Review of EFSA Opinion on the Reproductive Toxicity of Titanium Dioxide as a Food Additive
Introduction - Review of EFSA Opinion on the Reproductive Toxicity of Titanium Dioxide as a Food Additive
In this guide
In this guideThis is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Introduction
1. Titanium dioxide is an authorised Food Additive (E171) in the EU and under UK Food Law it is used in food as a colour to make food more visually appealing, to give colour to food that would otherwise be colourless, or to restore the original appearance of food. Titanium dioxide has been the subject of multiple safety evaluations.
2016 EFSA evaluation
2. In 2016, EFSA evaluated the safety of E171 and determined that it consisted mainly of micro-sized titanium dioxide particles, with a nano-sized (< 100 nm) fraction less than 3.2% by mass. Uncertainties around the identity and characterisation of E171 were highlighted, noting that no limits for the particle size of E171 were set. Similarly, with regard to toxicity, uncertainties around the identity and characterisation of E171 were also highlighted.
2019 EFSA re-evaluation
3. Specifications of E171 titanium dioxide were reviewed again in 2019. Based on the fraction of nanoparticles present in E171, it was determined that the food additive fell under the scope of the EFSA guidance on nanotechnology for “a material that is not engineered as nanomaterial but contains a fraction of particles, less than 50% in the number–size distribution, with one or more external dimensions in the size range 1–100 nm”. Thus, a recommendation for re-assessment of the safety of titanium dioxide was proposed and as a result a new EFSA Opinion was published in May 2021.
4. In this opinion, the EFSA Panel considered that some findings regarding immunotoxicity and inflammation with E171 as well as neurotoxicity with TiO2 nanoparticles may be indicative of adverse effects. They also considered that there are indications of the induction of aberrant crypt foci (ACF) with E171 and that no studies appropriately designed and conducted to investigate the potential carcinogenicity of TiO2 nanoparticles were available. Overall, on the basis of the currently available evidence along with all the uncertainties, in particular the fact that the concern regarding genotoxicity could not be resolved, the EFSA Panel concluded that E171 can no longer be considered as safe when used as a food additive.
COT and COM comments on the EFSA re-evaluation
5. Following the publication of the EFSA Opinion, the UK’s COT and Committee on Mutagenicity of Chemicals in Food, Consumer Products and the Environment (COM) considered the EFSA findings, and an interim position paper was published (COT, 2022). Overall, it was observed that the percentage of absorption was reported to be higher in the 2021 opinion than in the previous evaluation (EFSA, 2016), based on the same dataset. Additionally, the COT also questioned the conclusions with regards to the ability of TiO2 to induce ACF. Furthermore, the findings of the studies on neurotoxicity were considered inconsistent by the COT. It was noted that the Extended One Generation Reproduction Toxicity (EOGRT) study did not report any effects and that most of the other studies on this endpoint were of nanomaterials. They considered that had the test material in the EOGRT study been dispersed and stabilised in the nano form, some effects could possibly have been observed. The COT, as previously, questioned the relevance of such dispersion to real world use. Members noted that the histopathology tests performed for the EOGRT study were standard and were not sensitive enough in comparison to other studies on this endpoint that performed specific neuro-histopathology testing.
6. With regards to genotoxicity, the COT were in agreement with the COM’s view and further noted the large discrepancy between the underlying dataset and the conclusions drawn by EFSA. They further highlighted the inconsistencies between the outcomes of the 2020 SCCS Opinion discussed in detail in paragraph 45, where it was determined that the genotoxic effects of titanium dioxide manifest either via a thresholded or secondary mechanism, and the outcomes of the 2021 EFSA evaluation, where the EFSA Food Additives and Flavourings (FAF) Panel concluded that it was unclear if a threshold mode of action could be assumed. Regarding the genotoxicity of the nanoparticles, the COT considered that this could either be a concentration effect leading to oxidative damage or a stress effect, however, it was unclear as the results in different cell lines were equivocal and inconsistent. It was also noted that in some tests titanium dioxide had shown less reactivity.
7. On balance, the Committee considered that the weight of evidence did not support the conclusions drawn by EFSA. The COT also agreed with the comments of the COM with regards to risk communication that “As it stands the conclusion is highly risk adverse based on the weak evidence available, and it might create unnecessary concern to the public.” The COT suggested that the COM should independently review the database on genotoxicity and apply their Guidance on determining thresholds. When considering whether they agreed with EFSA’s conclusion that no differentiation could be made with regards to size/form of titanium dioxide and different aspects of toxicity, the COT took the opinion that nanoparticles were driving the toxicity. COM are currently in the pre-draft stages of re-assessing the genotoxicity of TiO2.
8. The full interim position paper is available here: Link to the TiO2 interim position paper. Considering the outputs of the discussions from the COT and the COM, the FSA has decided to launch their own review of the safety of titanium dioxide as a food additive.
Aim of this paper
9. The current paper presents the data from the EOGRT study as well as information from the literature; a second paper will be presented to the Committee at a future meeting. The aim of these papers is to present the data underlying the main changes in the 2021 Opinion, conclusions on toxicokinetic and absorption data, reproductive toxicity and ACF, developmental immunotoxicity and neurotoxicity from the recent EOGRT study and a revised literature search covering the period from 2015-2021, which the COT questioned in their review of the newest EFSA Opinion and enable the COT to independently assess the safety of titanium dioxide.
Background - Toxicity of Titanium Dioxide as a Food Additive
In this guide
In this guideThis is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Titanium Dioxide - Background
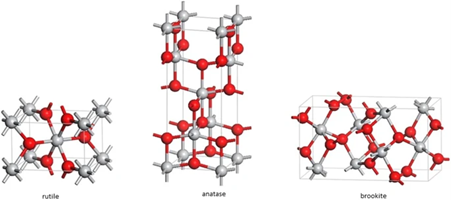
10. Titanium dioxide (TiO2) is an inorganic compound which exists in nature in
different crystalline forms - the anatase and rutile being the two most important (see Fig 1).
Chemical Abstracts Service (CAS) Registry number: 13463-67-7.
European Inventory of Existing Commercial Chemical Substances (EINECS) number: 236-675-5.
Colour Index (C.I.) number: 77891.
11. Titanium dioxide is an authorised Food Additive (E171) in the EU in accordance with Annex II to Regulation (EC) No 1333/2008 in both anatase and rutile forms (Commission Regulation (EU) No 231/2012) and under GB Food Law (retained EU law Regulation No 1333/2008 on food additives).
12. The uses of titanium dioxide include:
- As a colour to make food more visually appealing.
- To give colour to food that would otherwise be colourless.
- To restore the original appearance of food.
It is also widely used in cosmetics and medicines (EFSA, 2016).
Fig 1: Forms of TiO2
Previous Evaluations
Evaluations pre 2016
13. Titanium dioxide has been the subject of numerous evaluations by various scientific bodies.
14. The EU Scientific Committee on Food (SCF) evaluated titanium dioxide on a number of occasions (SCF, 1975 and 1977). In 1975, the SCF did not establish an Acceptable Daily Intake (ADI) for titanium dioxide based on the 1969 JECFA assessment concluding the lack of significant absorption and tissue storage in several species including humans. In 1977, the SCF included titanium dioxide in the category ‘colours for which an ADI was not established but which could be used in food’.
15. The Joint FAO/WHO Expert Committee of Food Additives (JECFA, 1969) - JECFA allocated an ADI ‘not limited except for good manufacturing practice’.
Evaluations from 2016 - to date
16. The use of food additives is regulated under the European Parliament and Council Regulation (EC) No 1333/2008 on food additives. Since titanium dioxide (E 171) was permitted in the EU before 20 January 2009, it belongs to the group of food additives which are subjected to a new risk assessment by the European Food Safety Authority (EFSA), according to Commission Regulation (EU) No 257/2010, and in line with the provision of Regulation (EC) No 1333/2008.
