Developing COT guidance
Introduction
In this guide
In this guide1. The COT has agreed to update its guidance on toxicity testing and its supporting principles. The previous COT guidelines date from 1982 but were superseded by guidance from the former Scientific Committee on Food (SCF) and the European Food Safety Authority (EFSA), and so have not been updated since then. To start this work, the COT held a workshop in May 2023. The workshop aimed to focus on identifying the principles that would underpin the development of the guidance. It was considered that the COT should identify existing frameworks and guidance and use or adapt these as necessary, and that it should integrate new approach methodologies, using the best science available.
2. The purpose of this paper is to start the work developing new COT guidance, and seek the Committee’s agreement on the way forward, including whether establishing a working group would be the most appropriate approach.
COT workshop: Evolving Our Assessment & Future Guiding Principles
In this guide
In this guide3. In May 2023, the COT held a workshop to start the work on new COT guidance. The workshop report is at Annex A. It was noted that the starting point for the process should be existing frameworks and guidance, but with the aim of including innovative improvements, where possible. The workshop aimed to identify the key areas that need to be considered in developing the guidance, and these included reviewing fundamental risk assessment principles, current guidance on risk assessment and what can be learned from it, integration of new approach methodologies (NAMs), exploring hazard versus risk, and weight of evidence. The overall objective of the workshop was to discuss how the Committee moves forward in a new era of risk assessment.
4. Topics discussed at the workshop included models and tools, assessment methods, data sources and quality, uncertainty, NAMs, training and skills, validation, regulatory interfaces, and the role of scientific advisory committees.
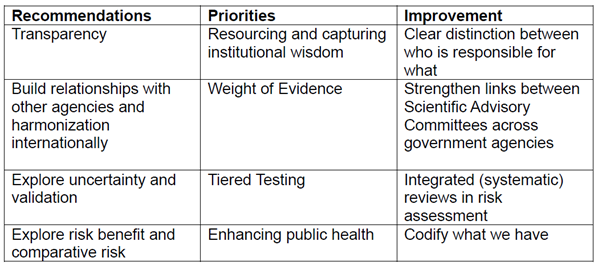
5. The workshop’s recommendations, priorities and proposed improvements to guidance are shown in Table 1.
Table 1. Recommendations, priorities, and proposed improvements to the COT guidance from the “Evolving our assessment and future guiding principles” workshop.
6. Following the workshop, the COT reviewed the draft workshop report at its October 2023 meeting, and identified the most important “must,” “could” and “should” points. These are shown below.
7. The Committee noted that assessment of benefits was not within the terms of reference of the COT and thought should be given as to how COT advice could be best aligned for this to be undertaken when needed or appropriate.
8. The Committee noted that to take the guidance forward, establishing an initial framework would be important. This could then be expanded and linked to other guidance as necessary. There were considered to be two parts to the work, to codify what the Committee currently do, and then to provide guidance on areas where the approach was not yet codified such as benchmark dose modelling.
9. The Committee agreed that it would be important to work with the policy colleagues from the relevant Government departments and not re-invent the risk analysis process. In particular, the required levels of protection needed for consumers should be considered.
Existing guidance
In this guide
In this guide10. EFSA’s Scientific Committee and panels have produced a large number of guidance documents. These include cross-cutting areas of guidance as well as sector specific guidance. These are listed in Annex B. The cross-cutting areas of guidance address a wide range of topics of relevance to the COT, including benchmark dose modelling, risk assessment of mixtures, weight of evidence, biological relevance and risk-benefit assessment, amongst others. The COT responded to public consultations by EFSA on the draft versions of many of the cross-cutting areas of guidance. Consideration of sector-specific guidance by EFSA, e.g. on food additives and consumer risk assessment of feed additives, may be of value in developing the overarching approach. In general, the guidance documents for food additives, feed additives and food contact materials take tiered approaches to the data requirements, but there are differences between them.
11. Also listed in Annex B are relevant guidance documents published by the United States (US) Food and Drug Administration (FDA) and Environmental Protection Agency (EPA).
12. EHC 240, the International Programme on Chemical Safety’s “Principles and methods for the risk assessment of chemicals in food” was developed and published in 2009, and chapters and sections on genotoxicity, dose-response assessment and derivation of health-based guidance values, dietary exposure assessment and enzymes were updated in 2020. This is available with the updated chapters at Principles and methods for the risk assessment of chemicals in food.
13. Other guidance which the COT may wish to consider includes the REACH guidance on chemical safety assessment (Guidance on Information Requirements and Chemical Safety Assessment - ECHA) and VICH guidelines for veterinary medicines (Guidelines).
Proposal on the potential structure of COT guidance
In this guide
In this guide14. Based on the COT’s discussions to date it is proposed that the guidance take the form of a main guidance document, which contains the overarching principles, and then separate guidance documents on specific topics which link to this. The starting point for the guidance would be existing guidance. It is suggested that the guidance should:
- Take a tiered approach.
- Be flexible.
- Take into account the 3/6 Rs (replacement, reduction and refinement of animal testing, but also recently extended to include the reproducibility, relevance and regulatory acceptance of alternatives).
- Specify the information needed rather than the specific studies.
- Be futureproof.
- Include NAMs and how they can be used in risk assessment or to support risk assessment.
- Include the integration of evidence.
15. Aspects that have been noted that may need to be taken into account include:
- Regulatory requirements / sector specific risk assessment needs.
- General chemical risk assessment versus assessment of applications for regulated products.
- The roles of COC and COM, how to interlink with or cross-reference their guidance and possible areas of joint working.
- The joint COT/COC reports on synthesising epidemiological evidence (SEES) and synthesising and integrating epidemiological and toxicological evidence (SETE) reports.
