expulsion by reducing crystalline transitions of the lipidic components and increasing intermolecular spaces (Müller et al., 2002; Kharat and McClements, 2019). Three types of structural characteristics of NLCs have been described which indicate the phase of the lipid interior (Tamjidi et al., 2013): imperfect type, in which the core consists of irregularly spaced solid and liquid lipids; amorphous type, in which the lipidic core congeals as a non-ordered solid; and multiple type, in which liquid nano-compartments exist within a solid matrix.
30. Table 2 summarises the key features of the lipid-based delivery systems introduced above, whilst Figure 1 provides a schematic overview of their structures.
31. Table 2. Overview of lipid-based formulations of supplement compounds.
|
Formulation type |
Structural features |
Components* |
Size range |
Refs |
|
Microemulsion |
Thermodynamically stable oil in water droplets; composed of lipophilic cores stabilised by polar surfaces. |
Surfactant (Co-surfactant), Oil, Water. |
Often smaller than nanoemulsions; often <100 nm |
McClements, 2012; Aswathanarayan and Vittal, 2019 |
|
Nanoemulsion |
Similar to microemulsions but thermodynamically unstable. |
Surfactant (Co-surfactant), Oil, Water. |
20 – 200 nm |
Choi and McClements, 2020 |
|
S(N/M)EDDS |
Isotropic mixtures of oils and surfactants that spontaneously emulsify upon agitation in aqueous environments (i.e., GIT). |
Surfactant (Co-surfactant), Oil. |
100-300 nm <50 nm (SM/NEDDS)
|
Neslihan and Benita, 2004 |
|
Micelles |
Spherical structures: inward facing hydrophobic core and outward facing hydrophilic head; no internal bulk phase (i.e., oil). |
Amphipathic surfactants, Water. |
up to 100 nm |
Huang et al., 2010 |
|
Solid lipid nanoparticles |
Lipid droplets with solid crystalline interior. |
Surfactant (Co-surfactant) Oil, Water. |
20-200 nm |
Yao et al., 2014 |
|
Nanostructured lipid carriers |
Lipid droplets with partially crystalline/two state/amorphous interior. |
Surfactant (Co-surfactant) Oil, Water. |
20-200 nm |
Yao et al., 2014 |
|
Liposomes |
One or more concentric shells composed of lipid bilayers. |
Phospholipids Water. |
25->1000 nm |
Sharma and Shamra, 1997; Liu et al., 2020 |
32. *Components will also include the bioactive molecule of interest; S(N/M)EDDS: self (nano/micro) emulsifying drug delivery system.
33. Figure 1. Schematic diagram of lipid-based formulation systems. SLN = solid. lipid nanoparticle. NLC = nanostructured lipid carrier. Figure based on the text and generated by the Secretariat.
Mechanisms of enhanced bioavailability in lipid-based delivery systems
Physicochemical parameters
34. Preparation of lipophilic molecules with amphiphilic and/or oil/lipidic components aids their solubility and dispersion within aqueous media. The solubility of a specific molecule will be formulation dependent and a function of the target molecule, (co)surfactant molecules, and/or oil/lipid phase and their relative concentrations (Choi and McClements, 2020). For instance, the encapsulation efficiency of a lipophilic molecule into lipid carriers/vesicles/particles, its partition coefficient, and its tendency to leach out of the lipid phase into the aqueous phase, dynamically affects solubility in these formulations. The structural characteristics of the preparation itself may exhibit a degree of instability, for example through the tendency to aggregate, swell or burst, which will also affect solubilisation prior to oral administration (Choi and McClements, 2020).
35. Formulation with lipid carriers and particles may protect labile molecules from environmental degradation. As many supplements may lose their functional features by oxidation, degradation, and reaction with other materials during processing and storage (Shin et al., 2015), encapsulation within emulsions, micelles, liposomes, or lipid particles has the potential to shield supplement compounds from degradative reactions and increase their stability (Li et al., 2021). As with solubilisation, the ability of a lipidic formulation to enhance the stability of a cargo molecule is a function of the physicochemical properties of the preparation. For instance, larger emulsion droplets led to enhanced stability of curcumin due to a smaller oil-water interfacial area at which the molecule could be degraded (Zou et al., 2015). The size of emulsion droplets also influences the penetration of light, thus modifying the stability of light-sensitive cargo molecules (Choi and McClements, 2020).
