Annex A to TOX/2025/26 - First draft statement on the risk for T-2 and HT-2 mycotoxins in food
Background
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
1. The mycotoxins T-2 and HT-2 were previously assessed by the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) in 2018 (COT, 2018) and 2021 (COT, 2021), reviewing their presence in the diet of infants and young children and the potential implications of combined mycotoxin exposure, respectively.
2. In 2020, the European Commission (EC) proposed establishing maximum levels (ML) for the mycotoxins T-2 and HT-2 in foods, which were lower than the current indicative levels set out in the European Commission Recommendation 2013/165/EU. Following the proposal, maximum legislative levels came into force in the European Union (EU) on the 1st of July 2024. These maximum levels were established for the sum of T-2 and HT-2 toxins only. Maximum levels were not established for the modified forms of T-2 and HT-2 (such as neosolaniol (NEO) or 4,15-diacetoxyscirpenol (DAS)) due to limited occurrence data, and the absence of a suitable routine method available for their analysis.
3. In light of the new EU maximum levels, the COT was asked by the Food Standards Agency (FSA) to assess the risk to UK consumers from T-2 and HT-2 in foods. As part of this work, the COT considered “the existing health-based guidance values (HBGVs) for T-2 and HT-2 mycotoxins set by the European Food Safety Authority (EFSA) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA)” in February 2023 (TOX/2023/04).
4. To assist the COT with the assessment of the risk of T-2 and HT-2 from food, the FSA and Food Standards Scotland (FSS) undertook a call for evidence from July 2023 to October 2023. The data call focussed on the collection of data from the cereals supply chain, from field to retail level. While T-2 and HT-2 have been detected in products of animal origin (POAO), likely as a result of contamination of feed (EFSA, 2017c), this data call did not include occurrence data for meat and dairy products and hence they have not been included here. A discussion paper, focussing on the exposure from T-2 and HT-2 was presented to the COT in July 2024 (TOX/2024/24) and in March 2025 (TOX/2025/14), following feedback from the Committee.
5. This statement discusses the risk for T-2 and HT-2 mycotoxins in food, focussing on the exposure from the consumption of cereal grains and, where available, products thereof.
Introduction
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Type A trichothecenes
6. T-2 and HT-2 are type A trichothecenes which are produced by a variety of Fusarium and other fungal species. Fusarium species grow and invade crops and produce T-2 and HT-2 under cool, moist conditions prior to harvest. They are found predominantly in cereal grains, and in particular oat grain, barley grain and wheat grain and products thereof (JECFA, 2016).
7. The chemical structures of T-2 and HT-2 are shown below in Figure 1.
Figure 1. Chemical structures of T-2 and HT-2 (PubChem, 2025).
Occurrence data
8. As part of this assessment, occurrence data on T-2 and HT-2 in food were acquired through a nationwide call for evidence (FSA, 2023). This call was issued by the FSA and FSS in July 2023 and officially closed in October 2023. However, the FSA and FSS continued to receive data up until February 2024. The data call focussed on cereals both pre- and post- cleaning/dehulling and finished products including, where possible, data that spans multiple years to reflect any annual variability of T-2 and HT-2 levels. The data received covered the UK harvest seasons from 2004 to 2023. Sampling data at retail level were also submitted for 2013 (n=60), 2014 (n=60) and 2024 (n=90).
9. The FSA and FSS received occurrence data on T-2 and HT-2, either as a sum or as individual mycotoxins. The level of detail provided by the respondents and the format varied, but the data included occurrence levels in processed and unprocessed cereal grains, cereal products and a small number of Ready to Eat (RTE) foods. The occurrence data submitted to the FSA and FSS were predominantly on unprocessed/raw materials, which were yet to undergo any cleaning. Occurrence data on grains submitted by industry as ‘already processed’ refers to grains that have been dehulled and cleaned, but remain as a commodity, that is they have not been incorporated in any RTE foods. Submitted data on RTE foods included biscuits, rusks and cookies, extruded cereal seed or root-based products, cereal bars, infant formula milk-based powder, oat porridge, muesli, mixed breakfast cereals, bread and rolls.
10. The data were collated, cleaned and assured within the FSA Exposure Assessment and Trade (EAT) team. The quality assurance (QA) methodology aligned with the main principles outlined in the aqua book (UK HM Treasury, 2015) and the guidelines in the government data quality framework (UK Governement Data Quality Hub, 2020) on data quality rules.
11. Prior to the data cleaning, a verification exercise was undertaken by the FSA to account for missing limit of quantification (LOQ) and/or limit of detection (LOD) values and sample type categorisation. For these amendments, assumptions were made based on the descriptors and values included by the submitters, such as the descriptors provided for commodity types based on the sample identification codes. The following criteria were applied to include data without compromising scientific integrity. Data were included when all of the following criteria were met:
a. Datapoints with reported LOQ > 0.
b. Datapoints where the FoodEx (EFSA, 2025) code could be defined.
c. Sample codes referring to products destined for human consumption (not feed).
12. For grains, only data on the sum of T-2 and HT-2, which were analytically determined in samples, were considered in the exposure assessment to allow for a direct comparison with the group HBGV (which is for the sum of both mycotoxins). For RTE products all reported values were considered, including individual T-2 or HT-2 occurrence data, due to the limited data available.
13. To estimate the median lower bound (LB) sum of T-2 and HT-2, values that were at or below the LOQ were assumed to be zero. To estimate the median upper bound (UB) occurrence levels, values that were at or below the LOQ were assumed to be at the LOQ; values above the LOQ were used as reported.
Seasonal variability
14. The presence of T-2 and HT-2 in crops is dependent on the weather at key growth stages such as flowering and can demonstrate large annual variability. While there are good agricultural practices deployed to manage the presence of mycotoxins in general, they have not proven to be effective for T-2 and HT-2, given the large dependence on climate/weather. Similarly, reliable rapid testing is not currently available. Currently, liquid chromatography mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS) are the primary techniques employed for testing T-2 and HT-2 contamination levels. However, recent assessments by industry see large variability between the methods developed, and performance characteristics such as limits of detection (LODs) and limits of quantification (LOQs) are often lacking (Safefood, 2024). This makes it difficult to reliably detect these toxins in samples. In addition, currently available test kits would not be ‘fit for purpose’ (Safefood, 2024) as rapid tests must be accurate, reproducible and provide the required sensitivity for regulatory compliance.
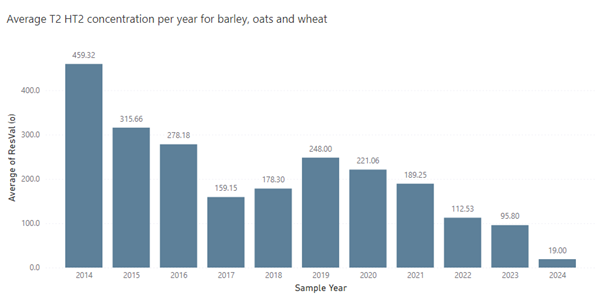
15. The data from the call for evidence covers the years 2004-2024, which spans a period either side of the EU recommendation from 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products (Commission Recommendation 2013/165/EU). Generally, the highest average levels of the sum of T2 and HT-2 from the data call were reported in the years 2008 to 2014, with lower levels being detected thereafter. The year 2014 is still recognised as a year with a particularly high prevalence of T-2 and HT-2, which could be attributed to seasonal variation, highlighting the importance of reviewing levels across a longer period of time.