17. The re-evaluation of titanium dioxide (E 171) as food additive was published on 14 September 2016. The EFSA Food Additives and Flavourings (FAF) Panel concluded, on the basis of the available evidence that titanium dioxide could be used as a food additive (E 171). EFSA recommended that additional reproductive toxicity testing could be performed to enable EFSA to establish a health-based guidance value for titanium dioxide (E 171). On the basis of the data available, the Panel concluded that the absorption and oral bioavailability of titanium dioxide was low, independent of size. For endpoints other than genotoxicity, the Panel identified a no-observed adverse effect level (NOAEL) of 2,250 mg/kg bw/d based on a study in rats. Compared to the exposure based on reported use levels and analytical data, the use of E171 was not considered to be of concern.
18. The Panel did not establish an Acceptable Daily Intake (ADI) due to the lack of an extended 90-day toxicity study or a multi-generation or extended one generation reproduction toxicity study with E171. This is because possible adverse effects were identified in the reproductive system in some studies conducted with test substances that were non-food grade or with inadequately characterised nanomaterial.
19. Overall, the Panel concluded that once definitive and reliable data on the reproductive toxicity of E 171 were available, the full dataset would enable the Panel to establish a health-based guidance value (ADI). They further recommended that:
- In order to enable the Panel to establish a health-based guidance value (ADI) for the food additive TiO2 (E 171), additional testing would be required. An extended 90-day study or a multigeneration or extended-one generation reproduction toxicity study according to the current OECD guidelines could be considered. Further studies should be performed with TiO2 (E 171) complying with the EU specifications and additionally including a characterisation of the particle size distribution of the test material. However, in deciding on actual testing, considerations of animal welfare need to be balanced against the improvement in the toxicological database within a tiered testing approach.
- The EU specifications for TiO2 (E 171) should include a characterisation of particle size distribution using appropriate statistical descriptors (e.g. range, median, quartiles) as well as the percentage (in number and by mass) of particles in the nanoscale (with at least one dimension < 100 nm), present in TiO2 (E 171) used as a food additive. The measuring methodology applied should comply with the EFSA Guidance document (EFSA Scientific Committee, 2011).
- The maximum limits for the impurities of the toxic elements (arsenic, lead, mercury and cadmium) in the EU specification for TiO2 (E 171) should be revised in order to ensure that TiO2 (E 171) as a food additive will not be a significant source of exposure to those toxic elements in foods.
20. In January 2017, a call for data was published requesting business operators to submit new reproductive toxicity data for titanium dioxide (E 171), as well as data addressing other recommendations concerning the specifications for titanium dioxide (E 171). Data from a new extended one-generation reproduction toxicity (EOGRT) study was submitted.
21. On 4 April 2017, the French Agency for Food, Environment and Occupational Health and Safety (ANSES) published an opinion on dietary exposure to nanoparticles of titanium dioxide assessing, in particular, the study of Bettini et al. (2017) and concluded that the data available do not bring into question the risk assessment performed by EFSA.
22. On 22 March 2018, the European Commission (EC) requested the EFSA Food Additives and Nutrient Sources Added to Food (ANS) Panel to evaluate four new studies describing potential adverse health effect of titanium dioxide used as food additive (E 171). The ANS Panel opinion, published on 4 July 2018, concluded that the outcome of the four studies did not merit re-opening the existing opinion of EFSA related to the safety of titanium dioxide (E 171) as food additive.
23. In that opinion EFSA recommended that biomarkers for putative pre-cancerous lesions in the colon should be examined, as additional parameters, in the reproductive toxicity study recommended by EFSA in 2016. Business operators followed this recommendation and published an EOGRT study to investigate these data gaps.
24. In 2019, ANSES published a review of the risk related to the ingestion of the food additive titanium dioxide (E 171) to include recent scientific studies published after their 2017 opinion. ANSES emphasised the lack of scientific data able to resolve the uncertainties regarding the safety of the additive E171. It reiterated recommendations to obtain data for characterising the different physico-chemical forms of E171 and additional toxicological data on the potential effects associated with their ingestion. Pending a better toxicological characterisation of E171, ANSES restated its previous general conclusions on nanomaterials aimed at limiting the exposure of workers, consumers and the environment as part of a gradual approach, in particular by promoting safe products that are equivalent in terms of function and effectiveness, and that do not contain nanomaterials. In addition to the 2017 opinion. The EC requested EFSA to assess the ANSES review in order to 1) highlight major findings showing that food additive titanium dioxide (E 171) is of safety concern 2) indicate whether it overrules the conclusion of the previous EFSA evaluation and 3) highlight any additional uncertainties that could be addressed in the ongoing follow-up work from the 2016 EFSA opinion.
25. EFSA published a statement on the risk related to the exposure to the food additive titanium dioxide (E 171) performed by ANSES (May 2019). EFSA concluded that the ANSES opinion does not identify any major new finding that would overrule the conclusions made in the previous EFSA scientific opinion on the safety of titanium dioxide (E 171) as a food additive. The ANSES opinion reiterated the previously identified uncertainties and data gaps, which are currently being addressed by recommendations follow-up activities requested after the 2016 EFSA review. EFSA considered that this recommendation should be revisited once the work on the physico-chemical characterisation of the food additive titanium dioxide (E 171) is completed.
26. The European Commission requested EFSA to assess new data addressing the uncertainties identified with respect to the characterisation of this food additive, including its particle size and particle size distribution provided by interested food business operators in response to the call for data published as a follow-up of the re-evaluation of titanium dioxide (E 171) (August 2018).
27. The new data assessment resulted in a scientific opinion on the proposed amendment of the specifications of titanium dioxide (E 171) with respect to the inclusion of the additional parameters related to its particle size distribution which was published in July 2019. EFSA indicated that the conclusions made, and the uncertainties identified, in the previous EFSA assessment (2016) of the food additive titanium dioxide (E 171) remain valid.
28. EFSA concluded that based on the proposed change in the specifications, the toxicological database on titanium dioxide (E 171) as a food additive should be revisited in line with the data requirements specified in the 2018 EFSA “Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain”.
Particle Size Considerations for TiO2 – 2018 review
29. One of the largest uncertainties in the 2016 EFSA evaluation was related to the composition of titanium dioxide. EFSA considered that E 171 mainly consisted of micro-sized titanium dioxide particles, with a nano-sized (< 100 nm) fraction less than 3.2% by mass. Uncertainties around the identity and characterisation of E 171 were however highlighted, noting that no limits for the particle size of E 171 were set in the EU specifications (EFSA, 2016).
30. Subsequently, in 2019, and following the evaluation of data submitted by interested operators, the Panel recommended that “the EU specifications for E 171 include the parameter of median minimum external dimension by particle number >100 nm (measured by electron microscopy), which is equivalent to less than 50% of constituent particles by number with a minimum external dimension <100 nm.”
31. The EFSA Scientific Committee published ‘Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health’ (EFSA Scientific Committee, 2018a) updating the 2011 Guidance Document on nanomaterials (EFSA Scientific Committee, 2011a), and clarifying that conventional materials containing a fraction of nanoparticles require specific risk assessment considerations.
32. A re-evaluation of E 171 was completed by the EFSA ANS Panel in 2018. The EC requested that EFSA assess a proposal for an amendment of the EU specifications for the food additive E 171 based on the data on particle size and particle size distribution that had been provided by the interested business operators in response to the first part of the European Commission call for data. This scientific opinion was adopted and published in June 2019. The ANS Panel recommended the inclusion of additional parameters related to the particle size distribution in the EU specifications for E 171 and concluded that the toxicological database should be revisited. The scope of the document covers engineered nanomaterials and materials containing a fraction of particles less than 50% in the number–size distribution, with one or more external dimensions in the size range 1–100 nm, a definition which could be applicable to the case of the food additive titanium dioxide (E 171).
Additional Evaluations - Toxicity of Titanium Dioxide as a Food Additive
In this guide
In this guideThis is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
33. Before expanding on the 2021 EFSA evaluation of Titanium dioxide following the 2019 recommendation, additional evaluations by other scientific bodies that were published prior to 2021 are discussed in the section below.
ANSES and ECHA (European Chemicals Agency)
34. Following a report by the French Authorities in 2016, and a proposal for evaluation of titanium dioxide the Committee for Risk Assessment (RAC) of the European Chemicals Agency (ECHA) concluded in June 2017 that titanium dioxide met the criteria to be classified as a substance suspected of causing cancer (category 2) if inhaled.