36. The characteristics of the interfacial layer between the surfactant and aqueous components of these preparations also effects physical stability and interaction with biological systems. For instance, “the electrical characteristics of the oil droplets [zeta potential] impact the physical stability of nanoemulsions by altering the colloidal interactions acting between the droplets, which impacts their tendency to aggregate with each other.” (Choi and McClements, 2020). The charge of the droplets/particles and their tendency to aggregate may also influence interaction with biological systems, for instance by modulating their adhesion to biological surfaces and the ability of enzymes to adsorb to their surfaces. Although less common in food/feed applications, coating of vesicles and micelles with ligand molecules can be used for targeting and precision delivery.
37. Encapsulation and solubilisation in lipid carriers may protect cargo molecules from degradation in the GI tract and increase their residence time, thus promoting absorption. For instance, certain liposomes may pass through the stomach relatively structurally intact due to the low activity of gastric lipases against phospholipids, thus protecting the liposomal cargo from the low pH of the stomach (Carrière et al., 1992; Liu et al., 2019; Liu et al., 2020). Following transit through the stomach, the liposomal cargo is released under the conditions of the small intestine thus promoting absorption at this site (Tan et al., 2014).
38. Conversely, carrier systems may undergo various physicochemical/structural changes as they transit through the mouth, oesophagus, and stomach with resultant impacts on the bioavailability of their associated cargo. The physical stress of mastication and torsional stomach contraction, coupled with changes in pH and ionic strength may induce particles to aggregate, burst, or swell. Particles may clump/aggregate (also referred to as flocculation), coalesce/partially coalesce back to a two-phase system, undergo Ostwald ripening (large particles grow and small ones shrink due to molecular diffusion of oil molecules through the aqueous phase), be digested, or solubilised (Yao et al., 2014). Similarly, the surface properties of the particles/droplets may undergo various changes through interaction with surface-active components of the GI fluid. These changes include adsorption, co-adsorption, multilayer formation, and digestion/degradation. The nature and extent of such events occurring to a specific formulation has the potential to impact on downstream bioavailability.