16. Figure 2 provides time-trend analyses for the sum of T-2 and HT-2 in three cereal grains (barley, oats and wheat, both unprocessed grain and processed grain) from data submitted via the call for evidence. The average values in this graph are the averages of the median values per year. The year-on-year variability and seasonal trend provides an indication of the degree to which the presence of mycotoxins was impacted by climatic events at key stages of crop growth. To get a more representative, yet still retrospective analysis of current exposure patterns, only the last 10 years of residues data are included in this figure (2014- 2024); this excludes the data from before 2013, the year the initial food safety recommendation came into force. The Committee agreed that the temporal trend analysis of T-2 and HT-2 residues from 2004 to the present demonstrated an overall decline in the levels of these mycotoxins in unprocessed and processed cereal grains (oats, wheat and barley).
*ResVal(o) – concentration in µg/kg.
Figure 2. Average sum of T-2 and HT-2 concentration per year for ‘all grains’ (processed and unprocessed grains of barley, oats and wheat).
Reduction factors for unprocessed cereal grains
17. Unprocessed oat grains intended for human consumption comprise of an outer hull which is the part of the grain which is often most contaminated. However, this outer hull is removed during processing, and this so-called ‘de-hulling’ process therefore significantly reduces the level of contamination.
18. A literature search was conducted to identify any information on the reduction of T-2 and HT-2 mycotoxins in cereal grains during processing. A ‘reduction factor’, when used in exposure calculations, takes into account the expected decrease in T-2 and HT-2 levels in unprocessed cereal grains once they are processed, i.e. de-hulled. Applying reduction factors would therefore allow for a more accurate representation of consumer exposure to T-2 and HT-2 and result in a more realistic exposure assessment. Several reduction factors for the sum of T-2 and HT-2 for oat grains were identified in the scientific literature ranging from 66 to 100 % (Meyer et al., 2022; Schwake-Anduschus et al., 2010; EFSA, 2011a; Pettersson 2008). For this assessment, a reduction factor of 85 % from Meyer et al. (2022) was applied; this means that all T-2 and HT-2 occurrence values for unprocessed grains were reduced by 85 %.
19. The factor of 85 % was chosen as it was the most scientifically robust as well as from the most recently conducted study. Although the reduction factor of 85 % was specifically for large oat kernels, Meyer et al. (2023) noted that “milling oats are traded to contain less than 10 % of thin oats below 2 mm slotted hole sieve”; therefore, this reduction factor was considered to be of relevance for this exposure assessment.
20. As some cultivars of oat and barley are hulless, Polišenská et al. (2020) noted that “special attention should be paid to the risk of their contamination by Fusarium mycotoxins, as the rate of mycotoxin reduction during processing could be much lower than that for hulled cereals”. However, in the UK, hulless cultivars of oats are typically used for animal feed and not for human consumption.
21. No reduction factors were identified for maize or barley. The limited information available suggested that starting levels and incidence of T-2 and HT-2 in wheat and maize were very low and hence limited data were available on their fate or how their levels change during manufacturing of retail products (Scudamore, 2009). One publication by Pascale et al. (2011) calculated an overall reduction of T-2 and HT-2 toxins by 54 % following the processing of durum wheat. However, the samples used in this study were artificially inoculated with Fusarium, and as such the high concentrations of T-2 and HT-2 in this study are unlikely to reflect concentrations under natural conditions. Furthermore, the percentage reduction might not be linear and might be less at lower levels of expected contamination. Given the limited information it is therefore unclear whether, or to which percentage, processing reduces T-2 and HT-2 contamination in wheat, maize or barley under natural conditions, though it is expected to be negligible.
Toxicity
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
22. The toxicity of T-2 and HT-2 has been reviewed previously by EFSA (2011a, 2017c), JECFA (2002, 2016, 2022) and the SCF (2002). All Committees agreed that these trichothecenes had both acute and chronic effects. An acute refence dose (ARfD) is the estimated amount of a substance in food or drink that can be ingested in a single meal or day without appreciable health risk to the consumer. The tolerable daily intake (TDI) is the estimated amount of a substance that can be ingested daily over a lifetime without posing a significant health risk.
23. The primary chronic effects of T-2 and HT-2 toxicity are haematotoxicity, immunotoxicity and reduced body weight gain. The primary acute effect of T-2 and HT-2 toxicity is emesis, where the effect is Cmax-dependent (related to peak concentration). Both acute and chronic effects occur in a similar dose range (1.8 – 3.3 µg/ kg bw), but the perceived difference in sensitivity to acute or chronic effects arises from different uncertainty factors which have been applied when deriving the corresponding HBGVs.
24. T-2 and HT-2 also demonstrated dermal toxicity, developmental and reproductive toxicity, and neurotoxicity, however these effects occurred at lower concentrations.
Toxicokinetics
25. The toxicokinetics of T-2 and HT-2 have been reviewed previously by JECFA (2001) and EFSA (2017a). In summary, there is very little information on the in vivo absorption of T-2 and HT-2 in animals after oral administration. Rapid absorption has been confirmed by the excretion of total radioactivity in rats within 48 hours after oral gavage. T-2 radioactivity was rapidly distributed to the liver, kidney and other organs without accumulation in any organ in orally dosed rats and mice. When tritiated T-2 was administered directly into the small intestine of male rats, 40 to 57 % of radioactivity was found in bile and blood suggesting an extensive hydrolysis to HT-2 and other metabolites during the rapid intestinal absorption of T-2 (EFSA, 2017a).
26. The metabolism of T-2 and HT-2 in humans and other species is complex and was previously reviewed by EFSA (2011a). In brief, phase I metabolites arise from either hydrolysis of ester groups, hydroxylation, or de-epoxidation. These reactions may also occur in combination. In 2017, EFSA decided to review new relevant data on T-2 and HT-2 and noted that glucuronides are the most prevalent mammalian phase II metabolites of T-2 and HT-2 (EFSA, 2017a).
27. In 2022, EFSA reviewed the toxicokinetics and fate of T-2 and HT-2 in ruminants. The Panel noted that: “Results of in vivo studies with cows point to a rapid absorption, an extensive biotransformation to several less toxic metabolites and a rapid excretion of the parent compound and its metabolites, with negligible tissue accumulation and transfer to milk” (EFSA, 2022). Therefore, accumulation of T-2 and HT-2 in animal tissues and milk is not expected to occur at a level of significance.