35. The main mechanism to explain the effects induced by titanium dioxide, in common with effects seen with other substances, was inflammation and an indirect genotoxic effect through production of reactive oxygen species (ROS) arising from the biopersistence and insolubility of all forms of titanium dioxide particles. However, a direct interaction with DNA could not be excluded, since titanium dioxide was found in the cell nucleus in various in vitro and in vivo studies.
36. This was in line with the International Agency for Research on Cancer (IARC) evaluation which concluded that “titanium dioxide is possible carcinogenic to humans (Group 2B) based on sufficient evidence in experimental animals and inadequate evidence from epidemiological studies. ” This was with relation to exposure via inhalation. However, in the same report by the French Authorities, ANSES concluded that there was no carcinogenic concern after oral or dermal administration.
Dutch Office for Risk Assessment
37. In 2018, the Dutch Office for Risk Assessment and Research held a workshop on the “potential health effects of the food additive titanium dioxide (E171)”, the results of which were published in 2019 , where overall the need for further studies to further investigate the effects of titanium dioxide exposure- particularly for the endpoints of colon tumours and immunotoxicology based on the data gaps and study limitations of the available database at the time was highlighted. Furthermore the need to better characterise the composition of E171 was noted.
38. In 2020, a review was published that summarised the outcomes of this workshop and additionally aimed to identify and evaluate recent toxicological studies on food-grade titanium dioxide and nano-sized titanium dioxide in ex-vivo, in-vitro, and in-vivo experiments along the gastrointestinal route, and to postulate an Adverse Outcome Pathway (AOP) following ingestion.
39. Adverse effects were identified including the generation of ROS, alterations of the gut microbiota, persistent inflammation, and other effects on the immune system. It was noted that findings were inconsistent between the different species and independent research groups.
40. With regards to the animal studies which reported positive effects with respect to precancerous lesions/tumour formation, it was noted that those were mainly used as research models and a proper investigation of a dose-response relationship was not performed. Based on the available information, it was not possible to carry out a risk assessment.
41. When considering the mode of action, it was postulated that it was closely related to the ability of titanium dioxide to induce ROS formation and promote inflammation. The potential key events were considered to be persistent inflammation and ROS generation that can result in oxidative stress as well as persistent epithelial cell injury and potentially lead to DNA damage and exert a tumour-promoting effect of E171 seen in some of the studies.
42. Finally, it was noted that it is generally assumed that the round and spherical crystal forms of TiO2 contribute to a lower extent to the induction of adverse effects, when ingested and similarly that titanium dioxide nanoparticles are suspected to induce more adverse effects than other particle sizes. However, a study by Proquin et al.(2017) was also mentioned, that demonstrated that a mixture of nano- and micro-sized TiO2 particles, as they are present in E171, induce more adverse effects than the single fractions alone.
43. The authors further expanded on possible interactions of E171 with its direct environment as well as other factors that could potentially affect agglomeration for example and discussed how these could directly affect the properties of titanium dioxide.
44. Therefore, they considered that “it is important to carefully examine and analyze the physicochemical characteristics of TiO2 particles in its vehicle, as well as in its surrounding matrix as their final milieu, to guarantee a profound assessment of potential adverse health effects of E171 and to adequately compare different studies in the process of risk assessment.” (Bischoff et al.,2020).
Scientific Committee on Consumer Safety (SCCS)
45. The EU Scientific Committee on Consumer Safety (SCCS) assessed titanium dioxide used in cosmetic products that lead to exposure by inhalation. With regards to mutagenicity and genotoxicity, the SCCS noted that in the 2010 evaluation, IARC concluded that that most of the in vitro genotoxicity studies with titanium dioxide exposure were negative despite the high rate of false positives and that the EFSA Panel in 2016 considered that the positive genotoxicity results may have been due to experimental conditions associated with the induction of oxidative stress.
46. The SCCS also noted that studies showing a positive association between the so-called group of Poorly Soluble Low Toxicity (PSLT) particles exposures and genotoxicity are generally consistent with the mechanism that sub-toxic concentrations of PSLT particles can cause inflammation and oxidative stress, which may lead to mutations.
47. Oxidative stress is considered to be the underlying mechanism of the proliferation and genotoxic responses to PSLT particles including titanium dioxide and thus there is a large body of evidence that titanium dioxide has no direct genotoxic potential.
48. The SCCS was of the opinion that “The genotoxic effects of titanium dioxide most probably manifest through an indirect mechanism (oxidative stress), or secondary mechanisms (e.g. oxidative stress and inflammation caused by immune cells).
49. The SCCS therefore considered it plausible that there is a practical threshold for this mode of action and therefore a risk assessment could be carried out for its use in cosmetic products.” They concluded that when used in cosmetic products titanium dioxide does not pose a genotoxic risk. (SCCS, 2020). Genotoxicity is not considered further in this paper.
EFSA Re-Assessment of Titanium Dioxide (E 171) , 2021
In this guide
In this guideThis is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
50. The following section of this paper discusses the EFSA re-evaluation. It briefly addresses the data considered by EFSA and presents the main conclusions. The underlying data on the endpoints of toxicokinetics and absorption, developmental and reproductive toxicity and aberrant crypt foci are further discussed in detail in paragraphs 81 onwards to allow the COT to independently assess them. While a comment has been included on the conclusions around genotoxicity, these will not be considered further in this paper.
51. Concerning absorption and toxicity of TiO2 particles that are present in E 171, the Panel concluded that:
- The absorption of TiO2 particles is low but they may accumulate in the body due to their long half-life.
- No studies appropriately designed and conducted to investigate the potential carcinogenicity of TiO2 nanoparticles were available.
Data & Methodology of the EFSA 2021 Opinion
52. The assessment was conducted in line with the principles described in the EFSA Guidance on transparency in the scientific aspects of risk assessment (EFSA Scientific Committee, 2009), and relevant existing Guidance from the EFSA Scientific Committee, including the Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health (EFSA Scientific Committee, 2018a).
53. The 2021 EFSA evaluation is based on the following data:
- Information from publications retrieved in the literature search (see Annex B for criteria).
- Data submitted in response to the call for data from European Commission as follow-up of there-evaluation of E 171.
- Toxicokinetic studies considered in the re-evaluation of titanium dioxide (E 171).
- Exposure data available in the re-evaluation 2016 and additional relevant information published since that time.
- In-vitro and in-vivo studies reported in the OECD dossier (2016) and submitted to EFSA.
54. Food consumption data used to estimate the dietary exposure to titanium dioxide (E 171) were derived from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database 1). Dietary data from the UK were included in the EFSA Comprehensive European Food Consumption Database for the period in which UK was a member of the European Union.
55. The Mintel’s Global New Products Database (GNPD) was used to verify the use of titanium dioxide (E 171) in food and beverage products and food supplements within the EU’s food market. The Mintel’s GNPD is an online database that contains the compulsory ingredient information present on the label of numerous products.
56. With regards to toxicity, a literature search was performed following the approach and information on the criteria for inclusion and exclusion of publications based on information from the abstract and title, and material used in the study is described in Annex B. Toxicokinetic and toxicity studies considered ‘included’ were assessed for their relevance and reliability. The Panel further assessed the EOGRTS data submitted by industry, which also included an endpoint investigating aberrant crypt foci induction, following the Panel’s recommendations in the 2016 evaluation.
57. Nanoscale considerations for the assessment of the study design and study results in toxicity studies classified with reliability 1 and 2 (see Annex B for criteria).
Dietary Exposure Data
58. Dietary exposure to E 171 from its use as a food additive was estimated combining the food consumption data available within the Comprehensive Database with reported use levels submitted to the EFSA ANS Panel (2016) and information extracted from a report of the Netherlands National Institute for Public Health and the Environment (RIVM) (Sprong et al., 2015). The exposure was estimated according to different exposure scenarios (EFSA ANS Panel, 2017). Uncertainties in the exposure assessment were identified and discussed. The current paper does not expand on this information due to the fact that, because of the Panel conclusions on genotoxicity, the exposure information was not further considered in the risk assessment.