Biological mechanisms
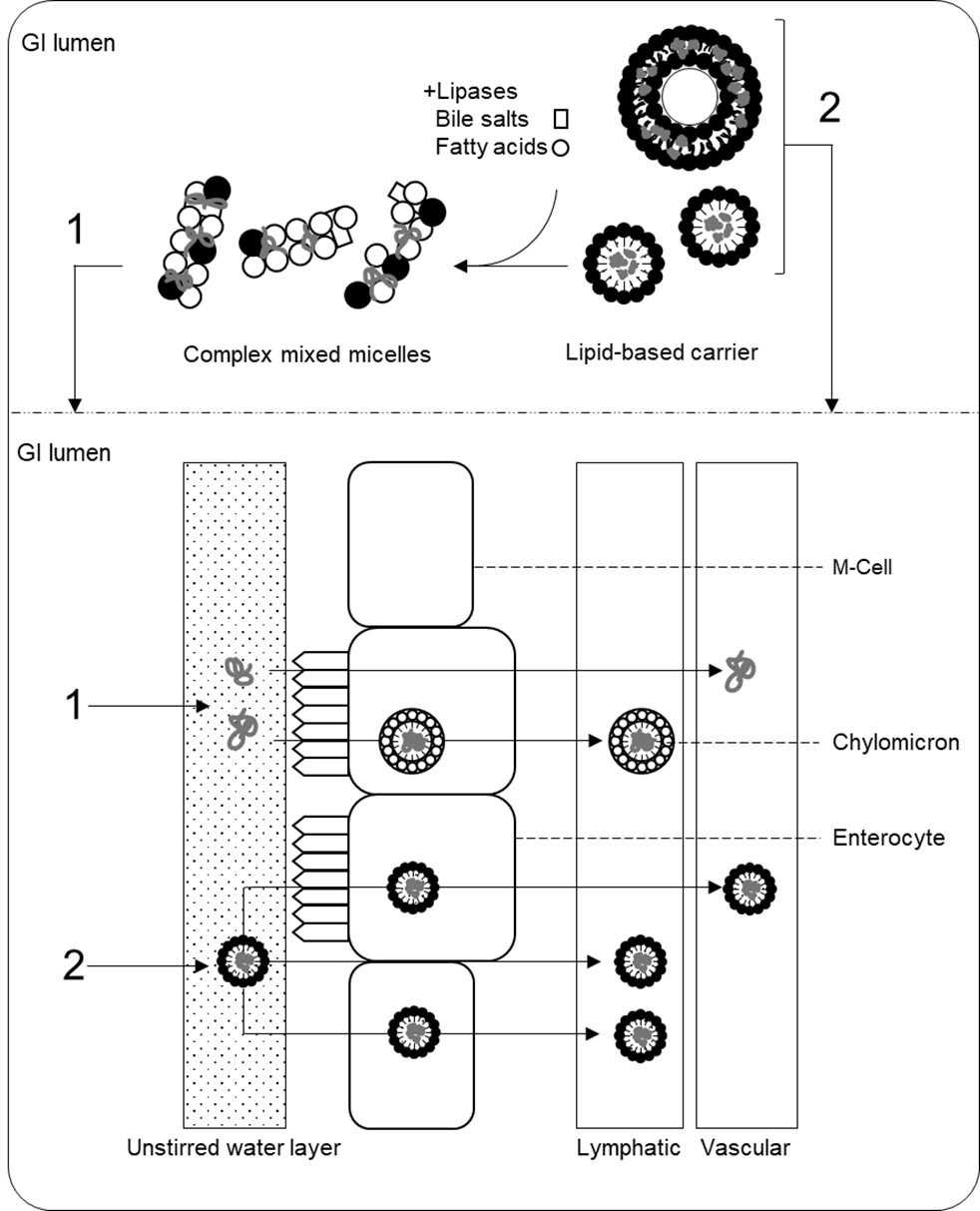
39. There are three key pathways by which the lipidic components of colloidal emulsions, micelles, liposomes, and lipid particles may modulate the absorption, bioavailability, and disposition of target molecules following oral administration. These are: “the alteration of the composition and character of the intestinal milieu, the recruitment of intestinal lymphatic drug transport, and the interaction with enterocyte-based transport processes” (Porter et al., 2007). Some of these pathways are general and are shared between different formulations, whereas others depend on the size and/or structural and molecular characteristics of specific particles. The following paragraphs summarise the general mechanisms of increased bioavailability in lipid-based delivery systems and notes some pathways specific to certain formulations (e.g., liposomes and solid lipid nanoparticles). Figure 2 provides a schematic overview of the absorption mechanisms via lipid-based formulations.
Solubilisation in the GI tract
40. Following entry to the small intestine, the large surface area-to-volume ratio and low interfacial tension of microemulsions and other micro- and nano- lipid-based formulations promotes their interaction with components of the intestinal milieu (Fanun, 2012). Here, lipid-based preparations undergo lipolysis by pancreatic lipases; triacylglycerols are converted into free fatty acids (FFAs), monoacylglycerols (MAGs), and diacylglycerols (DAGs), which then leave the droplet/particle and participate in the formation of mixed micelles with endogenous fatty acids and bile salts. Whilst ‘simple mixed micelles’ are formed from endogenous bile salts and phospholipids in the small intestine, in the presence of exogenous lipids, ‘complex mixed micelles’ are formed from the digestion products of these exogenous lipids (MAGs and FFAs), in addition to bile salts, and phospholipids, and contribute to the encapsulation of co-present lipophilic bioactive molecules (Yao et al., 2014).