HBGVs
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
EFSA’s group acute reference dose (ARfD)
28. In 2017, EFSA established a group ARfD of 0.3 μg/kg body weight for T-2, HT-2, and neosolaniol (NEO), based on a study by Wu et al. (2016) examining emesis in mink. Minks were selected as a suitable model for human vomiting due to their similar response to emetics like emetine. In the study, fasted female mink were administered varying oral and intraperitoneal doses of T-2 and HT-2, and emetic responses were monitored. The lowest oral dose causing vomiting was 0.05 mg/kg bw (75 % affected), with a NOAEL of 5 μg/kg bw, LOAEL of 50 μg/kg bw, and ED50 of 20 μg/kg bw.
29. EFSA performed a benchmark dose (BMD) analysis using PROAST software and selected a BMDL10 of 2.97 μg/kg bw. Applying an uncertainty factor of 10 for intraspecies variability (but none for interspecies differences due to similar emetic sensitivity between mink and humans), EFSA derived a group ARfD of 0.3 μg/kg bw. NEO was included based on equipotency data in ducklings. EFSA assumed dose additivity between T-2, HT-2, and their modified forms but noted the possibility of antagonistic or, less likely, synergistic effects of their co-occurrence.
30. The COT agreed with EFSA’s ARfD in 2018 but raised concerns about the wide BMD confidence interval, lack of interspecies factor for toxicokinetics, and the exclusive use of female mink. A later update to the PROAST software generated a higher model-averaged BMDL10 of 12.2 μg/kg bw, but due to uncertainties around model averaging, the more conservative EFSA value was retained.
EFSA’s group TDI
31. In 2017, EFSA established a group tolerable daily intake (TDI) of 0.02 μg/kg body weight (bw) for the sum of T-2, HT-2, and NEO toxins. This decision was based on their structural similarities, similar toxicological profiles, and the fact that HT-2 is a direct metabolite of T-2. EFSA applied relative potency factors (RPFs) of 1 for T-2 and HT-2, and 0.3 for NEO, using mainly in vivo data and a conservative rounding approach.
32. The TDI was derived using data from a 90-day rat study by Rahman et al. (2014), in which male Wistar rats were fed T-2 at doses of 0, 45, 68, or 90 μg/kg bw/day. The study reported dose-dependent reductions in white and red blood cells and platelets, along with clinical signs of toxicity. EFSA selected total leucocyte count as the critical endpoint and derived a BMDL10 of 3.3 μg/kg bw/day, applying a total uncertainty factor of 200 (10 for interspecies and 10 for intraspecies variability, and 2 for subchronic to chronic extrapolation).
33. EFSA had previously proposed a TDI of 100 ng/kg bw/day in 2011 based on a pig study by Rafai et al. (1995), but the Rahman study was considered more relevant due to its longer duration and clearer haematological effects. EFSA also included phase I metabolites in the group TDI, assuming dose addition, and applied RPFs accordingly.
34. EFSA however noted a number of uncertainties including the use of a subchronic study to set a chronic TDI, the lack of repeated-dose studies on HT-2, and the unspecified purity of the test material.
35. The COT endorsed EFSA’s group TDI in 2018 during their review of infant and young child exposure.
JECFA’s group ARfD
36. In April 2022, JECFA agreed that emesis is a common effect of acute trichothecene exposure in both humans and experimental animals. On this basis, the Committee established a group ARfD for T-2, HT-2 and DAS. JECFA applied the lower 95 % confidence limit on the benchmark dose for a 10 % response (BMDL10) of 2.6 μg/kg bw for emesis in mink following acute gavage exposure to T2 or HT2 as the point of departure (POD). Based on the available evidence, the Committee decided that an uncertainty factor of 8 (2.5 for interspecies variability in toxicodynamics and 3.16 for intra-human variability in toxicodynamics) was sufficiently protective.
37. Based on the above, JECFA established a group ARfD for T2, HT2 and DAS of 320 ng/kg bw (rounded down). Considering the highly comparable nature of the methods used in studies concerning the emetic effects of T2, HT2 and DAS in mink, the Committee recommended a relative potency factor of 0.2 for acute exposure to DAS.
JECFA’s group TDI
38. In April 2022, JECFA established a group TDI of 25 ng/kg bw for T2, HT2 and DAS, alone or in combination. JECFA concluded that the most sensitive, reliable and reproducible effects observed following repeated dietary exposure were reported in a 3-week toxicity study in juvenile pigs (Rafai et al., 1995). This study adequately characterised the test material and background exposure to common mycotoxins detected in feed and examined critical toxicological effects at relatively low doses (<25 µg/kg bw per day). JECFA also noted that juvenile pigs have been identified previously as a species sensitive to the emetic and haematotoxic effects of trichothecenes. Dose-response analysis of body weights, daily body weight gain and daily feed intake were conducted, and a BMDL10 of 1.8 µg/kg bw per day based on reduced daily body weight gain was selected as the most appropriate POD for establishing a group TDI. Considering that the critical effect (i.e. nausea-induced reductions in feed intake resulting in decreased body weight gain) is likely to be dependent on Cmax (the maximum concentration in plasma), and given JECFA’s low confidence in the overall toxicological database, a composite uncertainty factor of 72 was considered appropriate (8-fold for the group HBGV, 3-fold for extrapolation from subacute to chronic exposure, and 3-fold for other uncertainties in the database).
39. Although comparative longer-term data on T2, HT2 and DAS are not available, JECFA concluded that the relative potency factor of 0.2 for DAS was applicable for exposure durations longer than acute, (due to the similar critical effects observed following acute and repeated oral exposures), and hence should be applied in comparing dietary exposure to DAS with the group TDI.
COT HBGVs
40. In February 2023, in reviewing the existing HBGVs for T-2 and HT-2 mycotoxins set by EFSA and the Joint FAO/WHO Expert Committee on Food Additives (JECFA), the COT was content to continue applying EFSA’s HBGVs for future risk assessments.
Exposure Assessment
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
41. Exposures of T-2 and HT-2 in the population were estimated from consumption of cereal grains in the diet. However, as the occurrence data were predominantly from unprocessed grains, the approach to assessing exposure in foods as consumed is described below.
Methodology
42. Exposure assessments were conducted on a survey population basis using food consumption data and the corresponding LB and UB median occurrence values. Median occurrence levels were calculated for the sum of T-2 and HT-2 toxins (µg/kg) to avoid skewing the overall exposure, due to the wide concentration range of the reported occurrence levels. This was applied to all grains and the exposure “scenarios” for i) oat grains only, ii) all grains (oats, wheat, and barley), and iii) RTE foods.
43. A single food group was created in the National Diet and Nutrition Survey (NDNS) for estimating exposure to the sum of T-2 and HT-2 from consumption of oat grains only. Exposure to the sum of T-2 and HT-2 from this food group was estimated from NDNS consumption data, using occurrence estimates under the following scenarios:
- unprocessed oat grains,
- unprocessed oat grains after application of a reduction factor of 85 %,
- processed oat grains (submitted by industry as ‘already processed'); and,
- “oats combined” (the amalgamation of the occurrence data described in the second and third bullet points above).