Toxicity
59. With regard to the genotoxicity studies, combining the available lines of evidence, the FAF Panel concluded that “TiO2 particles have the potential to induce DNA strand breaks and chromosomal damage, but not gene mutations. No clear correlation was observed between the physico-chemical properties of TiO2 particles – such as crystalline form, size of constituent particles, shape and 7 agglomeration state – and the outcome of in vitro or in vivo genotoxicity assays” (i.e a cut-off value for TiO2 particle size with respect to genotoxicity could not be identified). The Panel also concluded that “several modes of action (MOA) may operate in parallel and the relative contributions of the different molecular mechanisms resulting in the genotoxicity of TiO2 particles are unknown. Based on the available data, no conclusion could be drawn as to whether the genotoxicity of TiO2 particles is mediated by a mode (s) of action with a threshold(s)”. Therefore, the Panel concluded that a concern for genotoxicity of TiO2 particles cannot be ruled out. The underlying data on genotoxicity is currently being reviewed by the COM, as part of the UK independent review of the safety of TiO2.
60. With regards to other endpoints the FAF Panel concluded “that the absorption of TiO2 particles is low, however they can accumulate in the body due to their long half-life; studies on general and organ toxicity, including the newly performed EOGRT study with E171, did not indicate adverse effects up to a dose of 1,000 mg/kg bw per day. In addition, no effects were seen in literature studies employing TiO2 NP > 30 nm up to the highest dose tested of 100 mg/kg bw per day. No effects on reproductive and developmental toxicity up to a dose of 1,000 mg/kg bw per day, the highest dose tested, were observed in the EOGRT study with E171. No other reliable studies were found in the literature addressing these effects with E171; some findings regarding immunotoxicity and inflammation with E171 as well as neurotoxicity with TiO2 NPs may be indicative of adverse effects. They also considered that there are indications of the induction of aberrant crypt foci in the small intestine with E171 and that no studies appropriately designed and conducted to investigate the potential carcinogenicity of TiO2 nanoparticles were available.”
Uncertainty
61. The Panel identified uncertainties related to the following points:
- The size distribution of the particles in marketed E171 that consumers are exposed to, related to the different types of E171, as presented in the EFSA ANS Panel (2019) opinion.
- The processes used by industry when using E171 in food and to what extent these processes may affect the degree of agglomeration and thus internal exposure.
- The state of agglomeration i.e. presence of ‘free’ (non-agglomerated) particles of tested material in gastrointestinal tract of the animals and its effect on absorption.
- The representativity of different tested materials used in toxicity studies for the food additive E171 when used in food.
- Differences in the physico-chemical properties of the different tested materials and the extent of their impact on the observed results.
- Interference in the measurements of Ti/TiO2 in blood, tissues or organs with the most widely used analytical technique, i.e. ICP-MS, and its impact on the reliability of tissue concentration data.
- Confidence in the limited kinetic data as the basis for estimating half-lives and accumulation and for assessment of internal exposure and, related to that, the extent of systemic availability based on the proposed amendment for EU specifications of titanium dioxide.
- None of the rodent studies were sufficiently long to cover the time needed for reaching the steady state for accumulation and this impacted the interpretation of the study results.
62. The Panel identified uncertainties regarding the EOGRT study with respect to its validity to fully identify all potential adverse effects of E 171 when used as a food additive:
- The extent to which the particle size distribution of the E 171 used in the EOGRT study is reflective of the particle size distributions of E 171 when added to foods.
- The extent to which the particle size distribution of E 171 in transit through the gastrointestinal tract in the EOGRT study was affected by the concentration in the diet (i.e. dose).The selected test material was representative of E 171 containing a large proportion (around 50% by number) of constituent particles below 100 nm (E 171 sample E reported in EFSA FAF Panel, 2019).The particle size distribution of the E 171 in samples of the test diet was also analysed after applying a sample dispersion protocol that aims to extract E 171 particles from the feed matrix and the results show that the particle size distribution of the constituent Safety assessment of the food additive titanium dioxide (E 171) was similar to that of pristine E 171 after dispersion (EFSA FAF Panel, 2019; Verleysen et al.,2020). However, neither of these procedures were considered by the Panel to reliably determine the particle size distribution of E 171 in the feed.
63. The Panel acknowledged that the methods for determining particle size distributions in complex foods and feeds in-situ are not currently available. Accordingly, the Panel considers that the extent to which the particle size distribution of the E 171 used in the EOGRT study is sufficiently reflective of the particle size distributions of E 171 when added to foods remains uncertain. The interested business operator considered that mixing of two dry components (feed and E 171) was the best possible option to retain the particle size distribution properties of the original E 171 sample, and that the use of liquid dispersion would add further superfluous unknowns.
64. The Panel considered that E 171 has a broad size distribution of constituent particles (from about 40 to 250 nm); considered that in dry form, this size distribution of the constituent particles is expected to be stable and further, that homogenous mixing of E 171 with dry diet is a pragmatic approach to adopt in terms of performing an animal study over an extended time frame such as the EOGRT study. The Panel considered this approach to be representative of some uses of E 171 in food (e.g. E 171 in confectionary coatings and fillings and in ready to use sauces. However, the Panel also noted that this approach may not be fully representative for all uses of E 171 in food since liquid dispersion of E 171 was reported to be used, potentially along with additional processes, to reduce the formation of agglomerates in suspension in some products (e.g. incorporation of E 171 into a tablet coating or capsule.
65. The Panel considered that investigations of TiO2 levels in tissues would have reduced uncertainty regarding dose dependency of internal exposure. However, the Panel noted that the EOGRT study demonstrated unequivocally low levels of internal exposure to TiO2 in animals that were fed a diet prepared by addition of E 171 to dry feed. Dispersed Nanoparticles show a greater tendency to agglomerate when suspended in liquid media at higher concentrations. This concentration effect on agglomeration and/or resistance to de-agglomeration may also exist in the gastro-intestinal tract at high-dose levels The Panel therefore considered that there remains an uncertainty regarding the effects of dose levels/concentrations in feed and the extent to which agglomeration occurred in the gastrointestinal tract. However, the Panel considered the propensity for this agglomeration is likely reduced when exposure is via feed rather than through bolus gavage administration of E 171
Conclusion:
66. Considering all currently available evidence and uncertainties, the Panel concluded that E 171 can no longer be considered as safe when used as a food additive due to genotoxicity considerations. This applies to E 171 as described in Commission Regulation (EU) No 231/2012 and E 171 specified in the EFSA Food Additive and Flavourings Panel opinion in 2019.
COT comments on the 2021 EFSA opinion
67. The COT considered the EFSA Opinion on titanium dioxide at their July 2021 meeting. The Committee considered a summary of the EFSA opinion as well as the preliminary comments from the COM meeting; these are noted in the introduction and not considered further in this paper.
68. The COT also noted that in several parts of the Opinion, published papers were presented at face value, and there was no discussion of the results nor the overall Weight of Evidence to support the conclusions being made. They furthermore noted discrepancies and conflicts between the results of the studies reported and the overall conclusions.
69. Overall, the COT considered that there was a lack of internal consistency and of objective weighing of the evidence. While some of this might have been due to differences in the nature of the TiO2 tested, this was not clear in the Opinion.
70. Members also noted that it was difficult to draw any conclusions from the studies and a closer look in terms of material characterisation was needed in order to understand some of the effects reported. Members also considered that follow up was needed on the reproductive toxicity study as only the presence or absence of an effect was measured.
71. The large variation in the specifications of E 171 was also discussed based on the analytical data for pristine E 171 that indicated that more than 50% of the constituents were in the nano-range so the COT considered that more clarification was needed on the actual composition of E 171. It was noted that the EFSA definition of nanomaterials lacked clarity with regard to materials that were not engineered as nanomaterials but contained particles in the nano range. The possibility and plausibility of removing the nano fraction from E 171 in order to mitigate the risk was also discussed by the COT.
72. With regard to absorption, it was noted that there was no reason to believe that titanium dioxide particles behaved differently to other particles in the gastrointestinal tract.
73. Members were advised that newer studies used in the previous evaluation were re-considered (evidence from deceased humans and indications that titanium dioxide could cross the placenta). The duration of the animal studies was not sufficient to evaluate at which levels steady state would be reached and therefore it was considered that absorption had previously been underestimated.
74. The extended one generation reproductive toxicity (EOGRT) study provided indirect evidence for systemic exposure following administration of titanium dioxide. Members were informed that EFSA had indications that when used by industry, E171 was dispersed into nanoparticles by sonication and therefore also considered data on materials made solely of nanoparticles for the assessment. However, this was questioned by Members as it was noted that pure nano titanium dioxide would lose its technical function in the food (as it would not provide colour) and would therefore not be of use.
75. The COT also questioned the conclusions with regards to the ability of TiO2 to induce aberrant crypt foci. On this point, the Committee were advised that because of the above consideration by EFSA, only one study that used sonication of the material was considered, as the material tested was undispersed in the other available studies.
76. The findings of the studies on neurotoxicity were considered inconsistent by the COT. It was noted that the EOGRT study did not report any effects and that most of the other studies on this endpoint were of nanomaterials.
77. In the EFSA evaluation, the issue of the test material in the EOGRT not being dispersed was taken into consideration with regards to the conclusions on this endpoint, as they considered that had it been dispersed and stabilised in the nano form some effects could possibly have been observed. The COT, as previously, questioned the relevance of such dispersion to real world use. Members noted that the histopathology tests performed for the EOGRT study were standard and were not sensitive enough in comparison to other studies on this endpoint that performed specific neuro-histopathology testing.
78. On balance, the Committee considered that the weight of evidence did not support the conclusions drawn by EFSA. The COT also agreed with the comments of the COM with regards to risk communication that “As it stands the conclusion is highly risk adverse based on the weak evidence available, and it might create unnecessary concern to the public.” They considered that care should be taken when expressing the conclusions as they might cause unnecessary concern and they were uncomfortable with EFSA’s binary communication on a dataset with a lot of uncertainties. They highlighted that the COT does not follow the precautionary approach.
79. When considering whether they agreed with EFSA’s conclusion that no differentiation could be made with regards to size/form of titanium dioxide and different aspects of toxicity, the COT erred towards the view that nanoparticles were driving the toxicity. It was decided that an interim position paper, capturing the COT’s view and the proposed next steps should be published.
Detailed breakdown on studies considered by EFSA
80. This section expands on the underlying data of toxicokinetics and absorption, the EOGRT study and ACF. For context, the EFSA Panel conclusions have been included in the relevant sections, for the COT’s information.
Toxicokinetics and Absorption
81. The toxicokinetics of E 171 was addressed in five studies in total, three studies in mice and in two studies in humans.
E171 studies in mice
Talamini et al (2019)
82. The test material used was: E171 (food grade titanium dioxide), 99.3% pure anatase, 35% nanoparticles.
83. Groups of 8 week old male NRF mice (n=22/group) were treated with 5 mg/kg bw of E171 dispersed in water (no sonication or deagglomeration). The animals were treated for 3 days/week for 3 weeks receiving a total of 9 treatments in 21 days. The average daily dose of 2 mg/kg bw. The actual treatment concentration was verified by inductively coupled plasma mass spectrometry (ICP-MS). The test material: E171 or water (control) was slowly dripped with pipette in the mouths of the mice, allowing for each drop to be swallowed.
84. The animals were weighed at the beginning of the experiment and observed daily. No signs of general toxicity were observed. On day 21, the animals were sacrificed and the lungs, liver, stomach, spleen. Kidney, brain, testes and whole intestine were removed. The concentrations of titanium were determined in 4 animals.
85. In the brain, kidney and testes, titanium levels were <0.03 µg/g, the quantification limit of the analytical method for solid tissue samples. In lungs and spleen the levels were low, with a not statistically significant, but slightly higher deposition in spleen of E171 treated animals compared to the controls. The authors reported that “Titanium concentration was one order of magnitude greater in the small intestine compared to the above tissues and distinctly higher in the stomach, large intestine and liver”. The concentrations of titanium in treated animals were: 1.07 ± 0.38 µg Ti/g tissue in the large intestine and 0.94 ± 0.57 µg Ti/g tissue in the liver. These levels were 1.8 and 3.6 times higher compared to the controls, respectively.
Comera et al (2020)
86. The test material used was: E 171, > 95% anatase, 20–340 nm (transmission electron microscopy- TEM); 44.7% nanoparticles.
87. In the first round of experiments adult C57BL/6 mice (12–18 weeks) were treated with a single gavage dose of 40 mg/kg of E171 suspended in water and sonicated or water. In the second round of experiments, 300μg/mL of E171 was suspended in buffer and used to fill a closed mid- jejunal loop of 10cm, pre-treated with inhibitors of tight junctions, micropinocytosis, clathrin-mediated endocytosis or raft-dependent endocytosis.
88. Animals were sacrificed at 2, 4, 8 and 24 hours following treatment. Confocal microscopy and micro x-ray fluorescence imaging was used to analyse the existence of particles in both the first and second round of experiments, whilst ICP-MS was used to determine titanium concentration in blood and tissues (jejunum, ileum, colon). The jejunal or colonic intraluminal contents were recovered by gentle scraping.
89. In mice treated with a single dose of E171, the number of titanium dioxide reflective particles in the lumen of the upper intestine was significantly increased, with analysis of the particles suggesting that no further agglomeration of titanium dioxide occurred during its transit through the intestinal tract, and that as it moved in the distal intestine, there was a decrease in its agglomeration state (as indicated by smaller particle size in the colonic versus the jejunal lumen).
90. An increase in the reflective particle content was observed in the jejunal and ileal villi, Peyer’s Patches and colon crypts. The overall particle content in jejunal villi increased from 2 hours after gavage, peaked at 4 hours, and returned to basal values at 8 hours. A statistically significant increase (p < 0.001) 4 hours after E171 administration was observed in the titanium dioxide particle density in the jejunal mucosa (increased by 3.4-fold over the controls). A lower and non-significant trend of increased particle content was also observed at 4 hours in the ileum and colon, with the values decreasing close to control levels at the time 8 hours in all three intestinal sections. In the jejunum, the reflective TiO2 particle spots displayed a mean diameter of 700 ± 59 nm (n = 70) and were mostly observed in the lamina propria and in goblet cells (GCs) distributed in the epithelium, with some of them also found in enterocytes lining the gut lumen. Analysis by transmission electron microscopy coupled with energy-dispersive X-ray spectroscopy (TEM-EDX) indicated the presence of both Ti and O in particles detected in jejunal GCs and enterocytes. These appeared as primary particles or aggregates with respective sizes of 450 and 170 nm. . In the Peyer’s patches, a statistically significant increase in laser-reflecting particles was found only at 8 hours (increased by 5.4-fold over that of controls (p < 0.001)). In blood, the number of particles significantly increased by 3.5- and 4.1-fold at 4 and 8 hours, respectively, but the titanium concentrations remained below the limit of detection (LOD<0.02 ng Ti/kg) at all timepoints. From the content in the intestines and the weight of the mice tissues, the authors calculated that approximately 0.007% of the titanium administered was present in the entire intestine at the 4 hours timepoint. The authors concluded that titanium dioxide was absorbed predominantly in the ileum, partly in jejunum and that small amount absorbed in the colon. Based on the surface area information it was concluded that titanium dioxide is predominantly absorbed by the small intestinal villi and to a lesser extent through Peyer’s patches.
91. In the ex vivo experiment, under anaesthesia, a closed mid-jejunal loop (10cm) was isolated and pre-treated with either just PBS (control) or a PBS solution with inhibitors as described above for 30 minutes. The contents were then rinsed and replaced with PBS (control) or sonicated E171 and incubated for a further 30 minutes. A significant inhibition of TiO2 absorption (by 66%) was observed and the authors considered that the paracellular route is a major pathway governing transepithelial TiO2 passage. However, as the absorption was not totally blocked by paracellular pathway inhibitors, the authors concluded that besides a paracellular pathway, endocytosis could also be involved in the transport of titanium dioxide from the intestinal lumen to blood.
Riedle et al., (2020)
92. The test material used was: E171, anatase, 119nm (EFSA 2021).
93. C57BL/6 mice were treated with titanium dioxide doses of 0, 6.25, 62.5 and 625 mg/kg of diet. These were equivalent to 0 and approximately equal to 1, 10 and 100 mg E 171/kg bw per day.
94. The animals were sacrificed at 6, 12 and 18 weeks. Animals sacrificed at 18 weeks were also used to validate that the diet permitted uptake in the intestinal lumen. The basal regions of the Peyer’s patches were surveyed and reflectance confocal microscopy was used to determine the presence of titanium dioxide.
95. Reflectant foci, indicative of titanium dioxide presence, were found at the base of the Peyer’s patches at all dose groups. SEM coupled to energy-dispersive X-ray (EDX) confirmed that the tissue contained subsurface particles rich in titanium. In the low and mid dose groups, weak signals were detected in the impacted cells at the base of the Peyer’s patches, whereas higher signals were observed at the highest dose group.
E171 studies in humans
Pele et al., (2015)
96. The test material used was: E171, anatase, d50=250nm (EFSA, 2021)
97. Eight healthy volunteers (self reported) with normal intestinal permeability were given a permeability solution. At 7am, following an overnight fast baseline, urine samples were collected. After consumption of the solution, urine samples were collected for 5 hours.
98. Baseline blood samples were also taken at 9 am. Following that, the subjects received two tablets containing 50 mg of E171 (total dose 100 mg). Blood samples were collected at: 30 minutes, 1, 1.5, 2, 3, 6, 8 and 10 hours post E171 ingestion. Of the 8 volunteers, only 7 completed the study as blood could not be withdrawn from the cannula of 1 subject.
99. Dark field microscopy was used to identify titanium dioxide in the blood. Random areas were visualised and the estimation of particles within each field was based on four reflective grades: 0 (<5 particles/field), 1(5-10 particles/field), 2(10-20 particles/field), 3 (>20 particles/field). This analysis was only performed in 5/7 subjects due to blood clotting in two subjects. ICP-MS was used to quantify titanium in the blood for 0-10 hours, except in two subjects were samples could not be collected at 8hours (2 subjects) and 10 hours (1 subject).
100. Based on the results of the dark field microscopy, it was determined that some of the ingested titanium dioxide was absorbed directly into the blood. A significant increase in positive signals was observed from 2 hours onwards and both dark field microscopy and ICP-MS demonstrated a peak in absorption at 6 hours, reaching up to 11 ng/mL and decreasing to around 5ng/mL by 10 hours post exposure. Only the titanium levels from 6 hours post exposure onwards were significantly different than the baseline. A positive correlation between reflective grades and total titanium levels was observed.
101. The authors hypothesised that two routes of uptake in the gut were involved: one proximal (in the duodenum/jejunum) and one distal (Peyer’s patches in ileum). This was based on the fact that at two hours the uptake was visible in the dark field microscopy and the levels peaked at 6 hours as determined by ICP-MS (I.e. early absorption and late peak).
Guillard et al., (2020)
102. The test material used was: titanium dioxide particles with a mean particle size of 104.9 ± 44.9 nm and a particle size distribution ranging from 20 to 440 nm, with 55% of NPs by number.
103. Human placentae and meconium were collected at term from normal pregnancies. The samples were analysed using ICP-MS and scanning transmission electron microscopy (STEM) coupled to EDX spectroscopy for content analysis of titanium and analysis of titanium dioxide particle deposition, respectively. Transplacental passage of titanium dioxide was determined using an ex vivo placental transfusion model.
104. All placental samples (n=22) contained titanium with the total content ranging from 0.01 to 0.48mg/kg of tissue. STEM-EDX confirmed the presence of titanium and oxygen in the particle deposits seen by TEM, as well as aluminium, silicone, iron, zinc and tin trace elements. Most of the analysed titanium dioxide particles were below 100nm. Size particle analysis of all particles indicated that 50% were below 100nm in diameter.
105. In 50% of the meconium samples (total of 18 samples), titanium was detected (0.02-1.5 mg/kg). TEM-EDX analysis confirmed the presence of titanium and oxygen elements in the particle deposits, alongside silicone, aluminium, iron and zinc. Analysis of all particles indicated a diameter of 5-194nm, with 26/33 (80%) in the nano range.
106. In the transplacental passage experiment, of the 7 ex vivo isolated perfused placentae, round shaped or small particle aggregates of titanium dioxide were observed. Titanium dioxide particles were recovered in the syncytiotrophoblast microvilli and had translocated in deeper areas of the placental chorionic mesenchyme surrounding foetal vessels. The particles had a diameter of below 250 nm, with 17 of them in the nano range
107. The authors concluded that the results indicated the passage of titanium dioxide particles across the human placenta with potential local accumulation during pregnancy, depending on the individual. The findings of the perfused placenta experiment indicated, according to the authors, that the human placental barrier is unable to completely prevent the passage of titanium dioxide from dietary sources and protect the fetus.
108. Based on both experiments (results of perfused placenta study and the titanium levels in the placenta and meconium), the authors noted that there was a need to assess the risk of titanium dioxide nanoparticle exposure in pregnant women and warranted specific attention for oral exposure to the nanosized fraction of the E171 food additive.
Studies on Titanium dioxide other than E171, or with titanium dioxide nanoparticles
Rats
Disdier et al., (2015)
109. The test material used was: 75% anatase, 25% rutile titanium dioxide nanoparticles 21.5± 5nm.
110. Adult Fischer rats were treated with 1 mg/kg titanium dioxide or saline buffer (control); no dispersion protocol was applied for the in vivo experiment. The dose was administered intravenously and samples were taken at 30 minutes, 1, 2,6 and 24 hours and 7, 28, 90 and 365 days post treatment from blood, liver, brain, spleen, kidney and lungs. Blood and brain samples were additionally collected at 5 and 15 minutes post injection. Titanium concentrations were determined by ICP-MS.
111. The authors reported that titanium burdens in the liver, spleen and lungs of the treated group were significantly higher for all time points post injection, however the levels declined over time. Levels in the liver were higher than the spleen and lungs. Titanium burden after a year remained high, suggesting biopersistence (approximately 33% of the titanium burden of the early time points). The titanium burden in the kidneys increased significantly from 30 minutes to 24 hours, but decreased significantly 7 days after i.v. administration. No statistically significant results were reported for the blood samples. For the time points before 24 hours, there was a statistically significant increase if titanium concentrations in the brain . After 24 hours, titanium content did not differ from controls. No further details were given in the text, but graph 2 provides more details on the levels of titanium in the different organs. The authors estimated that they recovered approximately 44 % of the administered dose in the liver, 10 % in lungs and 2 % in spleen 6 hours post administration.
Kreyling et al., (2017a)
112. The test item used was: titanium dioxide anatase nanoparticles, 7-10 nm
113. Female Wistar- Kyoto rats were dosed with 10-20 μg of radiolabelled titanium dioxide given as a single i.v. injection of nanoparticles suspended in water.
114. The animals were sacrificed at 1, 4, 24 hours and 7 and 28 days post administration (n=4 per time point). All organs as well as blood and all excretions of the animals were collected. The results of a separate intravenous study performed to investigate the absorption and biodistribution of soluble ionic48V were used to correct48V release from [48V] titanium dioxide nanoparticle.
115. The highest [48V] titanium dioxide accumulations were found in liver (95.5%ID on day-1), followed by spleen (2.5%), carcass (1%), skeleton (0.7%) and blood (0.4%). Detectable nanoparticle levels were found in all other organs. The [48V]Titanium dioxide NP content in blood decreased rapidly after 24h while the distribution in other organs and tissues remained rather constant until day-28. Particularly, 4 hours post, administration, 99.5% of the radioactive dose was found in the liver and at 28 days 88.9% of the dose was detected in the liver. The spleen and the kidneys contained: spleen between 2.5% and 4%, and kidneys between 0.05% and 0.2%. All other tissues had lower contents. The bones (including the marrow) and the remaining tissues contained 1% and 0.7%. The radiolabelled compound was excreted in urine and within 28 days the excretion amounted to roughly 1%. Highest excretion occurred on day 1. Excretion by the faeces, indicative of biliary excretion, amounted to 3% over 28 days.
Kreylig et al.,(2017b)
116. A similar experiment to the one described above was also carried out, with the animals exposed via the oral route (a single dose of an aqueous, radiolabelled-nanoparticle suspension by intra-esophageal instillation) in female Wistar-Kyoto rats.
117. Titanium concentrations were determined at five retention time points 1 h, 4 h, 24 h, 7 d and 28 d after gavage in four rats for each time point. However, after observing in the seven-day experiment that fecal excretion of the test item was complete after 4–5 days, no further animals were sacrificed for a 28-day biodistribution.
118. Blood, all organs, tissues and excreta were collected and the concentration of radioactive titanium dioxide was measured. Most of the radioactivity was excreted in the faeces. Absorption was calculated as the fraction of the dose that could not be accounted for by the radioactive content of the intestinal tract plus faeces. Approximately 0.6% of the dose was absorbed within an hour post-treatment. Seven days post treatment, roughly 0.05% of the dose administered was still present.
119. The authors noted that the distribution patterns between animals were variable and that several data were below the LOD during the first 4 hours. The spleen, kidneys, heart and uterus contained detectable levels even after 4 hours post treatment. Maximum retention was reached in the spleen, kidneys and heart at 24 hours post-treatment. In the liver, lung and blood, nanoparticle retention declined from 4 hours to 7 days. In the brain, uterus and kidneys, the highest concentrations were observed at day 7. The peak concentration in liver and spleen was 12.5% (4 hours) and 2.6% (24 hours) of the absorbed dose, respectively. According to the authors, due the slow excretion kinetics, accumulation of systemically circulating particles in specific cells and organs is likely to occur in subjects chronically exposed to titanium dioxide nanoparticles. When comparing the biodistribution of the radioactive titanium dioxide nanoparticles retained after oral administration with the results obtained after intravenous injection (Kreyling et al., 2017a), the authors concluded that the kinetics patterns are very different and intravenous injection does not appear an adequate surrogate for assessing the biodistribution occurring after oral exposure to titanium dioxide nanoparticles.
Geraets et al., (2014)
120. In the EFSA 2021 Opinion, only the i.v experiment was discussed, however this paper also contains the result of an oral administration experiment.
121. The test item used was: NM-100, NM101, NM-102, NM-103 and NM-104
122. In the oral study, the single dose groups received a gavage dose of 2.3 mg/rat corresponding to 6.8-8.6 mg/kg bw. The repeat dose groups received five consecutive daily administrations of 2.3 mg titanium dioxide in one mL per rat resulting in a cumulative dose range of 34.1- 42.4 mg/kg bw for male rats and 54.5- 59.9 mg/kg bw for female rats.
123. In the i.v. study, the suspensions prepared contained 2.3 mg titanium dioxide/mL. The single i.v. dose treated rats received a dose of 8.4-9.8 mg/kg bw and 12.4- 14.1 mg/kg bw for male and female rats respectively, via the tail vein. The repeated dose treated rats received a cumulative dose that ranged from 42.3-49.4 mg/kg bw and 61.2-71.9 mg/kg bw for male and female rats, respectively. Thus the actual dose in mg/kg bw depended on the weight of the rats.
124. After repeated oral exposure (overall dose of 11.5 mg Titanium dioxide) titanium levels were near or below the detection limit in liver and spleen, indicating a very low absorption. In two out of 30 liver/spleen samples of exposed animals (for NM-102 and NM-103) titanium levels were above the LOD, whereas all mesenteric lymph nodes (MLN) samples (including controls) contained titanium amounts above LOD. Only a small increase in titanium content was observed, because the background levels in MLN were 2–3 times the LOD. MLN from control rats contained 0.14 μg titanium whereas the highest titanium average was 0.36 μg and was located in MLN from NM-104 exposed rats. This gives an increase of 0.226 μg titanium in MLN or 0.003% of the 6895 μg Titanium exposure in the dose. The total recovery of dosed titanium in all tested organs (expressed as % of the total dose) was estimated to be approximately 0.02%.
125. In the i.v. study, the highest levels were observed in the liver, but redistribution to the spleen was observed over the 90 day post-exposure period Day 2/Day 6 and Day 90). Redistribution to remaining tissues was not identifiable. The authors hypothesised that release of particles from liver and possibly other organs may be responsible for the increase in spleen levels. Titanium was detected in all investigated tissues in the present study, i.e. blood, liver, spleen, kidney, lung, heart, brain, thymus and reproductive organs.
126. Both after single and repeated i.v. exposure, blood titanium levels in blood decreased rapidly during the first minutes after which the titanium levels slowly decreased and approached the limit of detection at 24 hours post exposure.
127. Based on the available data, the authors concluded that elimination of total Titanium dioxide has a long half-life. For the liver which was considered the main target organ, the estimated half-life was 28–248 days.
128. The authors considered that the data showed that at the long run Titanium dioxide particles will accumulate in the spleen. Finally they noted that the expected accumulation with daily exposure as a consequence of the negligible elimination might indicate a potential concern for human health risk.
Tassinari et al., (2014)
129. The test material used was titanium dioxide nanoparticles (anatase, primary size <25nm, BET surface area 45–55 m2 /g, purity 99%).
130. Male and female Sprague-Dawley rats (7 rats/sex/dose) were treated with 0, 1, 2 mg/kg body weight (bw) per day by gavage. The controls received distilled water.
131. Twenty-four hours after the last treatment (day 6), male and female rats were anaesthetised and blood samples were. Subsequently, the animals were sacrificed and the uterus, ovary, testes, thyroid and adrenals were excised and weighted. Spleen was sampled both for histopathological examination and for studying tissue deposition of Titanium dioxide nanoparticles.
132. The titanium content in the samples was determined by ICP-MS using an Elan DRC II spectrometer. The highest titanium concentration was detected in the thyroid, however the levels was no statistical significance between treated animals and controls (higher dose, 2 mg/kg bw per day and at 0.24 ± 0.09 vs. 0.22 ± 0.04 mg/g in controls). A significant increase was observed at the higher dose in the ovary (0.28 ± 0.07 vs. 0.12 ± 0.04 mg/g fresh weight). Levels in uteri were low (0.051 ± 0.006 vs. 0.49 ± 0.04 mg/g fresh weight). A significant increase in the concentration of titanium in the spleen concentration was observed at a dose of 2 mg/kg bw per day.
134. Overall, the authors considered that these results indicated the potential for titanium dioxide bioaccumulation.
Hendrickson et al., (2016)
134. The test material used was: NM-101 (5-10nm) and NP-25 (20-25nm).
135. Male Sprague Dawley rats were treated with 250 mg/kg bw/d of either one of the test materials, dispersed in an aqueous starch solution containing 0.1% Tween-80 and sonicated via intragastrical administration for 28 days.
136. Within a day of the last exposure, the animals were sacrificed and blood samples as well as samples from the lungs, liver, spleen, testes, small intestine heart, stomach and kidneys were harvested.
137. For animals treated with NM-101, titanium dioxide nanoparticles were detected in all organs and tissues. The organs with the highest concentrations were the spleen (0.227 μg/g) and liver (0.147 μg/g). In the kidneys, small intestine and testicles similar amounts of nanoparticles were detected (0.092, 0.098 and 0.089 μg/g respectively). Titanium dioxide nanoparticles were detected at 0.028 μg/g in the heart, 0.04 μg/g in the lungs and 0.049 μg/g in the brain.
138. In NP-25 treated animals accumulation of titanium dioxide nanoparticles were detected in the small intestines and liver (0.29 μg/g in the liver) and at low levels (0.01 μg/g) in the kidneys. In the spleen, it was detected at levels of 0.29 μg/g of the organ. No titanium dioxide nanoparticles were detected in the lungs, brain, testicles, heart or blood.
139. The authors concluded that biodistribution differs between smaller (NM-101) and larger (NP-25) titanium dioxide nanoparticles, with the smaller ones showing a greater distribution spread and accumulation in all organs. The smaller particles exhibited reduced but similar tendency. The main difference was that the larger nanoparticles could not overcome the blood brain barrier and penetrate the brain.
140. Due to the fact that the detected levels accounted for less than 1% of the administered dose, the authors concluded that the data was evidence of the limited bioavailability and efficient excretion of titanium dioxide.
Ammendolia et al.,2017
141. The test material used was titanium dioxide nanoparticles (anatase, primary size <25nm BET) surface area 45-55 m2 /g, purity 99%, suspended; the suspensions were sonicated.
142. Sprague-Dawley rats (10/sex/group) were treated with 1 or 2 mg/kg bw/d titanium dioxide nanoparticles or vehicle only (ultrapure water) via gavage for 5 consecutive days.