41. Mixed micelles are a complex mixture of colloidal structures whose formation is driven by the hydrophobic effect. These structures include micelles, vesicles, and liquid crystals that have dimensions in the nanometre range (Müllertz et al., 2012), although their precise composition depends on the concentration of components in the system and the characteristics of ingested lipids and/or surface-active molecules. Many components of complex mixed micelles have bilayer structures, and lipophilic molecules may be entrapped in these layers, thus facilitating their solubilisation and protection in the GI tract.
42. Solubilisation with complex mixed micelles delivers lipophilic molecules across the unstirred water layer of the gut lumen to the apical membrane of enterocytes (Yeap et al., 2013). The unstirred water layer is an aqueous diffusion barrier adjacent to the intestinal membrane that is a potential barrier to the intestinal absorption of compounds. Delivery across this site via complex mixed micelles therefore aids the intestinal absorption of lipophilic molecules. Absorption then occurs via passive and active transport mechanisms (Iqbal and Hussain, 2009). The absorption of lipophilic molecules into enterocytes is also modulated by the co-presence of FFAs, MAGs, phospholipids, and cholesterol (Kotake-Nara and Nagao, 2012). By contributing to the solubilisation of lipophilic compounds in complex mixed micelles, lipid-based formulations fundamentally increase the bioaccessibility of encapsulated compounds – i.e., the fraction of a compound available in a suitable form for absorption – thereby contributing to enhanced bioavailability (McClements, 2018).
43. The formation of complex mixed micelles is one of the key factors driving the increased bioavailability of lipophilic bioactive components formulated in lipid-based delivery systems. The solubilisation capacity of exogenous-endogenous complex mixed micelles that entrap lipophilic bioactives can significantly surpass that of endogenous (‘simple’) mixed micelles alone (Kossena et al. 2004), and their solubilisation capacity is dependent on the type and amount of digestible lipids present in the formulation as well as the characteristics of the bioactive molecule. For instance, the bioaccessibility of carotenoids was enhanced when formulated with long-chain versus medium-chain fatty acids, but no difference was observed with curcumin between these two types of fatty acids (Qian et al., 2012; Ahmed et al., 2012). Carotenoids are too large to fit in complex mixed micelles composed of medium-chain fatty acids (because they are smaller and have reduced curvature), whereas curcumin is small enough to fit into both types, thus explaining this discrepancy (Yao et al., 2014).
44. The size distribution of the particles/droplets in the lipid-based colloids may also impact on the incorporation into mixed micelles and subsequent modulation of bioavailability. For instance, beta-carotene bioaccessibility was increased with decreasing droplet size in an oil-in-water emulsion (Wang et al., 2012). This is likely to be due to increased exposed surface area for digestion by lipases. In this respect, due to their nanoscale size and large surface area to volume ratio, NLCs may be efficiently digested, solubilised, and incorporated into mixed micelles, thus aiding their passage across the unstirred water layer for absorption by enterocytes (Kipp, 2004).
Interactions with enterocytes
45. Lipid particles and/or their digested components may physiologically interact with enterocytes to modulate the absorption of associated lipophilic molecules. For instance, surfactant molecules may directly enhance the permeability of cell membranes and cause the opening of tight junctions, thereby facilitating cellular absorption and paracellular transport of associated compounds, respectively (Benzaria et al., 2013; Zordan-Nudo et al., 1993). This effect is dependent upon the lipid composition of the formulation. For example, olive oil nanoemulsions more effectively increased trans-enterocyte transport of pterostilbene than flaxseed, and oil type also modulated intracellular pterostilbene metabolism in these cells (Sun et al., 2015).
46. Lipidic excipients may also influence the transport pathways into and across enterocytes by affecting the activity and expression levels of transporter proteins. For instance, some studies have suggested that the lipidic components of emulsions may inhibit the apical membrane efflux transporter P-glycoprotein, thereby facilitating the absorption of compounds which are substrates for this transporter (Constantinides and Wasan, 2007; Bogman et al., 2003).