44. Additional food groups were created for estimating exposure to the sum of T-2 and HT-2 from consumption of cereal grains other than oat grains, no scientifically robust reduction factors were identified for these cereal grains. In addition, median occurrence values from the data here were below the LOQ, hence the application of a reduction factor would not be expected to affect exposure estimates. The following scenarios were applied:
a) unprocessed wheat grains,
b) processed wheat grains,
c) unprocessed barley grains; and,
d) processed barley grains.
45. Acute and chronic exposures for all grains were estimated for the sum of T-2 and HT-2 (mean and 97.5th percentile).
46. For all RTE foods, the exposure assessments were on a consumer basis using mean and maximum occurrence levels as the datasets were not sufficient to calculate the median. Furthermore, for the majority of RTE foods, chronic and acute exposures to individual toxins (T-2 or HT-2 only) were calculated, as due to the data submitted by industry, occurrence data were only available for individual mycotoxins, but not their sum. The exception being infant cereal for which usable data were available for the sum of T-2 and HT-2 and hence estimated exposures to the sum of T-2 and HT-2 were calculated for this food group.
Results
47. While exposures from unprocessed oats only were very high, these exposure estimates are unlikely to reflect a real-life scenario. The very limited data from processed oats, as submitted by industry, showed significantly lower levels of T-2 and HT-2 than in unprocessed oats. Applying a reduction factor (85 %) to unprocessed oats (which constituted the majority of the data received from industry) significantly reduced the levels, and levels after application were similar to those from industry for processed oats. While this supported the use of the reduction factor of 85 %, it also supported combined oats (unprocessed oats plus reduction factor, and processed oats) as the most realistic exposure scenarios, for oats, besides RTE foods.
48. No reduction factors for unprocessed wheat or barley could be applied, and hence all grain exposure was based on the limited data available from processed wheat and barley, as submitted by industry, as well as oats combined. The data available showed that the overall exposure from grains, here, was driven primarily by exposures from oats.
49. Estimated exposures to the sum of T-2 and HT-2 from oats combined, all processed cereal grains (oats combined, wheat and barley), as well as RTE foods are presented in the following paragraphs. Full results of the exposure assessment can be found in the previous discussion paper (TOX/2025/14). Exposure estimates for T-2 and HT-2 in cereal grains were based on a commodity approach and calculated by using the median across the occurrence data. Exposure estimates for T-2 and HT-2 in RTE foods were calculated by using the mean and maximum occurrence level on a food-by-food basis, due to the limited number of samples. All exposure estimates used both the mean and 97.5th percentile consumption rates (across all age and food groups).
50. Exposures from i) all processed cereal grains (oats combined, wheat and barley) and ii) oats combined (only) were estimated for the following population groups: Infants (4-18 month-olds), toddlers (1.5-3 year-olds), children (4-10 year-olds), older children (11-18 year-olds), adults (19-64 year-olds), elderly (65+ year-olds), adult vegetarians (19-64 year-olds), and women of childbearing age (16-49 year-olds).
Oats combined and all processed grains
Chronic exposure
51. Across all population groups evaluated, the lowest chronic exposures for all processed cereal grains (oats combined, wheat and barley) occurred in older children (11-18 years), with mean and 97.5th percentile exposures of 0.0015-0.0039 µg/kg bw and 0.010-0.017 µg/kg bw, respectively while the highest chronic exposures were in infants (4-18 months) with mean and 97.5th percentile exposures of 0.0063-0.010 µg/kg bw and 0.039-0.052 µg/kg bw, respectively.
52. For oats combined, the lowest chronic exposures were in older children (11-18 years) with mean and 97.5th percentile exposures of 0.0015-0.0019 µg/kg bw (LB-UB) and 0.010-0.013 µg/kg bw (LB-UB), respectively, while the highest chronic exposures were in infants (4-18 months) with mean and 97.5th percentile exposures of 0.0063-0.0083 µg/kg bw (LB-UB) and 0.039-0.051 µg/kg bw (LB-UB), respectively. Toddlers (1.5-3 years) had similar exposures to infants.
Acute exposure
53. For all processed cereal grains (oats combined, wheat and barley), the lowest acute exposures were in women of childbearing age (16-49 years) with mean and 97.5th percentile exposures of 0.0033-0.0082 µg/kg bw and 0.020-0.034 µg/kg bw, respectively, while the highest acute exposures were in infants (4-18 months) with mean and 97.5th percentile exposures of 0.014-0.021 µg/kg bw and 0.078-0.10 µg/kg bw, respectively.
54. For oats combined, the lowest acute exposures were in women of childbearing age (16-49 years) with mean and 97.5th percentile exposures of 0.0033-0.0043 µg/kg bw (LB-UB) and 0.020-0.026 µg/kg bw (LB-UB), respectively. The highest acute exposures were in infants (4-18 months) with mean and 97.5th percentile exposures of 0.014-0.018 µg/kg bw (LB-UB) and 0.078-0.10 µg/kg bw (LB-UB), respectively. Toddlers have similar exposures to infants.
Exposure from ready to eat (RTE) foods
55. Consumer-based exposure estimates from RTE foods were generated for the following population groups: infants (4-18 months), toddlers (1.5-3 years), adults (19-64 years), and adult vegetarians/vegans (19-64 years).
56. The estimated exposures are the mean and 97.5th percentile exposures based on the mean and maximum concentration (mean-max concentration) of T-2 or HT-2 (separately) or the sum of both, where available. Exposures to T-2 or HT-2 (separately) were predominantly used as very few datapoints were available overall for RTE foods and even fewer on the sum of T-2 and HT-2.
Sum of T-2 and HT-2 exposure estimates
57. Data for the sum of T-2 and HT-2 were only available for infants’ cereals. In brief, the highest mean and 97.5th percentile exposures, both for chronic and acute were in infants (4-18 months). In infants, mean and 97.5th percentile chronic exposures were 0.36-0.71 µg/kg bw (mean-max concentration), and 1.5-2.9 µg/kg bw (mean-max concentration), respectively. Chronic exposure estimates in toddlers (1.5-3 years) ranged from 0.22 µg/kg bw (mean) to 1.4 µg/kg bw (97.5th percentile).
58. For acute exposure in infants, mean and 97.5th percentile estimates were 0.71-1.4 µg/kg bw (mean-max concentration), and 2.6-5.2 µg/kg bw (mean-max concentration), respectively. Acute exposure estimates in toddlers ranged from 0.52 µg/kg bw (mean) to 2.6 µg/kg bw (97.5th percentile).
T-2 or HT-2 exposure estimates (separately)
59. Where data on the sum of T-2 and HT-2 in RTE foods were too limited and did not meet the inclusion criteria, the data on individually reported levels of T-2 or HT-2 were used.
