Risk ranking method
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.22. In the absence of data to establish HBGVs and conduct a risk assessment, a numerical risk ranking method was deemed appropriate to provide a measure of risk that policymakers can use to inform decisions on whether legislative standards should be updated or changed.
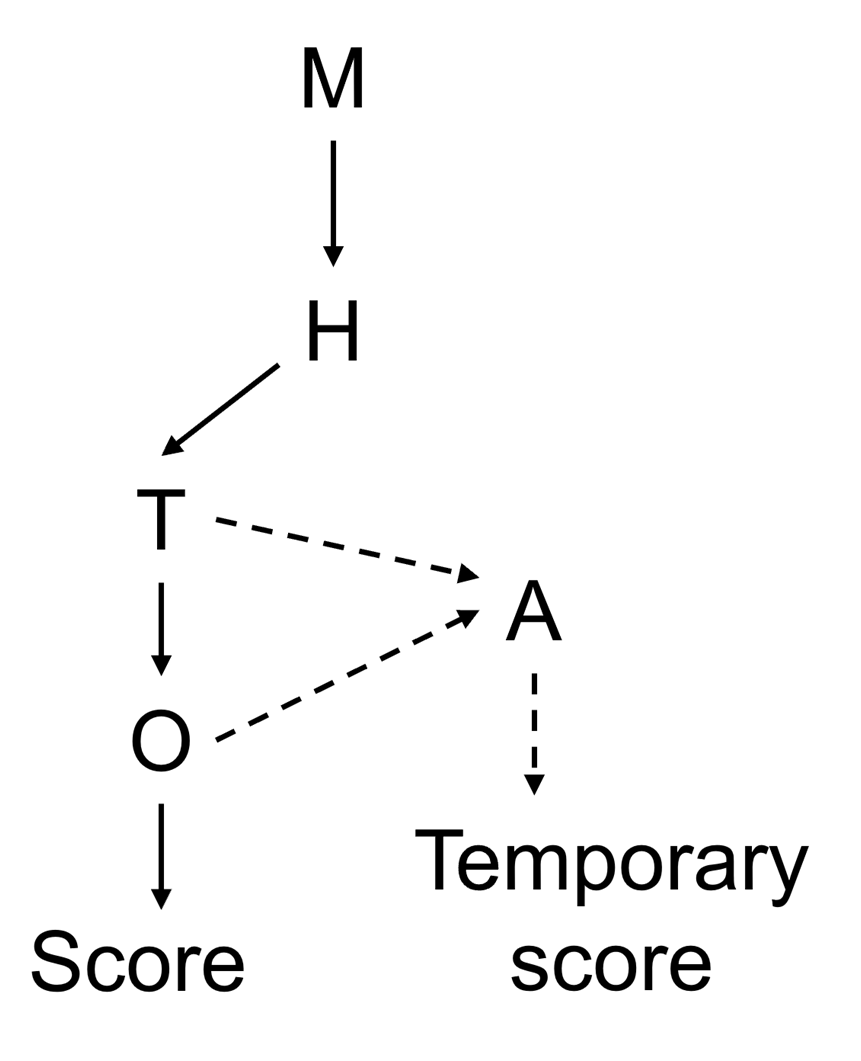
23. A decision tree was proposed to clearly depict the main considerations and to set out the amount of data available for each biotoxin, given the sometimes-limited database (Figure 1). Combining the decision tree with numerical scores for each step of the decision-making process clearly depicts the underlying considerations and the weighing of the data. The decision tree considered the following categories of information: monitoring, toxicological data, (i.e., human case reports and/or animal toxicity data), and occurrence data. Each group of emerging biotoxin was numerically scored on a scale of 1-5 for all categories generating a maximum score of 20 where higher scores represent a greater risk to public health. The considerations and weighing of evidence for each group of biotoxins were provided in tabular form. This was accompanied by a narrative explaining the underlying considerations and providing a transparent depiction of which data was driving the risk ranking.
24. An attempt at risk ranking novel AZAs, DA analogues and PtTX, groups which had insufficient data for any of the four categories, was attempted by comparison with structurally similar biotoxins (TOX/2025/15); however, the Committee concluded that this required further refinement before it could be used for the risk assessment of marine biotoxins. The read-across approach was retained in the decision tree (Figure 1) as a potential future method for creating temporary risk rankings for other biotoxins with limited information.
Figure 1. Risk ranking decision tree. M = monitoring; T = toxicity; H = human case reports; O = occurrence; A = analogue. Dashed lines represent the potential path for read-across using structurally similar toxins in the absence of data for T and/or O.
Monitoring
25. The score for Monitoring considered whether the toxins were included in any recent or ongoing official marine biotoxin monitoring programmes either in the UK or EU. Toxins which were extensively monitored were considered to have low risk and hence given the lowest score. Where only unofficial research monitoring programmes and monitoring in countries outside the EU was available, higher scores were allocated. Biotoxins for which monitoring was not conducted were considered to have the highest risk because the prevalence of the toxin, and therefore the risk, was unknown. In these instances, the highest score would be applied.
Human case reports
26. The score for Human case reports considered whether documented cases of human intoxication were available, their severity, and whether any fatalities had been reported. Higher scores were given to toxins for which both intoxications and fatalities have been reported and lower scores for toxins with reports of intoxications but no fatalities. The number of case reports was not considered because it was highly variable between toxins, and the information available, in general, was very limited. Toxins without information or reports of fatalities or intoxications were also given a score; however, please note that the absence of reports of intoxication/fatalities does not necessarily indicate that no intoxication (potentially even fatalities) have occurred. Underreporting has been noted as an uncertainty for marine biotoxins in general.
27. For this category there are only three scoring options because, due to the limited information available and associated uncertainties, it was not considered possible to distinguish them further. The scores have been designated 1, 3 and 5 to maintain an equal weighting of this category compared to the others.
Toxicity
28. The score for Toxicity considered the known adverse effects of each toxin, as identified from in vivo animal studies, usually in mice or rats. Neurotoxic effects were ranked highest followed by gastrointestinal effects and lastly mild effects such as weakness and general unwellness. A numerical score from 1-5 was applied to the endpoints described above and to the acute lethal dose (LD50). Whether a LD50 was considered ‘high’ or ‘low’ or rather ‘higher’ or ‘lower’, was, in this instance, determined qualitatively via the Committee’s judgement rather than quantitatively (i.e., specific LD50 ranges) due to the limited data available. The LD50s were considered to assist in differentiating toxicity profiles between biotoxins; however, they are based on a limited toxicological database leading to high uncertainty as to how much weight should be assigned to them.
Occurrence
29. The score for Occurrence considered documented cases of detection of these toxins either through official routine inspections, one-off incidents and/or research efforts. Detection in UK waters was ranked highest followed by Northern EU waters, as their temperature profile is similar to that of UK waters. Detection in Mediterranean EU waters was ranked lower because the water profile would be different to the UK’s however, this may change with climate change and increasing water temperatures. Detection outside the UK and EU was not considered here and would only be considered useful if no other data were available.
30. Scoring was conducted as follows:
- Monitoring (M):
o 5 points - no monitoring.
o 4 points - limited monitoring (in EU/UK).
o 3 points - moderately monitored (in some countries but not across all/UK).
o 2 points - extensive monitoring (EU/UK).
o 1 point - extensively monitored in the UK.
- Human case reports (H)
o 5 points - documented cases of human intoxications with fatalities.
o 3 points - documented cases of human intoxications without fatalities.
o 1 point - no documented cases.
- Toxicity (T):
o 5 points - causes severe neurotoxic effects with low LD50.
o 4 points - causes severe neurotoxic effects with relatively high LD50.
o 3 points - causes gastrointestinal effects with low to moderate LD50.
o 2 points - causes gastrointestinal effects with relatively high LD50.
o 1 point - causes mild other effects or high LD50 for other affects than the ones listed above.
- Occurrence (O):
o 5 points - frequently detected in UK waters or no data available.
o 4 points - occasionally detected in UK waters.
o 3 points - rarely detected in UK waters.
o 2 points - detected in Northern EU waters.
o 1 point - detected only in Mediterranean EU waters.
Read-across
31. Initially a read-across approach was proposed for scoring novel AZAs, DA analogues and PtTXs with no to very limited information available. The approach suggested using a structurally similar biotoxin (i.e., analogue) for which information was available to fill the data gaps and generate a temporary score. However, the Committee concluded that without evidence to show that, for example, the occurrence of one biotoxin directly relates to the occurrence of another, using analogues for all four scoring categories was associated with high uncertainty and would not result in a robust/appropriate score. The Committee suggested, however, that suitable read-across methods could be applied in the future, especially for the determination of hazard scores.
32. There was no to very limited data available for PtTX and novel AZAs in all categories; the Committee, therefore, considered it inappropriate to apply read-across here and did not include them in their final risk ranking.