Lymphatic transport and direct uptake mechanisms
47. Intact liposomes that have not been digested and incorporated into complex mixed micelles also interact with intestinal epithelial cells thereby influencing the absorption of their cargo molecules (Liu et al., 2020). For instance, liposomes may adhere to the cell membrane and release their contents for absorption by the cell (Blumenthal et al., 1977; Allen et al., 1981). Liposomes may also directly fuse with the cell membrane, thus delivering their contents intracellularly (Knoll et al., 1988), be intracellularly internalised by endocytosis (Straubinger et al., 1983), or become disrupted through exchanging their lipid components with cell membranes thereby leading to intracellular uptake of their contents (Sandra and Pagano, 1979). The pathways of cellular uptake of liposomes are size dependent: liposomes of approximately 98-160 nm are predominantly internalised by clathrin-dependent uptake, liposomes of approximately 70 nm by clathrin- and dynamin-dependent uptake, and liposomes of approximately 40 nm primarily by dynamin-dependent uptake mechanisms. The size of liposome also affects intracellular trafficking to endosomes and lysosomes (Andar et al., 2013) which therefore may exert important effects on the resultant bioavailability of cargo molecules.
48. Bioactives associated with lipid excipients may enter the lymphatic system after absorption by enterocytes, via packaging into chylomicrons. Within enterocytes the fate of bioactive lipophilic compounds is tied to the metabolism of the associated carrier lipids; whilst lipids with shorter chain lengths (C <12) pass into the portal vein, those with longer chains (C ≥12) are re-esterified into triacylglycerols. These are then packaged into chylomicrons and trafficked into the lymphatic system. Subsequently, the bioactive cargo molecules may be co-shuttled into this pathway (Yáñez et al., 2011; Trevaskis et al., 2008). The association of lipophilic molecules with chylomicrons evades first-pass metabolism and contributes to increased oral bioavailability. Packaging into chylomicrons may also protect associated the cargo molecules from metabolism within enterocytes, also contributing to increased bioavailability (Trevaskis et al., 2008).
49. Undigested (nano)emulsion droplets and lipid nanoparticles may also be absorbed intact, via paracellular and/or transcellular pathways or via M cells (Singh et al., 2017). The size of lipid particles influences their fate within the GI tract. For instance, SLNs may exhibit bio-adhesion to the intestinal wall thus increasing their retention time within the GI tract and potentially leading to increased absorption of encapsulated molecules (Ponchel et al., 1997; Li et al., 2009).
50. Smaller particles are also likely to have increased direct uptake within the GI tract and are absorbed into the lymphatic system via paracellular transport, thereby evading first pass metabolism (Kreuter, 1991). Absorption of SLNs have exhibited a biphasic distribution with early (1 -2 hr) and later (6 – 8 hr) peaks. These peaks may indicate rapid transport into the systemic circulation and release/distribution from organs into the circulation, respectively (Yuan et al., 2007).
51. Figure 2. Summary of absorption pathways from lipid-based delivery systems. (1) Lipid carriers are digested and incorporated into complex mixed micelles which deliver lipophilic bioactive molecules across the unstirred water layer for absorption by enterocytes. (2) Undigested lipid particles can be directly absorbed via paracellular and transcellular pathways. See the text for more detail. Figure generated by the Secretariat.
Summary
52. Overall, lipid-based formulation of lipophilic bioactive molecules facilitates their solubility within the GI tract and increases the bioaccessible fraction of the associated compound available for absorption. Although the physicochemical and structural features of these preparations, and therefore their fate within the GI tract, is subject to significant heterogeneity, the pathways leading to enhanced absorption depend on partial digestion of the lipid components, disassembly, and reassembly into complex mixed micelles. Mixed micelles contribute to the solubility of associated lipophilic bioactive molecules and deliver them to enterocytes for absorption via passive and active uptake mechanisms. A range of molecular interactions between the lipidic components of these formulations with enterocytes may also modulate uptake of associated bioactive molecules, for instance by enhancing cell permeability through physical and biological mechanisms. Importantly, formulation with lipidic excipients promotes the lymphatic transport of lipophilic molecules via chylomicron packaging in enterocytes and transport through M-cells. Lymphatic transport evades first pass metabolism, thus potentially increasing the fraction of bioactive molecule within the systemic circulation.