Chronic exposure estimates to T-2
60. The highest chronic exposure estimates to T-2 from RTE foods were from oat porridge in infants (4-18 months) with mean and 97.5th percentile exposures of 0.033-0.10 µg/kg bw (mean-max concentration), and 0.17-0.51 µg/kg bw (mean-max concentration), respectively. The lowest chronic exposure estimates to T-2 from RTE foods were from plain muesli in infants (4-18 months) with mean and 97.5th percentile exposures (of 0.00030-0.00043 µg/kg bw; mean-max concentration, and 0.001-0.0015 µg/kg bw; mean-max concentration, respectively).
Acute exposure estimates to T-2
61. The highest acute exposure estimates for T-2 from RTE foods were from oat porridge in toddlers (1.5-3 years), with mean and 97.5th percentile exposures of 0.11-0.34 µg/kg bw (mean-max concentration), and 0.27-0.85 µg/kg bw (mean-max concentration) respectively. The lowest acute exposure estimates to T-2 from RTE foods were from plain muesli in infants (4-18 months) with mean and 97.5th percentile exposures (of 0.00073-0.0011 µg/kg bw; mean-max concentration, and 0.0025-0.0036 µg/kg bw; mean-max concentration, respectively).
Chronic exposure estimates to HT-2
62. The highest chronic exposure estimates for HT-2 from RTE foods was from infants’ cereals, in infants (4-18 months), with mean and 97.5th percentile exposures of 0.70-0.71 µg/kg bw (mean-max concentration), and 2.9-2.9 µg/kg bw (mean-max concentration), respectively. The second highest chronic exposure estimates for HT-2 from RTE foods were from oat porridge, in infants (4-18 months), with mean and 97.5th percentile exposures of 0.057-0.27 µg/kg bw (mean-max concentration), and 0.28-1.4 µg/kg bw (mean-max concentration), respectively.
Acute exposure estimates to HT-2
63. The highest acute exposure estimates to HT-2 from RTE foods was from infants’ cereals, in infants (4-18 months), with mean and 97.5th percentile exposures of 1.4-1.4 µg/kg bw (mean-max concentration), and 5.2-5.2 µg/kg bw (mean-max concentration), respectively. The second highest chronic exposure estimates for HT-2 from RTE foods were from oat porridge, in infants (4-18 months), with mean and 97.5th percentile exposures of 0.13-0.61 µg/kg bw (mean-max concentration), and 0.46-2.2 µg/kg bw (mean-max concentration), respectively.
Risk characterisation
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
64. Trichothecenes, such as T-2 and HT-2 can cause chronic and acute adverse effects, with haematotoxicity and emesis being the critical effects, respectively. The COT confirmed in 2023 that they continue to apply the HBGVs established by EFSA: a group ARfD of 0.3 μg/kg bw for T-2, HT-2 and NEO and a group TDI of 0.02 μg/kg bw for T-2, HT-2 and NEO.
65. Following the EU’s decision to establish maximum levels for T-2 and HT-2 in specific foods, the COT was asked by the FSA and FSS to provide an assessment to determine the risk to human health in the UK from T-2 and HT-2 exposure from grains and grain products. To assist with the assessment the FSA and FSS undertook a call for evidence in 2024. NEO was not included in the call for evidence and has not been further considered here.
66. The statement provides an updated exposure assessment for UK consumers, following the call for evidence, data cleanup and the application of a reduction factor for unprocessed oat grains, to provide a more relevant exposure of UK consumers from oat grains, barley grains and wheat grains. The estimated exposures were compared to their respective HBGVs to assess acute and chronic health risks of UK consumers. The limited data on RTE foods were also included in the assessment.
67. It should be noted that the database for processed wheat and barley was relatively small and that processed oat grains here would be oats combined, i.e. unprocessed oat grains to which a reduction factor has been applied plus the limited data on processed oats industry submitted. The reduction factor of 85 % was selected from the literature and while supported by the limited data submitted by industry for processed oat grains, could significantly vary, potentially leading to an underestimation of risk, especially in hot spots of T-2 and HT-2 occurrence.
Oats combined and all processed grains
68. All chronic exposure estimates for oats combined were below the TDI of 0.02 μg/ kg bw/ day, with a few exceptions: high (97.5th percentile) exposure estimates for infants and toddlers (exceedance 2- to 3-fold; LB-UB), children aged 4-10 years (exceedance 2-fold; UB), whilst high (97.5th percentile) consumer vegetarians had exposures approximately equal to the TDI.
69. For all processed grains (i.e. oats combined, processed barley and processed wheat), mean exposures are below the TDI for all population groups assessed, indicating no health concern. However, 97.5th percentile exposure estimates for infants, toddlers and adults (exceedances of up to 3-fold of TDI; UB), and the elderly (exceedance of up to 4-fold of the TDI; UB) are of potential toxicological concern, while 97.5th percentile exposure estimates for children 4-10 years old and adult vegetarians (up to 2-fold; UB) are undesirable but unlikely to result in health concerns.
70. Acute exposure estimates for both oats combined, and all processed grains are below the ARfD across all population groups assessed, both at mean and high consumption, and are therefore not of toxicological concern.
Ready to Eat (RTE) foods
71. Data for the sum of T-2 and HT-2 in RTE foods were only available for infants’ cereals. Hence, exposure estimates were only calculated for infants (4-18 months) and toddlers (1.5-3 years). All chronic exposures exceeded the TDI, the lowest exceedance was 11-fold (mean occurrence with mean consumption rate) in toddlers, while the highest exceedance was 145-fold (max occurrence with 97.5th percentile consumption rate) in infants.
72. Chronic exposures to T-2 (only) were at the TDI in toddlers for high intakes (97.5th percentile) for wheat bread rolls, while mixed breakfast cereals resulted in exceedances up to 3-fold the TDI. Oat porridge exceeded the TDI in all groups and exposure scenarios, ranging from 2-fold (mean occurrence with 97.5th percentile consumption rate) to 8-fold (max occurrence with 97.5th percentile consumption rate) in adults and vegetarians, and 2-fold (mean occurrence with mean consumption rate) to 26-fold (max occurrence with 97.5th percentile consumption rate) in infants and toddlers.
73. Chronic exposures (97.5th percentile) to HT-2 (only) resulted in exceedances of the TDI in most RTE foods for infants and toddlers, and plain muesli and oat porridge in adults and vegetarians. Overall, exceedances in oat porridge were highest, with exceedances being 2-fold (mean occurrence with 97.5th percentile consumption rate) to 22-fold in adults and vegetarians (max occurrence with 97.5th percentile consumption rate), and 3-fold (mean occurrence with mean consumption rate) to 70-fold (max occurrence with 97.5th percentile consumption rate) in infants and toddlers.
74. Chronic exposures from RTE foods suggest a concern to consumer health, especially in infants and toddlers, however also for some foods in adults and vegetarians, mainly oat porridge. However, the submitted data on RTE foods is very limited; on average, sample numbers were <5, in the case of oat porridge <25. In addition, exposure estimates also depend on whether they were calculated using the sum of T-2 and HT-2 or individual mycotoxins. While the estimated exposures may be an indication of potential foods of concern, they were subject to a high degree of uncertainty. Hence, the exposures may not be representative. The large exceedances of the TDI that have been derived from RTE foods (22-, 26-, and 70-fold) only occur when using the maximum occurrence with the 97.5th percentile consumption rate. It is unlikely, that individuals would be exposed to foods at these levels continuously throughout their life, given the seasonable variability in T-2 and HT-2 occurrence levels. The mean occurrence level combined with the mean consumption rate may therefore be more appropriate for assessing a realistic chronic exposure; these exposure estimates are much lower, indicating a lower risk.
75. Acute exposures to the sum of T-2 and HT-2 from RTE foods exceeded the ARfD, ranging from 2-fold (mean occurrence with mean consumption rate) to 17-fold (max occurrence with 97.5th percentile consumption rate) in infants, and 2-fold (mean occurrence with mean consumption rate) to 9-fold (max occurrence with 97.5th percentile consumption rate) in toddlers. Data were only available for infants’ cereals, hence only these two age groups have been considered.
76. Acute exposures to T-2 (only) from RTE foods were all below the ARfD, except for oat porridge in infants and toddlers with exceedances of up to 3-fold the ARfD (max occurrence with 97.5th percentile consumption rate). For HT-2 (only), 97.5th percentile adult consumers (max occurrence) had exposures equal to the ARfD, whilst exposures of vegetarians exceeded the ARfD 3-fold (maximum occurrence with 97.5th percentile consumption rate). For HT-2 (only), infants and toddlers exceeded the ARfD by 2-fold (mean occurrence with 97.5th percentile consumption rate) to -7-fold (max occurrence with 97.5th percentile consumption rate) from oat porridge, whilst exceedances ranging from 3-fold (mean occurrence with mean consumption rate) to 17-fold (max occurrence with 97.5th percentile consumption rate) of the ARfD occurred from infants’ cereals.
77. While exceedances of the ARfD for adults, especially vegetarians are undesirable, it is unlikely that an occasional exceedance would result in a concern for health. Exceedances in infants and toddlers could potentially be of concern, if exposures were to occur at this level (in potential hotspots), however, the sample number for oat porridge was < 25 and may not be representative.
78. Comparing the exposure estimates from grains and RTE foods, RTE foods result in higher exposures to T-2 and HT-2, compared to processed oats or even unprocessed oats. However, given the small data set for RTE foods, the use of individual mycotoxins and mean and maximum occurrence levels adds significant uncertainty to these exposures. This stresses the need for a sufficiently large dataset to provide a reliable exposure assessment from RTE foods. The COT highlighted that the dataset for RTE foods here might not accurately reflect general exposure levels due to the limited number of data points and potential bias from targeted sampling where, for example, contamination was known or suspected.
Uncertainties and assumptions
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
79. The risk assessment for T-2 and HT-2 in food included a number of assumptions and uncertainties, which relate to the preparation of the occurrence data, the calculation of the consumption data and exposure assessment, as well as the risk assessment itself. These uncertainties are listed below in further detail.
80. Uncertainties associated with the preparation of the occurrence data:
a. When an LOD was not reported these data were included assuming all other acceptance criteria were met.
b. When a result value was not reported it was assumed to be equal to the LOQ (when LOQ > 0).
c. When a sample code description was not reported, the code was researched, and the description was filled in. Any changes to the codes over the years that the data covered would not be captured.
d. Food codes were grouped in food groups for the purpose of the assessment on the basis of the FoodEx descriptions of the codes. When in doubt assumptions were made as to which group the codes fitted best.
e. In the UK and Ireland, it is common for grain to be delivered to the mill ‘as harvested’ i.e. uncleaned and unprocessed with the husk still intact. Where mycotoxin contamination is associated with the outer layers of the grain this may exhibit higher levels of contamination. A large proportion of data submitted as part of the data call were from such unprocessed grains which therefore may exhibit higher levels of contamination compared to cleaned, processed grains. Thus, a reduction factor of 85% was applied to the sum of T-2 and HT-2 in unprocessed oat grains. It was assumed that this constitutes a realistic reduction, although different reduction factors have been reported in the literature, potentially over or underestimating the reduction and subsequent exposure.
f. No reduction factor was applied to unprocessed wheat and barley grains. The COT did not identify a scientifically robust reduction factor, however the occurrence data here for both unprocessed and processed forms also fell below LOQ. Hence the application of a reduction factor would not be expected to affect exposure estimates.
81. Uncertainties associated with the calculations of the consumption and exposure assessment estimates:
a. The description of food categories within the FoodEx food code system were not always aligned with the names given to similar foods in NDNS and DNSIYC. Therefore, some assumptions were made during the mapping of these foods to identify the closest match when searching the inhouse FSA recipes database for the most relevant food.
b. For the RTE food groups, in some cases, there are a limited number of consumers (<60) as well as a limited number of samples. This may lead to unreliable exposure estimates. Consumer numbers less than 60 (<60) should be treated with caution as they may not be true representation of the entire population.
c. Samples on sum of T-2 and HT-2 were only available for infant foods, for all other foods samples either T-2 (only) or HT-2 (only) were available.
d. For RTE food groups, there is uncertainty on whether concentrations were provided on a wet weight or dry weight basis, hence conversion factors were not applied while building the food groups. These include foods such as dried infant cereals and other dried food groups.
e. NDNS does not include pregnant or lactating women, therefore data for women of childbearing age (16-49 years) were used as a proxy and therefore may not be representative of the maternal diet.
f. The summation of exposures from individual grains, especially for acute exposures, is likely to overestimate actual exposure, particularly at the 97.5th percentile, as it is unlikely an individual would eat all grain foods in one single day, at that level.
82. Uncertainties associated with the risk assessment:
a. The exposure assessment only includes T-2 and HT-2 mycotoxins, however the group TDI and group ARfD established by EFSA also includes NEO. Uncertainty regarding the occurrence of NEO in cereal grains, as well as its exclusion from the exposure assessment might lead to an underestimation of total exposure and thus a possible underestimation of the corresponding health risk.
b. Exposure to T-2 and HT-2 were based on grains or products thereof only. Other potential sources of T-2 and HT-2, such as POAO were not considered.
c. For RTE foods T-2 or HT-2 only were compared to a HBGV based on the sum of both mycotoxins (plus NEO). While this may give an indication of exposure, it might not provide a realistic assessment may under-estimate the actual exposure.
d. T-2 and HT-2 occurrence in cereal grains is significantly influenced by climate and levels can vary significantly from year to year (as indicated in Figure 2). Year to year variability may mean that individuals could be exposed to high levels of T-2 and HT-2 in one year compared to other years.
Conclusions - Annex A to TOX/2025/26
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
83. The COT has been asked by the FSA and FSS to assess the risk to the UK population to T-2 and HT-2 statement from consumption of oat, wheat, barley and products thereof.
