Data from literature search
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.This is a discussion paper. It does not reflect the views of the Committee. It should not be cited.
112. As mentioned in paragraph 5, a literature search was carried out using the search string "Garcinia cambogia" AND "toxicity" in PubMed, Science Direct and Google Scholar. No filters or restrictions were used. These were in addition to the data reviewed by ANSES.
Toxicokinetics
113. van Loon et al., (2000) investigated the acute effects of ingestion of HCA (“6-30 times the reported dosage applied in human weight-loss studies) on plasma HCA availability. They further investigated whether systemic HCA availability altered fat oxidation rates and plasma metabolite concentrations at rest and during moderate-intensity exercise in “endurance-trained” humans by assessing total fat and oxidation rate calculations. Ten cyclists presumed to be male (sex based on information from a pilot study (n=3 males; these males were not included in the main study); aged 25± 2 years; BMI 22.1 ± 0.5 kg/m2) received a total of 0.5 g/kg bw of a liquid G. cambogia extract - Citrimax HCA-450-LS (48% HCA), which was divided over 4 boluses. This resulted in 18 ± 0.4 g HCA being ingested by every subject in the HCA trial. The placebo was water; the number of subjects in the control group were not detailed. It should be noted that beverages (HCA drink or water) were provided in a randomised order. A blood sample was taken at rest, and subjects were provided with either HCA or water to drink 45 mins before exercise. Blood samples were taken at 15 minutes intervals until t=0. Subjects received their second dose 15 minutes before exercise. Following a warm-up period (5 minutes), subjects started cycling at a moderate intensity of 50% Wmax for 2 h (t = 0–120). During exercise, subjects received another bolus of test drink at t = 30 and at t = 60. During exercise, blood samples were taken at 30-min intervals (t = 30 and 60).
114. In the pilot study, it was determined that plasma HCA concentrations increased over time after ingestion of a single dose of HCA solution 4.4g over a 3.5-hour period. Maximal values were attained after 60-90 minutes at 0.12 ± 0.03 mmol/L, after which the concentration decreased. No HCA was detected in the samples collected in the placebo trial.
115. In the main study, plasma HCA concentrations increased up to 0.08 ± 0.01 mmol/L (16.6 mg/L) after the ingestion of 4.4 ± 0.1 g HCA at t = -45 and t = -15 during resting conditions. Plasma HCA concentrations increased further up to 0.39 ± 0.02 mmol/L (82.0 ± 4.8 mg/L) after the ingestion of 4.4 ± 0.1 g HCA after 30 and 60 min of exercise. It was concluded by the authors that plasma HCA availability does not increase energy expenditure or stimulate skeletal fat muscle fat oxidation at rest or during exercise.
116. Loe et al., (2001) dosed fasting humans (n=4; 3 males and one female, subjects were anonymised; age range 21-42; weight range 52.3-86.2 kg) with 2 g of HCA (in the form of CitriMax HCA-600-SXP capsules, ~500 mg) to assess the [bio]availability in humans using a novel gas chromatography/mass spectrometry (GC/MS) method. The subjects were all “healthy, non-smokers that had no history of cardiovascular disease or diabetes”. Blood samples were collected 30 minutes after ingestion of the supplement and every 30 minutes thereafter over a period of 3.5 to 4 hours. A 50 mL blood sample from a control subject (who did not take the supplement) was also collected to construct a standard curve. The peak plasma HCA concentration was observed 2 hours after administration, measuring 8.4 µg/mL, which demonstrated that HCA absorption is relatively fast.
117. Cruz et al., (2021) aimed to determine the main pharmacokinetic parameters of G. cambogia extract/HCA in “healthy” women (n=16 fasted period and n=13/16 for fed period; ages 21-41 years with BMI 20.29–25.82 kg/m2), and to evaluate the food effects on HCA absorption. Subjects received 1,500 G. cambogia, of which 750 mg is HCA extract under 8 hours of fasting. In the fed period, a high-calorie breakfast (~600 calories) was given after dosing. Plasma HCA concentrations were significantly higher in the fasted state (1.21 µg/mL) compared to the fed state (0.40 µg/mL). Overall, plasma concentrations ranged from 0.05 – 2.74 µg/mL. Peak plasma concentration (Cmax) and area under the curve time concentration (AUC0-10hrs) were 3-fold and 2-fold lower (p<0.001, 0.01), in the fed-condition, respectively. The maximum concentration for both groups were similar with 2 hours as the median. In the presence of food, it was observed that, HCA elimination was reduced (5 hours vs 3 hours under fasted conditions). The authors further noted substantial inter-individual variation for the different pharmacokinetic parameters in both periods. The authors suggested that “HCA might suffer an active absorption uptake and intense adsorption on food.”
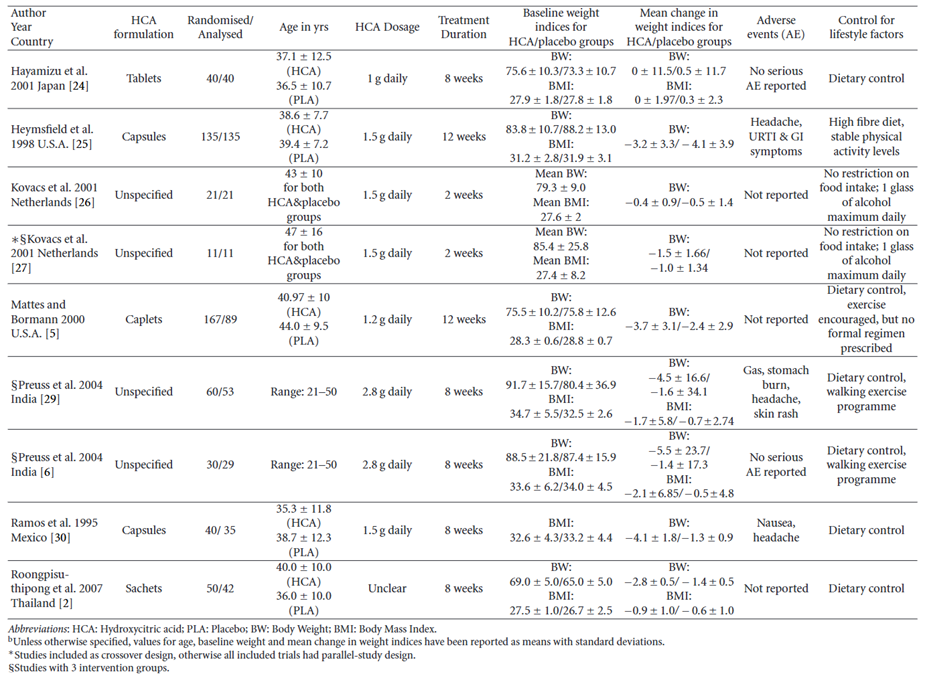
118. Heymsfield et al., (1998) evaluated the efficacy of G. cambogia for body weight and fat mass loss in “overweight but otherwise healthy adults” (aged 18-65 years; BMI range 27 - 38 kg/m2). The total daily dose of G. cambogia extract was 3,000 mg, of which 1,500 mg was HCA or a placebo was received. Both groups were prescribed a high-fibre, low energy diet. A total of 135 subjects were randomised to either active HCA (n=66) or placebo (n=69) groups; 42 (64%) in the active HCA group and 42 (61%) in the placebo group completed 12 weeks of treatment (P=.74). It was found that the co-administration of HCA with a high-fibre diet and low-energy may have inhibited the gastrointestinal absorption of HCA. There was no significant difference in group weight loss (mean [SD], 3.2 [3.3] kg vs 4.1 [3.9] kg; P=.14) between the exposed and placebo groups.