- The four nations of the UK and divergence, e.g. between Great Britain and Northern Ireland.
16. Views of the COT are sought, but areas where UK-specific guidance may be helpful may include:
- Risk assessment of mixtures.
- Integrated approaches to testing and assessment (IATA).
- Benchmark dose modelling.
- Exposure assessment.
- Novel forms of, for example, supplements ingredients.
- The use of artificial intelligence (IA).
- Risk-benefit approaches.
- Biological and statistical significance.
- Biomonitoring.
- Systematic reviews and literature techniques.
- Risks to infants <12 weeks of age.
17. Members are asked to consider where UK-specific guidance is required as it would be helpful to not diverge significantly from EFSA guidance where this is not necessary.
Discussion and Questions
In this guide
In this guideDiscussion
18. Members are asked to consider how they would wish to proceed with this work, for example, if they agree with establishing a working group, initially to develop the main guidance document. It would also be useful to consider where existing guidance could be used or signposted in the main COT guidance document. Members are also asked to consider what topics require UK-specific guidance.
19. It would also be helpful for Members to consider whether they would wish to develop sector-specific guidance or whether this should be considered by the JEGs once the main COT guidance document is finalised.
Questions on which the views of the Committee are sought
20. Members are asked to consider the following questions:
i. Are Members content with the proposal on the format of the guidance?
ii. Are there any other factors that need to be taken into account?
iii. On what topics should COT guidance be developed, and on what can existing guidance be endorsed or signposted?
iv. Does the Committee agree with establishing a working group to develop the main guidance document containing the general principles?
v. What is the priority order in establishing stand-alone guidance pieces?
vi. Would the COT wish to develop sector-specific guidance, or should this be considered by the JEGs once the main COT guidance document giving the general principles is finalised?
21. If the Committee agrees to establishing a working group, volunteers are sought to participate in this working group. It is anticipated that four meetings of the working group would be held in 2025, the first by March 2025.
Secretariat
November 2024
Abbreviations
In this guide
In this guide|
BMD |
benchmark dose |
|
BMDL |
benchmark dose lower confidence limit |
|
COC |
Committee on Carcinogenicity of Chemicals in Food, Consumer Products and the Environment |
|
COM |
Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment |
|
COT |
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment |
|
EFSA |
European Food Safety Authority |
|
EPA |
Environmental Protection Agency |
|
FDA |
Food and Drug Administration |
|
NAMs |
New approach methodologies |
|
REACH |
Registration, Evaluation, Authorisation and Restriction of Chemicals |
|
VICH |
Veterinary International Conference on Harmonisation |
Annex A
In this guide
In this guideEvolving Our Assessment & Future Guiding Principles Workshop Report (2023) DOI: https://doi.org/10.46756/sci.fsa.qpo647 the report can also be found at the web address: Evolving Our Assessment & Future Guiding Principles Workshop Report (2023) | Committee on Toxicity.
Secretariat
November 2024
Annex B
In this guide
In this guideLists of guidance documents by EFSA, the US FDA and US EPA
Secretariat
December 2024
EFSA Guidance
Cross-cutting guidance
Guidance on the use of the benchmark dose approach in risk assessment
Guidance on aneugenicity assessment.
Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment.
Genotoxicity assessment of chemical mixtures.
Clarification of some aspects related to genotoxicity assessment.
Guidance on uncertainty analysis in scientific assessments.
Guidance on the use of the weight of evidence approach in scientific assessments.
Guidance on the assessment of the biological relevance of data in scientific assessments.
Guidance on statistical reporting.
Guidance on Expert Knowledge Elicitation in Food and Feed Safety Risk Assessment.
Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment.
Guidance on risk–benefit assessment of foods.
Transparency in risk assessment carried out by EFSA: Guidance Document on procedural aspects.
Opinion of the Scientific Committee related to uncertainties in dietary exposure assessment.
Sector-specific guidance
Guidance for submission for food additive evaluations.
Scientific Guidance for the submission of dossiers on food enzymes.
Scientific Guidance for the preparation of applications on smoke flavouring primary products.
Guidance on the renewal of the authorisation of feed additives.
Guidance on the assessment of the safety of feed additives for the consumer.
Guidance on the assessment of the safety of feed additives for the target species.
Guidance on conducting repeated-dose 90-day oral toxicity study in rodents on whole food/feed.
FDA toxicology guidance documents for food ingredients
Preparation of food contact substance notifications (toxicology recommendations).
Toxicological principles for the safety assessment of food ingredients: Redbook 2000.
Toxicological principles for the safety assessment of direct food additives and color additives: 1993 Draft Redbook II. Sections of Draft Redbook II not yet finalized in Redbook 2000 are available.
Summary table of recommended toxicological testing for additives used in food.
EPA toxicology guidance
Framework for human health risk assessment to inform decision making.
Benchmark dose technical guidance.
Recommended use of body weight3/4 as the default method in derivation of the oral reference dose
Framework for metals risk assessment.
Guidelines for carcinogen risk assessment.
Supplemental guidance for assessing susceptibility from early-life exposure to carcinogens.
Guidelines for neurotoxicity risk assessment.
Guidance on cumulative risk assessment: part 1. planning and scoping.
Guiding principles for Monte Carlo analysis.
Guidelines for reproductive toxicity risk assessment.
Guidelines for developmental toxicity risk assessment.
Guidelines for mutagenicity risk assessment.
Guidelines for the health risk assessment of chemical mixtures.
Note: only clear guidance documents (not other scientific opinions, reviews, case studies or white papers) that are relevant to the remit of the COT have been listed here.
Guidance related to the regulatory risk assessment of pesticides has not been listed, though some relates to combined exposures and may be of relevance to the COT’s work. These are: