COT workshop: Evolving Our Assessment & Future Guiding Principles
In this guide
In this guide3. In May 2023, the COT held a workshop to start the work on new COT guidance. The workshop report is at Annex A. It was noted that the starting point for the process should be existing frameworks and guidance, but with the aim of including innovative improvements, where possible. The workshop aimed to identify the key areas that need to be considered in developing the guidance, and these included reviewing fundamental risk assessment principles, current guidance on risk assessment and what can be learned from it, integration of new approach methodologies (NAMs), exploring hazard versus risk, and weight of evidence. The overall objective of the workshop was to discuss how the Committee moves forward in a new era of risk assessment.
4. Topics discussed at the workshop included models and tools, assessment methods, data sources and quality, uncertainty, NAMs, training and skills, validation, regulatory interfaces, and the role of scientific advisory committees.
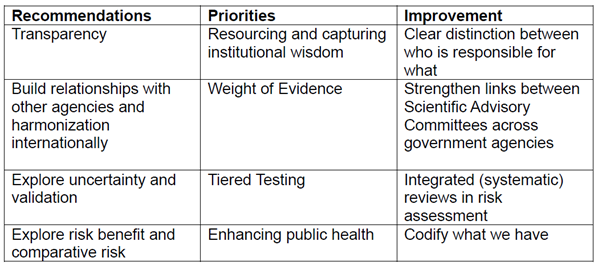
5. The workshop’s recommendations, priorities and proposed improvements to guidance are shown in Table 1.
Table 1. Recommendations, priorities, and proposed improvements to the COT guidance from the “Evolving our assessment and future guiding principles” workshop.
6. Following the workshop, the COT reviewed the draft workshop report at its October 2023 meeting, and identified the most important “must,” “could” and “should” points. These are shown below.
7. The Committee noted that assessment of benefits was not within the terms of reference of the COT and thought should be given as to how COT advice could be best aligned for this to be undertaken when needed or appropriate.
8. The Committee noted that to take the guidance forward, establishing an initial framework would be important. This could then be expanded and linked to other guidance as necessary. There were considered to be two parts to the work, to codify what the Committee currently do, and then to provide guidance on areas where the approach was not yet codified such as benchmark dose modelling.
9. The Committee agreed that it would be important to work with the policy colleagues from the relevant Government departments and not re-invent the risk analysis process. In particular, the required levels of protection needed for consumers should be considered.