119. In the literature, (2S,3S)-HCA has been described to inhibit the ATP-citrate lyase enzyme (Jakopin, 2019). This is the primary enzyme responsible for the synthesis of cytosolic acetyl-CoA in many tissues. The product, acetyl-CoA, serves several important biosynthetic pathways, including lipogenesis and cholesterogenesis. In nervous tissue, ATP citrate-lyase may be involved in the biosynthesis of acetylcholine (NCBI, 2025). Thus, it is believed that HCA is widely distributed.
120. No other data were available for distribution, metabolism and excretion. It is also unclear if different Garcinia species influences the bioavailability of HCA.
Review articles
121. Andueza et al., (2021) performed a review to investigate the effectiveness and side-effects of nutritional supplements based on G. cambogia to promote weight loss. The efficacy of other Garcinia species was also provided. They utilised the Cochrane and PubMed databases; 13/51 and 29/53 articles were selected for detailed review, respectively. They concluded that toxicity cannot be reliably attributed to Garcinia, as it is typically present in MIDS. The authors were of the opinion that case reports describing adverse effects usually reflect the associations between the observed toxicity and the intake of the dietary supplement, rather than causality. Furthermore, the authors note that the use of these supplements should be discouraged for pregnant and lactating women, since HCA can affect the production of fatty acids and cholesterol, which can directly influence the production of sterols and steroid hormones.
122. Data from the LiverTox database noted that studies in rats and other animal models have suggested that G. cambogia and HCA do not have significant toxicities, although testicular toxicity was found with high doses (Saito et al., 2005). In humans, Garcinia has been linked to rare reports of serotonin syndrome, rhabdomyolysis and hepatic toxicity; however, the role of Garcinia as opposed to other components of MIDS typically used in humans is yet to be fully elucidated. The frequency of hepatic adverse reactions was estimated to be 1:10,000 and it was concluded that consumption of G. cambogia is likely a rare cause of clinically apparent liver injury (NCBI, 2019).
123. Márquez et al., (2012) noted that caution should be exercised when interpreting the results from human studies as other randomized, placebo-controlled clinical trials have not reported the same outcomes. Furthermore, most studies in humans have been conducted on small samples and mainly in the short term. None of them have shown whether these effects persist beyond 12 weeks of intervention. Therefore, there is still little evidence to support the potential effectiveness and long-term benefits of G. cambogia extracts. Regarding toxicity and safety, it is important to note that except in rare cases, studies conducted in experimental animals have not reported increased mortality or significant toxicity. Furthermore, at the doses usually administered, no differences have been reported in terms of side effects or adverse events (those studied) in humans between individuals exposed to G. cambogia and controls.
124. Chuah et al., (2013) “critically assessed” the evidence from the in vitro, in vivo, and clinical trials on the safety of Garcinia/HCA as a dietary supplement for treating obesity. The methodology in which the authors collected and reviewed studies in the literature was not described. The following endpoints were considered: cytotoxicity, genotoxicity, acute toxicity (oral, dermal, dermal irritation and eye irritation), sub-chronic (90 days) toxicity and reproductive and teratogenic toxicity. The authors summarised that G. cambogia/HCA is generally safe and a NOAEL up to 1, 240 mg/kg per day based on a developmental toxicity study in rats by Deshmukh et al., (2008a) (see Developmental toxicity section). In experimental animal studies at up to 233x the human equivalency dose of HCA (1, 500 mg/day of HCA), toxicological studies revealed no death, significant body weight changes, or gross necropsy findings in Albino rats (Ohia et al., 2001). The authors note that G. cambogia extract has been a widely used anti-obesity herbal supplement for decades, around the world without a birth defect or reproductive problem, as such, they are of the opinion that HCA is unlikely to cause reproductive or developmental toxicity. However, they acknowledged that most randomised clinical trials (RCTs) have been conducted on a small scale and with short exposure duration.
Genotoxicity
125. Lee & Lee (2007) evaluated the genotoxicity of HCA isolated from G. cambogia using three tests: the Ames test, the in vitro chromosomal aberration test, and an in vivo micronucleus test. The test item was described as “a natural, highly water-soluble, calcium-potassium salt of 60% HCA extract”; commercially known as Super CitriMax HCA-600-SXS (HCA-SX).
126. The Ames Salmonella mutation test was used according to the plate incorporation procedure described by Maron and Ames (1983). The five strains of Salmonella typhimurium (TA98, TA100, TA102, TA1535, and TA1537) were provided by Prof. B. N. Ames (University of California Berkeley). The assay was performed with and without metabolic activation using an S9 mixture. Negative and positive controls were used for each strain. The positive controls performed without metabolic activation were: 2-nitrofluorene (1 μg per plate) for TA98, sodium azide (1.5 μg per plate) for TA100 and TA1535, mitomycin (1 μg per plate) for TA102, and acridine mutagen (1 μg per plate) for TA1537. The positive controls performed with metabolic activation was 2-aminoanthracene (1 μg per plate) for all strains. Six concentrations of HCA-SX were examined with triplicate plates per dose: 0, 20, 200, 500, 2,500, and 12,500 μM/plate. HCA-SX did not induce mutagenic activity in any of the five bacterial strains tested, under any of the activation conditions examined.
127. For the chromosomal aberration test, the Chinese hamster ovary (CHO) cell line was provided by the Cancer Research Institute, Seoul National University, Korea. CHO cells were maintained under monolayer conditions in Eagle’s minimum essential medium supplemented with 10% foetal bovine serum, with L-glutamine and ampicillin at 37°C in a 5% CO2 atmosphere. For each treatment, 3 × 105 cells were cultured in duplicate in 5 mL of culture medium in a 2.5-L flask. After the cells were incubated for 24 h, they were treated with an HCA-SX 10 μL reaction volume. A 5-hr pulse treatment was then carried out with and without the S9 mixture (used at a final concentration of 10% in the treatment medium). Benzo[a]pyrene (BaP) was used as a positive control in the presence of S9. Mitomycin C (MMC) was used as a positive control in the absence of S9 mixture. Chromosomal aberration percentages included by HCA-SC in the treated groups were >3% and 4%, respectively. In the positive control groups, the percentage of structural chromosome aberrations in the BaP and MMC-treated groups were >21% with S9 and >25% without S9, respectively. HCA-SX was not observed to induce any cytotoxic effect.
128. For the micronucleus test, 7- to 8-week-old ICR male mice (n=5/group) were acclimatised for at least 7 days prior to the start of the test. HCA-SX were dissolved in dimethyl sulfoxide (DMSO). The groups were as follows: DMSO (negative control), 20, 100, 500, 2,500 or 12,500 HCA-SX μmol/kg dissolved/suspended in DMSO, mitomycin C at 2 mg/kg (positive control). Animals were administered the treatments by intraperitoneal injection and were sacrificed by cervical dislocation 24 hours after the test substance administration. Numbers of micronucleated cells were determined by counting the number of polychromatic erythrocytes (PCEs) from at least 1,000 PCEs per animal. The micronucleated polychromatic erythrocytes (MNPCEs) that contained micronuclei were counted from at least 1,000 PCEs. No mortality was observed. MNPCE/1,000 PCEs were induced at the highest HCA-SX dose (12,500 μmol/kg) and PCE/(PCE + NCE) ratios decreased with increasing dose.
129. The authors concluded that HCA-SX was not found to be genotoxic by bacterial or by chromosome aberration testing. It demonstrated “a weak mutagenic effect by micronucleus testing but did not induce structural chromosomal aberrations or significantly induce MNPCEs at the doses used.
130. The article by Lee & Lee (2007) (as summarised in paragraphs 125-129) was refuted by Lau et al., (2008), stating that for the in vivo micronucleus test, the authors: i) selected an inappropriate route of administration intraperitoneal rather than the oral route; ii) the use of vehicle for the test item (DMSO) was not justified and varied to that of the positive control (water), as DMSO may react with the test compound to induce an adverse effect; iii) a dose-range finding test was not performed and the range of selected doses (separated by a factor of 5) differed from conventional dose levels used in toxicological studies; iv) discrepancy with reporting of results; v) “very weak” statistical analysis, where a t-test was utilised without performing a one-way analysis of variance previously; a regression or correlation analyses was also not performed.
131. Ghosh & Mukherjee (2017) evaluated the in vitro genotoxicity of HCA (50.9% HCA in calcium salt of HCA) in human lymphocytes. The following methods were used: trypan blue dye exclusion test, MTT assay, Comet assay and a DNA diffusion assay. The trypan blue and MTT assay were performed according to the test methods of Tennant (1964) and Mosmann (1983), respectively, with modifications by Sinha et al., (2014). The Comet assay was performed following the method of Tice et al., (2000), with modifications based on Sinha et al., (2014). Cells were exposed to HCA (0, 10, 20, 40 or 100 µg/mL) for 3 hours or 24 hours and processed for cytotoxicity and genotoxicity analyses. The effects of HCA on erythrocytes were determined by a haemolysis test using the same doses and exposure duration. Results from the trypan blue and MTT assay in human lymphocytes demonstrated the absence of significant induction of cytotoxicity when compared to the positive control groups. However, as observed in the Comet assay, HCA induced DNA damage that was statistically significant at concentrations of 40 and 100 µg/mL. The authors noted that these concentrations were “almost identical to and approximately double the maximum permitted dose [author does not detail if this the human equivalent dose] (i.e. 900 – 2,800 mg/day or 15 – 47 mg/kg/day, respectively).” Oxidative stress, as a potential mechanism of DNA damage was evaluated using DCFH-DA dye. A significant increase in reactive oxygen species (ROS) production was found at concentrations of 40 and 100 µg/mL at both time points compared to the respective controls. As for the effects on the erythrocytes, no haemolytic potential was observed. The authors concluded that HCA-induced genotoxicity may not lead to apoptotic/necrotic cell death. The observed DNA damage can be attributed to oxidative stress, which was independent of mitochondrial ROS generation.
Human data
Clinical data
132. Onakpoya et al., (2010) performed a systematic review and meta-analysis of RCTs on the use of Garcinia extract (HCA) as a weight loss supplement. Twenty-three eligible trials were identified of which twelve were further analysed. Nine of the twelve trials provided data for statistical pooling (see Figure 4). The dosage of HCA and the duration of the study were varied, ranging from 1-2.8 grams daily and from 2 to 12 weeks, respectively. The adverse effects reported in the RCTs included headache, skin rash, common cold, and gastrointestinal symptoms. In most of the studies there were no “major” differences in adverse events between the HCA treatment and placebo groups. Except for one trial where gastrointestinal symptoms were twice as frequent in the HCA group compared to the placebo group (Heymsfield et al., 1998).
Figure 4 - Results table for studies with adequate data for meta-analysis (reproduced from Onakpoya et al., 2011).
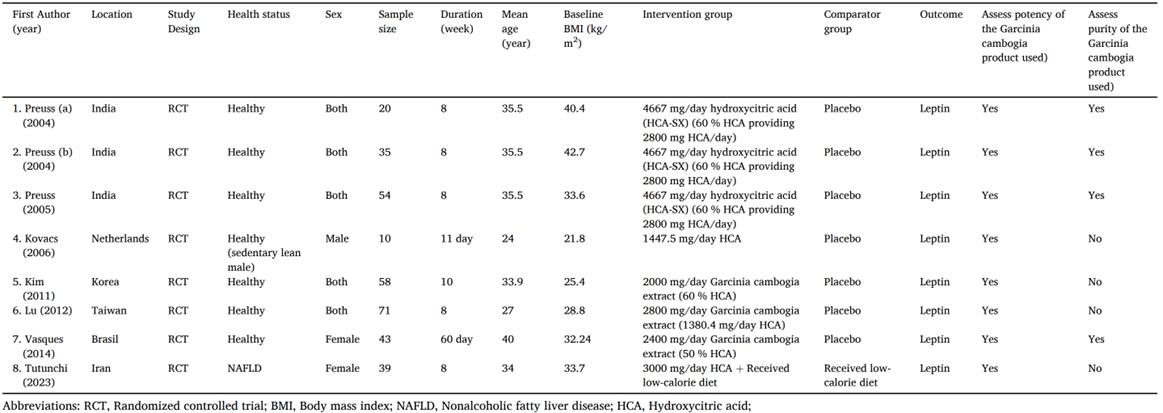
133. Amini et al., (2024) performed a systematic review and meta-analysis of RCTs on the use of G. cambogia (HCA) on serum leptin concentrations. Eight studies were included in the meta-analysis (see Figure 5). Leptin is a peptide hormone that is produced and secreted by adipose tissue. It plays a role in appetite control, immune system modulation, insulin sensitivity, blood pressure regulation and energy homeostasis. The authors observed that several of the included studies found no adverse effects of supplementation. In one study, 38.4% of participants reported the following side effects during treatment: gastrointestinal symptoms, thirst, dizziness and diuresis with gastric discomfort being the commonly reported (Vasques et al., 2014). It was noted by the authors that the treatment period in the selected trials ranged from 11 days to 10 weeks and the long-term side effects of supplementation requires further evaluation as several case reports observed liver injury following G. cambogia supplementation.
Figure 5 - Demographic characteristics of the included studies (reproduced from Amini et al., 2024).
Hepatotoxic case reports
DILIN network
134. The United States Drug-Induced Liver Injury Network (DILIN) have published several articles concerning herb related drug injury. Articles that referred to G. cambogia are summarised in chronological order.
135. In 2015, Navarro et al., noted that G. cambogia was implicated with herbal products in the DILIN database; however, the role of this herb in causing liver injury was difficult to assign since there was a lack of documentation of their chemical presence and purity, the possibility of contamination with other herbal products or mislabelling of the ingredients.
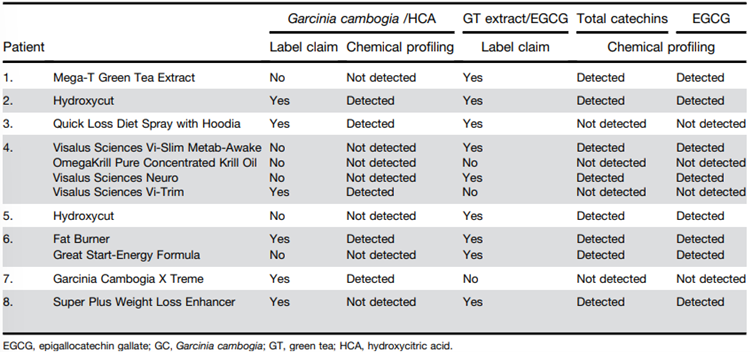
136. In 2022, Vuppalanchi et al., described the hepatotoxic effects of G. cambogia, either alone or in combination with green tea extract (catechins) consumption. Among the 1,418 patients enrolled in the DILIN from 2004-2018, it was identified that 22 cases of liver injury could be attributed to G. cambogia alone (n=5/22), in combination with green tea (n=16/22) or in combination with ashwagandha (n=1/22). Control groups consisted of 57 patients with liver injury from herbal and dietary supplements (HDS) containing green tea without G. cambogia and 103 patients from other HDS.
137. Patients who took G. cambogia (see Figure 6) were aged 17 and 54 years (n=22; 12 females and 10 males) with liver injury arising 13-223 days (median = 51 days) following the first dose. The doses for each product type were not made readily available, and it should be noted that although one of the products claimed the presence of Garcinia/HCA, it was not detected.
Figure 6 - Names and products linked to G. cambogia and green tea extract (catechins) induced liver injury (reproduced from Vuppalanchi et al., 2022).
138. Of these patients, one died, one required liver transplantation and 20 were hospitalised. The liver injury was hepatocellular with jaundice. Peak values of aminotransferases were significantly higher (2,001 ± 1,386 U/L) in the G. cambogia group (P < .018), the median time for improvement in total bilirubin (TB) was significantly lower compared with the control groups (10 vs 17 and 13 days; P = .03).
139. The presence of HLA-B∗35:01 allele was significantly higher in the G. cambogia containing HDS (55%) compared with patients because of other HDS (19%) (P = .002) and those with acute liver injury from conventional drugs (12%) (P = 2.55 × 10-6). The authors concluded that liver injury caused by G. cambogia and green tea was clinically indistinguishable and hypothesised that there is possible association immune-mediated mechanism of injury via HLA-B*35:01 allele.
LATINDILI
140. Bessone et al., (2025) published a “comprehensive analysis” of patients enrolled into the Latin American DILI Network over a decade. Chemicals suspected of causing DILI were classified according to the Anatomical Therapeutic Chemical classification. Causality was assessed using the Roussel Uclaf Assessment method. Overall, 468 idiosyncratic DILI cases were analysed, it was observed that 62% were women (mean age 49 years). Of the cases, 4.1% had a fatal outcome, and 24 patients (12%) developed chronic DILI. The most common drug classes were systemic anti-infectives (31%), musculoskeletal agents (12%), antineoplastic and immunomodulating agents (11%), and herbal and dietary supplements (9%). A total of 6 cases were attributed to G. cambogia.
Other literature
141. Al-Khazraji et al., (2020) [abstract only – poster for symposium] claimed to report the first human case report of G. cambogia induced autoimmune hepatitis. A 39-year-old female with no past medical history presented with fatigue and dark coloured urine. Clinical exam demonstrated a palpable liver and scleral icterus. The patient reported using a “slimming herbal tea supplement” containing pure GC for weight loss 5 weeks prior to presentation [no further information on dose or HCA content]. Elevated transaminase levels were observed and were considered significant: alanine transaminase (ALT) 1803 IU/L; aspartate aminotransferase (AST) 1026 IU/L; alkaline phosphatase (ALP) 139 mg/dL and TB 5.2 mg/dL. Further liver work up revealed an elevated anti-nuclear antibody (ANA) 1:160 titer; anti-smooth muscle antibodies (ASMA) 1:320; and immunoglobulin G 1,814. The normal range for these parameters were not detailed in the paper. A liver biopsy was performed, which demonstrated moderate mixed inflammatory cells including lymphocytes, plasma cells, neutrophils and “rare” eosinophils in the portal tracts. Interface hepatitis and cholestasis was also noted. The authors stated that findings were consistent with DILI with a background of autoimmune hepatitis. The patient was treated with steroids (Prednisone 40 mg orally, daily), upon tapering of her steroids; her liver function began to rise, and she was started on immunosuppressive therapy for long term maintenance with “good response”. The authors further stated that G. cambogia liver injury can last for 2–3 months with normalizing of liver function tests by 5 months.
142. Crescioli et al., (2018) presented four cases of acute liver failure in women taking G. cambogia extract for weight loss, and a literature review of clinical evidence about hepatic toxicity in patients taking dietary supplements containing G. cambogia extract.
143. For Case 1, a 61-year-old woman presented to the emergency department with symptoms of abdominal pain, nausea, progressive weakness, jaundice, dark urine, and acholic stools. The patient’s anamnesis denoted cholecystectomy, mixed dyslipidemia, and hypothyroidism in treatment with levothyroxine. There was no history of alcoholism or exposure to hepatotoxins; she also denied paracetamol abuse. She reported taking one envelope/daily of SUPER ANANAS SLIM®, for a period of 2 months to lose weight. This contained extracts of G. cambogia (HCA 60%), Ananas comosus (bromelain 334 GDU; gelatine dissolving units), and Ilex paraguariensis [Yerba mate] (caffeine 2%). Laboratory tests revealed that ALT, AST, TB, ALP, gamma-glutamyl transferase (GGT), direct bilirubin and albumin were higher than the normal total range at 1,629 U/L, 1,121 U/L, 22.5 mg/dL, 16.7 mg/dL and 2.2 g/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL, 0-0.25 mg/dL and 3.5-5 g/dL, respectively. Serology was negative for hepatitis viruses or autoantibodies. A check-up 3-months prior had found these parameters to be normal. The abdominal computed tomography scan revealed a small peritoneal effusion, perihepatic lymphadenopathy, and a hepatic biopsy which was consistent with cholestatic hepatitis. Four weeks after the cessation of the supplement intake, patient symptoms and liver function tests gradually improved, and the patient was discharged with no need for liver transplant. Four months later, the levels of the previously tested markers reverted to normal values.
144. For Case 2, a 39-year-old woman presented to the emergency department with symptoms of jaundice, asthenia, loss of appetite, and right hypochondrial pain. Her anamnesis denoted arterial hypertension, obesity (BM 44.9 kg/m2), and hiatal hernia. Her medication at the time of admission were methyldopa 500 mg/day, domperidone 20 mg 3 times/day and omeprazole two 20 mg capsules/day. She also reported taking two dietary supplements for weight loss recommended by her dietician. The first, OBLESS®, contained in each capsule: C. aurantium [bitter orange] 140 mg of extract 10% (14 mg of synephrine), G. cambogia (72 mg of HCA), Orthosiphon stamineus [cat’s whiskers or Java tea] (0.2 mg of sinensetin), and Griffonia simplicifolia [African black bean] (75 mg of 5-hydroxy-l-tryptophan). The second dietary supplement was a magistral preparation containing in each capsule: C. aurantium [bitter orange] 350 mg of extract 6% (21 mg of synephrine), Rhodiola rosea [Roseroot or Goldenroot] 150 mg extract, and O. stamineus [cat’s whiskers or Java tea] 200 mg extract. The patient declared that she had been taking the first dietary supplement for the previous month (1 capsule/day) and the magistral preparation for 15 days (1 capsule/day), simultaneously. Laboratory tests performed revealed that ALT, AST and TB were higher than the normal ranges at 1,554 U/L, 1,071 U/L and 15.2 mg/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL, respectively. Serology was negative for hepatitis viruses, cytomegalovirus, and varicella-zoster virus. Non-specific antinuclear antibodies and biliary antibodies were positive. Abdominal ultrasound was normal and did not reveal steatosis. Liver biopsy was not performed. Supplement intake was ceased. After 12 days of hospitalisation, the patient was discharged with no need of supplementary therapies, and a diagnosis of acute cholestatic hepatitis related to consumption of OBLESS® was made.
145. For Case 3, a 47-year-old woman was admitted to the emergency department with symptoms of severe abdominal pain (right hypochondrial). Her anamnesis denoted hyperthyroidism (treated with levothyroxine 100 µg/day), arterial hypertension (treated with enalapril 20 mg/day), and mild obesity. She also reported taking of THERMO GIALLO®, as self-medication for weight control. Each capsule contains 50 µg chromium and 400 mg G. cambogia of which 50% was HCA (200 mg), the patient reported that she had been consuming 2 capsules/day for a month. Laboratory tests revealed elevation of ALT, AST and TB at 299 U/L, 67 U/L and 0.7 mg/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL, respectively. Serology was negative for hepatitis or autoantibodies. No clinical evidence of steatosis was observed, and a liver biopsy was not performed. During the patient’s hospital stay, after cessation of weight-loss supplement, the levels of TB spontaneously declined, and her symptoms and liver function tests “rapidly improved”, without the need of therapies. The patient was discharged with a diagnosis of acute hepatitis.
146. For Case 4, a 52-year-old woman was admitted to the emergency department and was diagnosed with acute hepatitis. No “significant” diseases and medication therapies were reported in anamnesis. However, she was taking two JILL COOPER BE SLIM® products (1 capsule/day for each product) for weight control, containing 400 mg G. cambogia of which 60% was HCA (240 mg), and 400 mg green coffee of which 50% was chlorogenic acid (200 mg), respectively. These products were purchased via T-commerce and were used for a month prior to the emergency department visit. Laboratory tests revealed elevation of ALT, AST, and TB at 1,819 U/L, 1,442 U/L and 14.7 mg/dL, respectively. The normal range for each parameter is 0-35 U/L, 0-40 U/L, 0.2-1 mg/dL. Serologies for hepatitis viruses and autoantibodies were also negative. No clinical evidence of steatosis was observed, and a liver biopsy was not performed. During the following days [number of days not specified] and cessation of the supplement products, liver parameters “spontaneously declined” and acute hepatitis “completely resolved” with no need of supplementary therapies.
147. As previously mentioned, Crescioli et al., (2018) also performed a literature review related of four areas: i) general information for G. cambogia; ii) its use in humans for weight control; iii) the safety of G. cambogia, iv) G. cambogia dietary supplements. The criteria were filtered for human case reports and series only, published between January 2000 and October 2017. The final selection were 24 case reports and 8 case series reporting adverse effects in a total of 66 patients who consumed G. cambogia extract. Five studies reported single cases of myocarditis (Allen et al., 2014), cardiomyopathy (Joseph et al., 2014), serotonin toxicity (Lopez et al., 2014), hypoglycaemia (Roche et al., 2014), and thrombocytopenic purpura (Sikka et al., 2016). Two patients presented acute pancreatitis (Sidhu & Khehra, 2016) and diabetic ketoacidosis (Bystrak et al., 2017), three patients experienced rhabdomyolysis (Dehoney & Wellein, (2009); Hines et al., (2015); Mansi & Huang, (2004)), and in five studies the authors described adverse events of mania (Cotovio & Oliveira-Maia, (2017); Narasimha et al., (2013); Hendrickson et al., (2016)) and multiple psychotic symptoms (Nguyen et al., (2017); Wong et al., (2016)). The patients were mostly women (62%) with no relevant medical history. Seventeen out of the 32 studies described cases of acute liver injury, liver failure, and hepatotoxicity, observed in 50 patients who consumed G. cambogia dietary supplements or G. cambogia “pure extract” (Actis et al., (2007), Chen et al., (2010), Corey et al., (2016), Dara et al., (2008), Elinav et al., (2007), Fong et al., (2010), Jones & Andrew (2007), Kothadia & Olivera-Martinez, (2016), Lunsford et al., (2016), McDonnell et al., (2009), Melendez-Rosado et al., (2015), Schoepfer et al., (2007), Shim & Saab, (2008), Smith et al., (2016), Stevens et al., (2005), Stickel et al., (2009), Vitalone et al., (2011).
148. According to Crescioli et al., (2018), all patients with hepatic adverse effects consumed their food supplements according to the manufacturer’s recommendations and yet developed similar symptoms, including jaundice, weakness, abdominal pain, dark urine, nausea, and vomiting, which were the most reported. The duration of exposure varied with some patients taking supplements for a few days or weeks to more than one year. Of the 50 patients, the symptoms of 38 improved, while 8 required liver transplantation, 1 was diagnosed with liver cirrhosis and 2 died.
149.. Sharma et al., (2018) presented a case report with “severe” liver toxicity following exposure to Hydroxycut®.
150. A 19-year-old man with no “significant” past medical history presented to a community medical centre with 2-day history of fever (103ºF/39.4ºC on presentation), severe fatigue, myalgia, arthralgias and an erythematous rash over his lower extremities. He started using Hydroxycut® [product formulation or HCA content not specified] approximately one week prior to presentation for fat burning and muscle building. He denied any smoking or alcohol use and was taking no other prescription or over the counter medication apart from Hydroxycut® and Myoflex® cream. His blood test revealed an AST level of 23 U/L, ALT of 81 U/L, alkaline phosphatase of 298 U/L white blood cell count of 31x109/L, haemoglobin level of 12.7 g/dL and a normal platelet count. TB was 7.3 mg/dL and he had a prothrombin time of 16.7 seconds. The paper did not provide details of the normal range for these parameters. Blood cultures, urine analysis, chest X-ray, abdominal ultrasound, computed tomography scan and magnetic resonance cholangiopancreatography results were normal. His liver appeared normal in size and texture and there was no evidence of mass, vascular compromise, stone disease, ascites or biliary ductal dilatation. The patient continued to have rising bilirubin levels over the next 3 days and despite antibiotics (Piperacillin and Tazobactum) had a persistent fever. On day 4, the patient was transferred to a hospital for possible urgent liver transplant evaluation due to rising TB of 12.7 mg/dL and a prothrombin time of 21.7 seconds. His hepatic profile showed AST at 27 U/L, ALT at 24 U/L, alkaline phosphatase at 152 U/L, bilirubin at 12.4 mg/dL, a prothrombin time of 15.4 seconds, ammonia at 38 µg/dL, a white blood cell count of 34.8 x 10(9)/L (71 neutrophils and 24 bands), haemoglobin level at 11.2 g/dL, and a platelet count of 237,000/µL. Repeat blood cultures and sputum cultures, as well as urine analysis showed no evidence of infection. Serologies for hepatitis A, B, and C, anti-mitochondrial antibody, anti-nuclear antibody, anti-smooth muscle antibody, F actin IgG, liver kidney Microsomal antibody-1, cytomegalovirus, Epstein Barr virus, Herpes Simplex virus, Group A streptococcal antigen, Coxsackie virus, Monospot virus and leptospirosis were all negative. Ceruloplasmin and Alpha-1 anti-trypsin levels were normal. Human immunodeficiency virus and Rapid plasma reagin tests were negative. Alpha-fetoprotein level was normal at 1 ng/mL. Iron studies were notable for mild iron deficiency and a ferritin level of 463 ng/mL. A comprehensive urine toxicity screen was negative for any drugs except for opiates which he was administered at the hospital for pain management. A liver biopsy done on the 7th day of hospitalization showed acute cholangitis with scant micro vascular fatty changes (<5%) and no evidence of lobulitis, hepatocytes necrosis, cholestasis, fibrosis, parasite, ova, vasculitis, thrombosis, viral inclusions or neoplasm. Infectious disease consultation was obtained, and Vancomycin was added to his antibiotic regimen. No infectious aetiology could be determined and the patient continued to have hyperbilirubinemia. He was started on Ursodiol 600 mg orally twice a day. His bilirubin level peaked at 18.6 milligram/dL, (direct at 10.2) and started to decrease after day 6 of hospitalization. Peak alkaline phosphatase was on day 3 of admission at 298 units/L and peak AST/ALT levels were 110/142 units/L respectively on days 11 and 13. All abnormal levels gradually started to decrease. Antibiotics were discontinued after a total of 14 days of therapy. The patient was discharged from hospital on day 17 with a bilirubin level of 6.8 mg/dL (direct at 3.2), AST level of 68 U/L, ALT level of 108 U/L, alkaline phosphatase level of 160 U/L. The patient had gradual recovery of liver functions and at 14 weeks after initial onset of symptoms his liver function tests had returned to normal.
151. The authors acknowledged that “whilst causation is difficult to prove in any drug induced injury, the temporal relationship after Hydroxycut® exposure and gradual improvement after withdrawing the involved medication plus the absence of any other aetiologies despite comprehensive testing would point to Hydroxycut® being the most likely possible cause for hepatotoxicity.”
152. Lunsford et al., (2016) reported “the first known case” of fulminant hepatic failure associated with dietary intake of a “pure” G. cambogia supplement.
153. A 34-year-old Hispanic male presented with nausea, vomiting, abdominal pain, and dark urine. Testing revealed elevated transaminases and TB; however, imaging failed to demonstrate cirrhosis or anatomic abnormality. Hepatitis work-up, including testing for viral hepatitis, hemochromatosis, Wilson’s disease, and autoimmune hepatitis, was unremarkable with the exception of an elevated Ferritin level of 7,089 mg/dL. Genetic testing for hemochromatosis was negative. His medical history was only positive for occasional social alcohol use, and the drug toxicology testing was negative. He denied use of energy drinks, herbs, Chinese teas, or muscle milk. He was advised to discontinue alcohol use, which he did, and his symptoms initially seemed to abate. Six weeks later, the patient developed asterixis, jaundice, and confusion. Follow-up imaging was concerning for rapid onset of cirrhosis or infiltrative hepatocellular carcinoma. On admission, transaminases were elevated with AST 624 U/L, alanine ALT 520 U/L and TB of 34.7 mg/dL. Autoantibody titers demonstrated a positive antinuclear antibody, but no other positive autoantibodies. Evaluation of Wilson’s disease demonstrated normal ceruloplasmin and copper levels; however, 24-h urine copper was elevated. Serum ferritin but not transferrin was elevated. A liver biopsy was performed and demonstrated sub-massive necrosis with collapse of the hepatic architecture involving about 70% of the liver parenchyma. Mild lymphocytic inflammatory infiltration and minimal canalicular cholestasis were seen. No viral inclusions or other infectious agents were identified by histology or immunohistochemistry. No evidence of granuloma, tumour, or features of cirrhosis were demonstrated. Periodic acid-Schiff stain with diastase was negative for alpha-1 antitrypsin globules. Iron stain showed only mild iron deposition in Kupffer cells and hepatocytes. Quantitative tissue copper was within normal limits. Findings were suspected to be potentially related to drug-induced liver injury. After questioning, the patient confirmed intake of G. cambogia, purchased on the Internet. He was taking two 80 mg capsules of “Garcinia Cambogia 5:1 Extract” three times daily before meals for five months preceding initial presentation. Since not advised against intake, he continued the supplement after initial presentation. He denied any other medications or supplements and reported no alcohol intake for two months. An 80 mg tablet of a 5:1 concentrate of G. cambogia was determined to be equivalent to 400mg of standard preparation. Other listed ingredients include rice flour, gelatine, magnesium stearate, and silica. However, it was confirmed that the manufacturer does not perform assays to determine HCA concentration. The patient’s status declined and his mental status deteriorated; he was listed for liver transplantation and received an orthotopic liver transplant. Histopathologic examination of the explanted liver demonstrated near total hepatic necrosis with massive hepatocellular dropout and mixed inflammatory cell infiltrates, consistent with severe drug-induced liver injury.
154. The authors concluded that “while evidence from a case report rarely offers proof of causality, this case, in conjunction with known cases of hepatotoxicity and liver failure associated with other G. cambogia-containing supplements warrants a high index of suspicion.”
155. García-Cortés et al., (2016) reviewed the reported cases of hepatotoxicity by dietary supplements. Two case-studies were summarised relating to G. cambogia induced hepatotoxicity. Corey et al., (2015) reported a 52-year-old female requiring liver transplant after taking G. cambogia extract 936 mg (2 capsules per day) with 60% HCA (USA Nutra Labs) for 15 days. To note that the supplement also contained calcium (50 mg), chromium (200 µg), and potassium (50 mg) at the ingested amount.
156. Melendez-Rosado et al., (2015) reported a 42-year-old female with elevated transaminase levels (ALT at 1,277 U/L and AST at 2,792 U/L, which are 70 and 45 times the upper limit of normal, respectively) and coagulopathy. It should be noted that the woman had a medical history of hypertension, chronic kidney disease (stage V), diabetes mellitus type 2, chronic back pain, haemochromatosis, and obesity. Abdominal ultrasound showed mildly coarse hepatic echotexture with no intrahepatic or extrahepatic dilatation, and abdominal computed tomographic scan showed a surgically absent gallbladder and no hepatic parenchymal abnormalities. A week before presentation, the patient started taking “pure G. cambogia” for weight loss. The supplement was discontinued, and the patient was empirically treated with N-acetylcysteine due to concerns of acetaminophen toxicity. After several days of supportive care, the patient’s abdominal pain resolved, and liver enzymes recovered to baseline. The patient was discharged after 4 days. Four months later, the patient’s symptoms had not recurred, and normalisation of her liver function studies was noted. The authors were of the opinion that the development of acute abdominal pain with elevated liver enzymes in the setting of a baseline liver inflammation in combination with the use of acetaminophen and the introduction of a hepatotoxic herbal supplement makes the diagnosis of acute hepatitis secondary to G. cambogia “very likely”. No other information on the supplement, dose or exposure time could be obtained.
Animal studies
Subchronic effects
157. Shara et al., (2003) evaluated the dose- and time-dependent effects of HCA-SX in male and female Sprague-Dawley rats on body weight, hepatic and testicular lipid peroxidation, DNA fragmentation, liver and testis weight, expressed as such and as a percentage of body weight and brain weight, and histological changes over a period of 90 days. An animal research protocol (ARC# 0598) was obtained from Creighton University Medical Center. HCA-SX “a natural, highly water-soluble”, calcium-potassium salt of 60% HCA extract from G. cambogia – commercially known as SuperCitriMax HCA-600 SXS was dissolved in water and administered by gavage at 0, 0.2, 2.0 and 5% of feed intake. Control animals received the vehicle (water). Food and water consumption were measured 2-3 times weekly. Mortality/morbidity was assessed once daily throughout the study period. Clinical signs were evaluated once to twice daily. Body weights were recorded on day 1, twice weekly thereafter and before necropsy. Animals were sacrificed on days 30, 60 and 90 of treatment and the target organs were either processed immediately, preserved in 10% buffered formalin for histopathology, or stored at -80ºC. The number of animals per dose group was not explicitly stated by the authors; however, it was detailed that all result values were reported as a mean ± standard deviation from 5-7 samples.
158. By day 90, feed intake was reduced by 13.7, 26.7 and 25.6% in male rats following supplementation of HCA-SX at the aforementioned doses (0 (water), 0.2, 2.0 and 5% of feed intake), respectively, when compared to their corresponding controls. In females, the values were 16.3, 19.6 and 22.8%, respectively. Regarding changes in body weight, ~11.2, 12.4 and 15.8% reduction in body weight in male rats following supplementation at the tested dose levels, respectively. For females these values were ~11.1, 18.1 and 13.0%, respectively. These were considered as significant by the authors. Regarding changes in testicular weight (expressed as a percentage of body weight and brain weight), a small but not statistically significant increase in testis weight with increasing age was observed in control animals. The testis weights in the exposed groups were similar to the control animals. Regarding the effects of HCA-SX on hepatic and testicular lipid peroxidation, a time-dependent increase in hepatic lipid peroxidation was generally observed in all samples; however, these were not significant in the HCA-SX exposed groups. A small but non-significant increase in testicular lipid peroxidation was observed in the control group as well as the HCA-SX exposed group. Regarding the effects of HCA-SX on hepatic and testicular DNA fragmentation, the results showed that there were no significant HCA-SX treatment related effects on these parameters (in male rats for the latter) when compared with their respective controls. Histopathological analyses on the liver, brain and testis, revealed that HCA-SX exposure did not cause any morphological alterations in these tissues.
159. The authors were of the opinion that these results indicated that HCA-SX was “safe and efficacious in weight management” under the test conditions.
Reproductive toxicity
160. Deshmukh et al., (2008a) evaluated the effects of a novel calcium/potassium salt of HCA on the reproductive systems of male and female rats, the postnatal maturation and reproductive capacity of their offspring, and possible cumulative effects through multiple generations. The study was performed in compliance with a standard study protocol based on the US FDA Redbook Guidelines for Reproductive Studies IV.C.9.a, and Guidelines for Developmental Toxicity Studies IV.C.9.B., Feed Additive Safety (US FDA, 1993), and the OECD principles of Good Laboratory Practice.
161. The test article, HCA-SX, commercially known as Super CitriMax was mixed with powdered rodent diet to obtain three concentration levels. A small volume of diet premix was prepared, which was then mixed with the remaining portion of the diet in a mechanised ribbon blender for about 20 minutes to obtain the desired homogeneity of the test article concentration in diet. The experimental diets were prepared once a week based upon the results of the stability tests on HCA-SX. The diet preparation procedure was subsequently validated by conducting stability studies and homogeneity on exposed diets.
162. Sprague-Dawley rats (n=30/sex per dose group) were administered feed containing HCA at dose levels of 0, 1,000, 3,000, or 10,000 ppm for 10 weeks prior to mating, during mating, and, for females, through gestation and lactation, across two generations. The control group of animals were fed normal diet. The male and female rats of the F0 generation from each dose group were mated and allowed to deliver normally. At weaning, one male and one female pup from each litter from the control and treatment dose groups were selected for the F1 generation. The selected F1 animals were exposed to HCA-SX for 10 weeks before mating and then they were mated to produce a second generation F2a. During the period of study, animals were examined daily for signs of clinical toxicity and their body weight and feed consumption (g/rat/day) were recorded twice a week. N.B. A discrepancy was noted where the abstract states the latter was performed twice a week recording, whilst the methods section describes one weekly recording. For the parents (F0 and F1) and the offspring (F1 and F2a), reproductive parameters such as fertility and mating, gestation, parturition, litters, lactation, sexual maturity, and development of offspring were assessed. At termination, necropsy and histopathological examinations were performed on all animals.
163. Data on feed consumption by the parental male and female rats of both (F0 and F1) generations during the premating and mating periods, for both sexes, and during gestation and lactation in the case of female rats, did not reveal any “remarkable” treatment-related changes in the average daily feed intake by the male and female rats compared to the respective control groups, across the different dose levels for each of the F0 and F1 generations and also when compared across these two generations. Based on feed intake, the resulting dose of HCA-SX for the highest-dose groups of male and female was calculated as 813 and 1,205 mg/kg/day, respectively, for the F0 generation, while the same was respectively 1,018 and 1,524 mg/kg/day in the case of the F1 generation. The total daily dose of HCA-SX for all groups are provided in Table 2.
Table 2 – Daily dose of HCA-SX (mg/kg bw per day) during the premating period (reproduced from Deshmukh et al., (2008a).
|
HCA-SX level in diet (ppm) |
F0 Males (mg/kg bw per day) |
F0 Females (mg/kg bw per day) |
F1 Males (mg/kg bw per day) |
F1 Females (mg/kg bw per day) |
|
Control (0) |
0 |
0 |
0 |
0 |
|
Low (1,000) |
80 |
109 |
89 |
135 |
|
Mid (3,000) |
246 |
354 |
268 |
447 |
|
High (10,000) |
813 |
1,205 |
1,018 |
1,524 |
164. Dietary exposure to all animals and offspring at the F0 and F1 generations, did not reveal significant incidence of mortality or abnormal clinical signs. Compared to the respective controls, the HCA treatment groups at all dose levels, did not have different feed consumption or body weight. All deaths and abnormal clinical signs observed in the rats during F0 and F1 generations, such as transient/reversible spells of emaciation, abdominal breathing, respiratory rates, hypoactivity, circling disorder, and lacrimation, were considered to be incidental and not test substance related.
165. The average bodyweight and body weight gains on the parental F0 and F1 generations up to all stages including lactation of female rats of the exposed groups at all doses, did not reveal any significant differences when compared to the respective control groups. Although, a mild but significant lowering of body weight gain in F1 male offspring was noted in the highest dose level after they were weaned, between 3 to 7 weeks of their life. For the duration of the study, the difference in body weights persisted; however, it was not significant, and the percentage gain in body weights between control and exposed groups of male rats was found to be comparable on week 31 (it was slightly higher in the high dose group than the control group). The authors did not count this as an adverse effect while deciding the NOAEL, as it was considered to be a likely effect of the pharmacological activity of HCA-SX. There were other “occasional” instances of group mean values of exposed animals differing from those of the respective control; however, these were not considered of no toxicological significance due to the “small magnitude of variation”.
166. During the gestation period in all females exposed to HCA-SX, no treatment-related adverse effects on reproductive performance in terms of fertility and mating, gestation, parturition, and the litters born were observed. The values of male fertility indices for the exposed groups in the F0 and F1 generations did not differ significantly from those of the controls and also compared well with the historical control data at the test facility. The sperm motility of the F1 parents was lower than the F0 parents (in exposed groups). The authors did not consider this to be related to HCA-SX exposure, as the lowering was also observed in the concurrent control group of rats.
167. All the gross and microscopic findings of the parental organ weights, necropsy and histopathology were considered to be incidental as the incidence was found to be comparable among the control group and the treatment groups, without any dose-dependent trend.
168. Offspring observations are hereby summarised. The body weights of some of the pups selected as parents for the next generation were recorded at ~4 weeks after they were weaned at the end of their lactation; in male offsprings exposed at the highest dose (10,000 ppm), the group mean body weights were significantly lower than those of the control group; however, this effect was not considered as an adverse effect [was considered as a pharmacological effect]. When compared with their respective controls, data on survival and clinical observations recorded for the offspring of both generations F1 and F2a during the lactation period of 21 days did not reveal any remarkable differences. Nor were there any adverse effects on their litter sizes, sex ratio of litters, live birth indices and the viability of litters, which were calculated on days 4, 7, 14 and 21 of lactation from parental females exposed to HCA-SX at all dose levels. The sexual maturation [age at which there is balanopreputial separation in males and vaginal opening in females] was only measured for the F2a groups. It was observed that exposure to HCA-SX at any of the dose levels did not affect the age of sexual maturity of the offspring belonging to the F2a generation. Exposure of the parental animals of both the F0 and F1 generation to HCA-SX at the tested dose levels had no adverse effect on the physical development of their litters, compared to the respective control groups. The group mean values of absolute and relative weights of the brain, spleen, and thymus of the pups of the F1 and F2a generation did not significantly alter between the control and treatment groups. The study authors considered that all the gross and microscopic pathology findings in this study were accidental as the incidence was found to be comparable among the control group and the treatment groups, without any dose-dependent trend. HCA-SX treatment did not cause any significant histopathological changes in any organ.
169. The authors noted that exposure to HCA-SX did not affect reproductive performance as evaluated by sexual maturity fertility and mating, gestation, parturition, litter properties, lactation, and development of the offspring. Nor did it induce any systemic toxicity in the parental rats and their offspring at the tested concentration levels. Based on the results of this study, the authors determined a NOAEL to be greater than 10,000 HCA ppm in the diet or equivalent to 1,018 and 1,524 HCA mg/kg bw per day in male and female rats, respectively.
170. Saito et al., (2005) investigated the ability of G. cambogia extract to suppress body fat accumulation in developing male Zucker obese (fa/fa) rats. 6-week-old rats (n=6 per dose group) were fed diets containing HCA powder (41.2 wt%: ratio of free to lactone form was 36.6 to 63.4) at 0, 2, 10.1, 20.1 and 30.2 g HCA/kg diet for 92 or 93 days. All groups had free access to water. Each diet group was pair-fed to the 30.2 g HCA/kg diet group. On the last experimental day, the rats were allowed to consume three-quarters of the food intake of the previous day and were then killed by cardiac puncture. Liver, kidney, spleen, spleen, testis, and epididymal fat pads were excised, washed with isotonic saline and weighed. The liver, spleen and testis were fixed with 10% formalin neutral buffer solution, pH 7.4 and histopathological examinations were performed after haematoxylin-eosin staining. At the highest dose, there was significant suppression of epididymal fat accumulation in developing male Zucker obese rats, compared with the other groups. The higher dose groups (20.1 and 30.2 g HCA/kg diet) caused “potent” testicular atrophy and toxicity, whereas diets containing 10.1 g HCA/kg diet or less did not. The authors derived a NOAEL of 10.1 g HCA/kg diet equivalent to 389 mg HCA/kg BW per day.
171. Burdock et al., (2005) performed a “critical review” of the article by Saito et al., (2005) (as summarised in paragraph 170) and raised the following comments: (1) the form of HCA and toxicity; (2) experimental study design and results; (3) Zucker rat model and testicular toxicity; and (4) dietary ingredients and testicular toxicity. The form of HCA can vary its toxic profile, dietary supplements containing HCA consist of various salts, including HCA–sodium, –calcium, –potassium, –magnesium, or combinations thereof. Depending on the salt(s) used and the extraction process, the solubility, bioavailability, bioefficacy and lactone content can vary considerably.
172. Limitations on the experimental design were noted: the amount of food received by pair-fed animals was approximately 10% less (compared to ad libitum fed control, which was not included). Burdock et al., further state that HCA is known to affect satiety, effectively preventing consumption of food at high levels. Secondly, the investigators reported severe diarrhoea at the highest use levels (1,244 mg HCA/kg/day), which may have further affected the feed intake in this group and other groups (as a result of pair feeding). Thirdly, Saito et al., (2005) stated that Zucker obese rats (or models with higher lipogenic properties) may be insensitive to HCA at usual dietary levels; however, a biphasic effect of HCA on fat accumulation in the liver was noted. The selection of Zucker rat model was thought to be inappropriate since serum concentrations of testosterone in obese male Zucker rat at the age of 2-, 3- and 4-months were lower when compared to lean male Zucker rats. Testes morphology of Zucker rats was also found to be different from lean rats.
173. It was not made clear by Saito et al., (2005), whether dietary imbalance or nutritional imbalance (use of high levels of the extract, pair feeding) may have contributed to the observed testicular toxicity at two highest doses of G. cambogia extract.
Developmental toxicity
174. Deshmukh et al., (2008b) conducted a follow-up study (summarised in paragraphs 160-169) to evaluate maternal toxicity and effects on the developing embryo in Sprague-Dawley rats (effects included death, structural abnormalities, and altered or retarded growth) when exposed to HCA-SX. The study was performed as per the previous protocol in Deshmukh et al., (2008a). A total of 30 males and 30 female pups per dose group (except the 3,000 ppm (mid) dose group where 25 of each sex were available), including the concurrent control group. The animals in this study were selected randomly after weaning from each F2b litter of the F1 generation from the two-generation reproductive toxicity study (as summarised in paragraphs 160-169). Therefore, the rats in the treatment group were exposed directly to HCA-SX via feed, prior to which they had indirect exposure to HCA during lactation. The dietary exposure levels were the same as those employed for the two-generation reproductive toxicity study: 0, 1,000, 3000, or 10,000 ppm. Following mating at maturity, the pregnant rats were observed twice daily for clinical signs of adverse effects, and body weight and feed consumption were recorded. On day 20 of gestation, animals were subjected to a necropsy and caesarean section to examine the uterus, ovaries, and foetuses for assessment of different parameters of pregnancy and embryo-foetal defects.
175. The daily amount of HCA-SX consumed at dietary feed levels of 1,000, 3,000 or 10,000 (ppm) (equivalent to 0.1%, 0.3% and 1.1%) were calculated as 103, 352, or 1,240 mg/kg bw per day, respectively.
176. Comparison indices of sperm-positive females (mating behaviour), maternal deaths during pregnancy, number of pregnant/non-pregnant females, pregnancy rate (%), and females with resorptions (%) were evaluated between the control group and the HCA-SX exposed groups. At the dose levels administered, no adverse effects were observed. Maternal body weight changes during gestation were recorded for the following periods: days 0, 7, 14 and 20. The group mean values of body weight gain for each period did not show any significant differences between the exposed and control groups. On the 20th day of gestation, a significant decrease (by 13%) in mean body weight of the rats maintained on the highest dose (10,000 ppm) was noted. During the study, no treatment-related clinical effects were observed in any of the groups. However, the following incidences were reported: i) wryneck and a transient period of emaciation were observed in females during the course of the study [was deemed to be not dose related by the authors]; ii) on day 17 of gestation, one pregnant female from the highest dose group died – profuse haemorrhage [by vaginal bleeding] was identified as the probable cause of death. No other mortalities were noted in any of the groups during the course of the study. Observations made on the gravid uteri of females euthanised on day 20 of gestation did not reveal any “remarkable” alterations indicative of adverse effects of HCA-SX on absolute uterus weight, number of: corpora lutea, implantations, live and dead implants, early and late resorptions, and post-implantation losses (%). Observations made on the litters of females euthanised on day 20 of gestation did not reveal any remarkable treatment-related alterations in litter size, number of foetuses, sex ratios and foetal weights. The group mean litter size from the highest-dose group of HCA-SX was significantly lower than the control group (p <0.05). However, these observations deemed to be not of biological significance by the authors as the changes were smaller in magnitude compared to the variation observed in the historical control data.
177. Observations in the foetal groups are summarised. The number of foetuses examined in the control, 1,000, 3,000 and 10,000 ppm dose groups were 226, 227, 158 and 160, respectively. The only “major malformation” was an omphalocele [birth defect, where the intestine or other abdominal organs stick out of the belly button], which was found in two foetuses: one in the control and high-dose groups. This was considered incidental by the authors. Two other “minor malformations” were observed. An incidence of a small haematoma at the tip of the tail in three foetuses: one in the control, low-dose and mid-dose groups. One foetus in the high-dose group was small (runt). No treatment-related significant incidence of soft tissue alterations in foetuses of dams exposed to HCA-SX at the tested doses were noted (n=108, 106, 74, 76 for the control and each HCA-SX dose groups, respectively). Small incidences of “minor anomalies” were observed including, globular heart and unilateral enlargement of the ventricle of the heart, mottled lungs, unilateral displacement of adrenal, and unilateral hypoplasia of kidney in foetal soft tissues. These were considered as incidental and of no toxicological significance by the authors. The only abnormal finding classified under “major anomalies” was unilateral cerebral hypoplasia observed in one of the foetuses in the low-dose group; this was considered to be incidental by the authors, in light of its isolated incidence (0.94%). There was no incidence of any significant and treatment related skeletal abnormalities in foetuses of dams exposed to HCA-SX. Any abnormalities were considered incidental or of no toxicological significance due to either being a normal variant, minor in nature or the incidence was in isolation and/or comparable to the control dose group.
178. The authors concluded that HCA-SX was not found to be teratogenic in Sprague-Dawley rats at dietary exposure levels of 1,000, 3000, and 10,000 ppm, equivalent to the dose levels of 103, 352, or 1,240 mg/kg/day, respectively. Based on the results of the study, the NOAEL of HCA-SX was determined by the authors at 1,240 mg/kg per day.
Mode of action
179. The cause of hepatotoxicity from G. cambogia is unclear. In vitro studies suggest that HCA may be toxic to the liver in high doses, but the rare instances of acute liver injury that occur with G. cambogia suggest an idiosyncratic form of injury. The possibility of mislabelling or adulteration with hepatotoxic herbal products has been identified as an issue in herbal related injury.
180. In the literature review by Crescioli et al., (2018), it was noted that HCA mechanism of toxicity is not clearly defined; however, HCA increases hepatic collagen accumulation, lipid peroxidation, mRNA levels of genes related to oxidative stress (superoxide dismutase and glutathione peroxidase), and inflammatory responses (tumour necrosis factor- α and monocyte chemoattractant protein-1). It was suggested that certain patients could have genetic predisposition leading to hepatotoxicity, such as cytochrome P450 polymorphisms promoting toxic accumulation of metabolites. The information suggested that there is a potential causal relationship between G. cambogia product exposure and development of herb induced liver injury (HILI); however, these were limited by the lack of data on factors influencing the severity of HILI, especially for cases derived from the literature.