84. Based on the data received through the FSA and FSS call for evidence, chronic exposures to oats (combined) at the 97.5th percentile consumption rate were of toxicological concern in infants and toddlers, while exposures in children and vegetarians were undesirable but unlikely to result in health concerns. Chronic mean exposures and all acute exposures were not of toxicological concern. This is in line with the COT’s conclusion on the risk of T-2 and HT-2 in the infant diet (COT, 2018). Based on a 2015 mycotoxin survey of oat-based products (FSA, 2015), acute exposures were all below the EFSA group ARfD and therefore not of toxicological concern, while for chronic exposures the EFSA group TDI was exceeded. Hence, an effect on health could not be entirely excluded. This conclusion relates to the information described under “Oats combined and all processed grains” (paragraphs 68-70).
85. Chronic exposures from RTE foods suggest a significant concern to consumer health, especially in infants and toddlers, however also for some foods in adults and vegetarians, mainly oat porridge. While acute exposures in adults, especially vegetarians were undesirable, exceedances of the ARfD for infants and toddlers were of potential concern, if they were to occur at the levels reported. However, the estimated exposures were based on very limited data and were subject to a high degree of uncertainty. In addition, samples on sum of T-2 and HT-2 were only available for infant foods, for all other foods samples either T-2 (only) or HT-2 (only) were available. This conclusion relates to the information described under “Ready to Eat (RTE) foods” (paragraphs 71-78).
86. The exposure estimates from RTE foods are significantly higher than exposure estimates from processed oat grains, unprocessed oat grains, or all processed grains (oats combined, wheat, and barley grains). It is unclear why this is the case and may have been influenced by several factors and uncertainties in the data:
a) in terms of year-to-year variability, it was not possible to link the submitted data on RTE foods to an identifiably ‘bad’ year for T-2 and HT-2 levels,
b) the relatively small data set and use of mean and maximum occurrence values,
c) the assessment of individual mycotoxins rather than the sum; and,
d) RTE foods potentially being targeted samples for reports of high occurrence levels.
This conclusion relates to the information described under “Ready to Eat (RTE) foods” (paragraph 78).
87. The COT noted that industry testing from raw commodities to RTE foods, or more generally on finished products, would be useful to provide a more comprehensive dataset and more reliable exposure assessment. This conclusion relates to the information described under “Ready to Eat (RTE) foods” (paragraph 78).
88. Exposures to processed grains were based on a commodity approach and calculated by using the median across the occurrence data, while exposures to RTE foods, due to the limited number of samples, were calculated on a food-by-food basis and mean and maximum occurrence level. RTE foods only provide a very limited snapshot of exposures to final food products and direct comparison to exposures from all grains was therefore not possible. The analytical method used may further add to the uncertainties in the exposures from RTE foods, where a low level/non-detect was determined to be below LOQ, the LOQ was used as the occurrence level to estimate exposures. As some methods may not have been sensitive enough, with high LOQs this would have resulted in relatively high “occurrence levels”. Using the mean and max as well as individual mycotoxins, rather than the sum of T-2 and HT-2 added further uncertainties. The large exceedances of the TDI that have been derived from RTE foods (22, 26, 70-fold) only occur when using the maximum occurrence with the 97.5th percentile consumption rate. As such, it is unlikely that a consumer would be exposed to this level chronically. The mean occurrence level combined with the mean consumption rate would most likely be a more realistic exposure scenario with exposure estimates being lower, indicating a lower risk. This conclusion relates to the information described under “Ready to Eat (RTE) foods” (paragraphs 71-78).
89. While year-to-year variability of T-2 and HT-2 occurrence in cereal grains (as shown in Figure 2) may potentially affect acute exposures due to hot spots or a particularly bad year leading to occasional high exposures, chronic exposures to the sum of T-2 and HT-2 from grains were calculated on a commodity basis. Consumption was modelled based on all foods containing the grains and occurrence was calculated at the LB and UB median. Therefore, these were the most representative estimates of chronic exposure. This conclusion relates to the information described under “Methodology” (paragraphs 42-46).
90. Overall, based on the occurrence data provided via the call for evidence for processed grains (oat, barley and wheat) and the limited number of RTE foods, a health concern arising from chronic exposures, especially for infants and toddlers cannot be excluded. However, given all the uncertainties, the estimated exposures for RTE foods may not be reliable and not representative of RTE foods. To confirm this, the Committee recommended the acquisition of a larger dataset for the sum of T-2 and HT-2 in RTE foods. This conclusion relates to the information described under the sections “Risk characterisation” (paragraphs 64-78) and “Uncertainties and assumptions” (paragraphs 79-82).
Secretariat
July 2025
Abbreviations
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
|
ARfD |
Acute reference dose |
|
DAS |
4,15- diacetoxyscirpenol |
|
DNSIYC |
Diet and Nutrition Survey of Infants and Young Children |
|
ELISA |
Enzyme-Linked Immunosorbent Assays |
|
FPIA |
Fluorescence Polarisation Immunoassays |
|
HBGV |
Health-based guidance value |
|
HT-2 |
HT-2 toxin |
|
LB |
Lower bound |
|
LC-MS |
Liquid chromatography mass spectrometry |
|
LFDs |
Lateral Flow Devices |
|
LOD |
Limit of detection |
|
LOQ |
Limit of quantification |
|
Max |
Maximum |
|
ML |
Maximum levels |
|
NDNS |
National Diet and Nutrition Survey |
|
NEO |
Neosolaniol |
|
QA |
Quality assurance |
|
RTE |
Ready to eat |
|
RPC |
Raw primary commodity |
|
T-2 |
T-2 toxin |
|
TDI |
Tolerable daily intake |
|
UB |
Upper bound |
|
COT |
Committee on the Toxicity of Chemicals in Food, Consumer Products and the Environment |
|
EAT |
FSA’s Exposure assessment team |
|
EC |
European Commission |
|
EFSA |
European Food Safety Authority |
|
EU |
European Union |
|
FSA |
Food Standards Agency |
|
FSS |
Food Standards Scotland |
|
JECFA |
Joint FAO/WHO Expert Committee on Food Additives |
References - Annex A to TOX/2025/26
In this guide
In this guideThis is a paper for discussion. This does not represent the views of the Committee and should not be cited.
Bates B.D., Collins K., Jones P., et al. (2020) National Diet and Nutrition Survey Results from years 9, 10 and 11 (combined) of the Rolling Programme (2016/2017 to 2018/2019). Survey, London: Public Health England. NDNS: results from years 9 to 11 (2016 to 2017 and 2018 to 2019) - GOV.UK.
Bates B., Lennox A., Prentice A., et al. (2014) National Diet and Nutrition Survey: Results from Years 1, 2, 3 and 4 (combined) of the Rolling Programme (2008/2009 – 2011/2012). London: Public Health England. Main heading.
Bates B., Cox L., Page S., et al. (2016) National Diet and Nutrition Survey Results from Years 5 and 6 (combined) of the Rolling Programme (2012/2013 – 2013/2014). London: Public Health England. Main heading.
COT (2018) Statement of T-2 toxin (T2), HT-2 toxin (HT2) and neosolaniol (NEO) in the diet of infants aged 0 to 12 months and children aged 1 to 5 years. cotstatement-t2ht2andneosolaniol.pdf.
COT (2021) Statement on the potential risk(s) of combined exposure to mycotoxins. Combined exposure to mycotoxins report.
Croucher D. (2023) United Kingdom Oat Supply in the context of the Food Standards Agency / Food Standards Scotland Call for Data on T-2 and HT-2 Toxins. A Science & Evidence Based Review including additional data on UK Milling Barely. Confidential.
Department of Health (2011) “Diet and Nutrition Survey of Infants and Young Children, 2011.” Diet and nutrition survey of infants and young children, 2011 - GOV.UK.
EFSA (2011a) Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA Journal 9(12): 2481 Scientific Opinion on the risks for animal and public health related to the presence of T‐2 and HT‐2 toxin in food and feed.
EFSA (2011b) Use of BMDS and PROAST software packages by EFSA Scientific Panels and Units for applying the Benchmark Dose (BMD) approach in risk assessment. EN-113. pp190. Use of BMDS and PROAST software packages by EFSA Scientific Panels and Units for applying the Benchmark Dose (BMD) approach in risk assessment | EFSA.
EFSA (2017a) Appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA Journal 51(1): 4655.
EFSA (2017b) Update: use of the benchmark dose approach in risk assessment. EFSA Journal 15(1): 4658.
EFSA (2017c) Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA Journal 15(8):4972.
EFSA (2022) Assessment of information as regards the toxicity of T-2 and HT-2 toxin for ruminants. EFSA Journal 20(9): 7564.
EFSA (2025) Food classification standardisation – The FoodEx2 system. Food classification standardisation – The FoodEx2 system | EFSA.
FAO/WHO (2001) WHO Food Additive Series: 47. Safety evaluation of certain mycotoxins in food. T-2 AND HT-2 TOXINS (JECFA 47, 2001).
FSA (2015). Retail survey of T-2 and HT-2 toxin levels in oat based products. Executive Summary: fs102126execsum.pdf.
FSA (2023) Call for data: T-2 and HT-2 toxins in food Call for data: T-2 and HT-2 toxins in food | Food Standards Agency.
Gordon G. (1985) Ipecacuanha induced emesis in the treatment of self-poisoned adults. Archives of Emergency Medicine 2: 2. https://doi.org/10.1136/emj.2.4.203.
JECFA (2022) Summary of Conclusions of 93rd meeting of JECFA. 93rd Joint FAO/WHO Expert Committee on Food Additives (JECFA) - Food additives. Summary and conclusions. 2022.
JECFA (2023) Evaluation of certain contaminants in food: ninety-third report of the Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain contaminants in food: ninety-third report of the Joint FAO/WHO Expert Committee on Food Additives.
JECFA (2024) Safety evaluation of certain food contaminants: prepared by the ninety-third meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Geneva: World Health Organization and Food and Agriculture. Safety evaluation of certain contaminants in food: prepared by the ninety-third meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA).
Meyer J.C., Birr T., Hennies I., et al. (2022) “Reduction of deoxynivalenol, T-2 and HT-2 toxins and associated Fusarium species during commercial and laboratory de-hulling of milling oats.” Food Additives & Contaminants: Part A 39(6): 1163–1183.
Nathanail A.V., Varga E., Meng-Reiterer J., et al. (2015) Metabolism of the Fusarium Mycotoxins T-2 Toxin and HT-2 Toxin in Wheat. J. Agric. Food Chem. 63: 7862–7872
Pascale M., Haidukowski M., Lattanzio V., et al. (2011) Distribution of T-2 and HT-2 Toxins in Milling Fractions of Durum Wheat. Journal of Food Protection 74(10): 1700-1707.
Percie du Sert N., Holmes A.M., Wallis R., et al. (2012) Predicting the emetic liability of novel chemical entities: a comparative study. British Journal of Pharmacology 165: 1848-1867.
Pettersson H. (2008) T-2 and HT-2 toxins in oats and oat products. 5th EC Fusarium-Toxin Forum, Brussels, 10-11 January 2008.
Polišenská I., Jirsa O., Vaculová K., et al. (2020) Fusarium Mycotoxins in Two Hulless Oat and Barley Cultivars Used for Food Purposes. Foods, 9(8), 1037.
PubChem (2025) PubChem database. National Institutes of Health (NIH) | (.gov).
Rafai P., Tuboly S., Bata A., et al. (1995a) Effect of various levels of T2 toxin in the immune system of growing pigs. Vet. Rec. 136: 511-514.
Rafai P., Bata A., Vanyi A., et al. (1995b) Effect of various levels of T2 toxin on the clinical status, performance and metabolism of growing pigs. Vet. Rec.136: 485-489.
Rahman S., Sharma A.K., Singh N.D., et al. (2014) Clinico-haematological changes in T2 toxicosis in Wistar rats. Indian Journal of Veterinary Pathology. 38: 22-28.
Roberts C., Steer T., Maplethorpe N., et al. (2018) National Diet and Nutrition Survey Results from Years 7 and 8 (combined) of the Rolling Programme (2014/2015 to 2015/2016). Survey, London: Public Health England. National Diet and Nutrition Survey.
Safefood (2024) Mycotoxin control in cereals: safeguarding food. Technical Project Report. Mycotoxin control in cereals: safeguarding human food.
Schwake-Anduschus C., Langenkämper G., Unbehend G., et al. (2010) Occurrence of Fusarium T-2 and HT-2 toxins in oats from cultivar studies in Germany and degradation of the toxins during grain cleaning treatment and food processing. Food Additives and Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment 27: 1253-1260.
SCF (2002) Opinion of the Scientific Committee on Food on Fusarium toxins. Part 6: Group evaluation of T2 toxin, HT2 toxin, nivalenol and deoxynivalenol. Opinion of the Scientific Committee on Food on Fusarium toxins. Part 6: Group evaluation of T-2 toxin, HT-2 toxin, Nivaleno...
Ueno Y., Kenji I., Norio S, et al. (1974) Toxicological approaches to the metabolites of Fusaria. VI. Vomiting factor from moldy corn infected with Fusarium spp. Japanese Journal of Experimental Medicine 44: 123–127.
UK Government Data Quality Hub (2020) “The Government Data Quality Framework.” The Government Data Quality Framework - GOV.UK.
UK HM Treasury (2015) “The Aqua Book: guidance on producing quality analysis for government.” The Aqua Book: guidance on producing quality analysis for government.
Wu W., Zhou H., Bursian S.J., et al. (2016) Emetic responses to T2 toxin, HT2 toxin and emetine correspond to plasma elevations of peptide YY3-36 and 5-hydroxytryptamine. Archives of Toxicology. 90: 997-1007.
Zhang F., Wang L., Yang Z-H., et al. (2006) Value of mink vomit model in study of anti-emetic drugs. World J. Gastroenterol. 12(8): 1300-1302.