143. A day (24 hours) after the last dose, the animals were sacrificed and the small intestine was excised. A piece of jejunum was used for histological analysis and the remaining part of small intestine was sampled for studying either tissue accumulation of titanium dioxide nanoparticles, determined as titanium by ICP-MS.
144. Titanium was detected at in small intestine tissue at 0.08±0.02lg/g in the control, 0.09 ± 0.02l g/g in the low dose group and at 0.13 ± 0.03lg/g at the high dose group.
Hendrickson et al., 2020
145. The test material used was titanium dioxide nanoparticles, rutile, rod/ needle like shape, 5930 nm.
146. Wistar rats were treated with 50 mg/kg bw titanium dioxide using an isolated intestinal loop technique.
147. Three hours post treatment, the isolated loop was cut out. The liver and spleen were collected.
148. The presence of particles in tissues was studied by TEM and diffraction analysis. Loose agglomerates (100 nm and larger) were detected. Diffraction analysis was used to confirm that the particles were titanium dioxide. Titanium dioxide nanoparticles were detected on the surface and between the microvilli of the mucosal cells of the small intestine and also in the mucosal tissue. Nanoparticles were detected in the Peyer’s patches, both as single nanoparticles and agglomerates of sizes ranging between 20 and 60 nm. In the liver, parenchymal tissue aggregates of titanium dioxide nanoparticles (150–200 nm) and up to 300 nm were seen. In the spleen red pulp, single nanoparticles (20–30 nm), agglomerates (up to 100 nm) and conglomerates (up to 800 nm) were observed.
Chen et al.,2020a
149. The test material used was titanium dioxide nanoparticles anatase, 29 nm (SEM).
150. Sprague- Dawley rats were dosed with 0, 2, 10, 50 mg/kg bw by oral gavage for 90 days. The test material was sonicated prior to treatment.
151. The tissue distribution of the titanium dioxide nanoparticles was evaluated by determining the titanium content in blood and tissues including liver, stomach, small intestine, colon, spleen, heart, lung, kidneys and testicles by high resolution ICP-MS.
152. Significantly increased titanium dioxide nanoparticle levels were only detected in the colon of rats exposed to 50 mg/kg test material, compared with the control group. There was no dose-response relationship, however. The authors hypothesised that the significant increase of titanium dioxide in colon tissue was due to the titanium dioxide nanoparticles attaching on the surface of the colonic mucosa tissue and not in mucosa cells. As most of orally ingested test material was excreted through feces, it resulted to long-term retention in large intestine. The titanium dioxide nanoparticles did not enter the colon epithelial cells and were mainly deposited in the intestinal cavity or between villi. The content of Ti in all tissues was very low, which was approximately 0.0001%-0.00001% or 100-1000 ng/g tissue, except for the colon of the high dose group. All spleen and heart tissue samples from rats contained very low titanium levels, which were below the limit of detection (LOD) of 0.032 μg/g. Finally, they concluded that the results indicate that the absorption and distribution of titanium dioxide nanoparticles was very low after low-dose and long-term oral administration.
153. The EFSA evaluation also cites a second study by Chen et al¸(2020b), however it has not been described in the current paper as, following evaluation by the Secretariat it appears to investigate effects on lipid metabolism rather than addressing toxicokinetics/absorption.
Human studies
Heringa et al (2018)
154. Titanium was measured using high resolution ICP-MS in liver and spleen from 15 deceased human subjects (nine women and six men) who had donated their bodies for research and educational purposes. The LOD of the method was 10 ng/g tissue.
155. TiO2 particles were detected in 7/15 liver and 13/15 spleen samples. The number-based TiO2 particle size distributions in liver and spleen were comparable and had a size range of 85–550nm and 85–720nm, respectively. In the tissues, 24% of the TiO2 particles in the number-based size distribution was < 100 nm, but this fraction may be underestimated considering that the smallest titanium dioxide particle that could be detected with the method used was 85 nm.
156. The particle mass concentration in liver ranged from 0.01 to 0.3 mg titanium/kg tissue. In the spleen, the concentration ranged from 0.01 to 0.4 mg titanium/kg tissue. The average concentration in samples where titanium could be determined was 40 ng/g in the liver and 80 ng/g in the spleen.
157. Small tissue grains of liver and spleen from two subjects were analysed using SEM-EDX to visualize the titanium dioxide particles. The observed particles were composed of titanium and oxygen and were present as an aggregate or agglomerate, consisting of smaller primary particles of 75–150 nm. Presence of titanium was also confirmed semi-quantitatively by EDX analysis in dry-ashed liver and spleen samples.
Peters et al (2020)
158. Post-mortem human liver, spleen, kidney, jejunum and ileum samples were analyzed from 15 human subjects, 7 male and 8 female, who died at the age of 64–98 years. From these persons, written informed consent was obtained during life that allowed the use of their entire bodies for educational and research purposes.
159. The total titanium concentration in the organs ranged from 0.01 to 2.0 mg titanium/kg tissue with an average value of 0.17 mg titanium/kg tissue and a standard deviation of 0.33 mg/kg. The authors considered that this was an indication of large differences between subjects and organs. The highest concentrations were detected in the jejunum and ileum (average of 0.34 and 0.43 mg titanium/kg respectively), followed by the kidney, spleen and liver (0.08, 0.06 and 0.03 mg titanium/kg respectively).
160. The particle sizes were measured by spICP-MS and ranged between 50 and 500 nm in the different tissues (50 nm was the lower size detection limit). The titanium dioxide particle concentrations were considered by the authors to represent about 80% of the total titanium concentration.
EOGRT Study - Toxicity of Titanium Dioxide as a Food Additive
In this guide
In this guideThis is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
161. The Extended One Generation Reproductive Toxicity (EOGRT) study was commissioned by interested business operators to address the data gaps identified in 2016. The protocol was later amended to accommodate the investigation of additional parameters related to the occurrence and titanium dioxide-related induction of aberrant crypt foci (ACF) in the colon (preneoplastic lesions that had been reported by Bettini et al. (2017). Methodology: Test Material, Doses, Administration of Treatment.
162. The test material: Titanium dioxide E171-E, Particle size (ECD); (number measurement, primary particle size) x10 = 0.070 µm x50 = 0.110 µm x90 = 0.180 µm via the diet.
163. The doses used were: Group 1: 0 mg/kg b.w./day, Group 2: 100 mg/kg b.w./day, Group 3: 300 mg/kg b.w./day, 4: 1000 mg/kg b.w./day 20 male and 20 female rats evaluated. The concentration of the test item in the diet was adjusted based on the mean group food consumption per sex. The concentration was adjusted weekly using the food consumption values from the previous week.
164. The test item was administered in graduated doses to several groups of males and females prior to, during and after mating until weaning of the F1 and F2 Generation. The F1 Generation was dosed in the same way as the F0 Generation after weaning. Until weaning, the exposure of the F1 Pups to the test item was indirectly through the breast milk, however the pups additionally received the test item directly when commencing feeding by themselves during the last week of the lactation period. The duration of dosing depended on the requested endpoints for the different cohorts of the F1 Generation. Cohort 1B animals were maintained on treatment beyond PND 90 and bred to obtain an F2 Generation. Detailed examination of key developmental endpoints, such as offspring viability, neonatal health, developmental status at birth, and physical and functional development until adulthood, was performed to identify specific target organs in the offspring. Possible endocrine disruptor effects of the test item were also examined.
Evaluation of Sexual Function and Fertility
Male fertility:
165. An overview of the results for male fertility parameters is reported in Annex A. No statistically significant or dose-related effects on sperm motility, total spermatids/gram testis, percentage of abnormal spermatozoa and male mating index were observed in the F0 generation. The slight decrease in the number of successful matings at doses of 300 and 1,000 mg/kg bw per day appears unrelated to the male partners, as all males that failed to impregnate their females showed normal sperm motility and sperm counts. Only one of the high-dose males was found to have a lower testicular spermatid content (50% of the group mean), a finding that was also associated with a slightly lower testis weight (85% of the group mean). The number of abnormal sperm was low in all dose groups and remained below 2% in the few males in which abnormal sperm were found.
166. The Panel noted that the epidydimal sperm parameters were not evaluated but that this deviation has no effect on the final conclusion of the study. There were no effects on any of the sperm endpoints in the cohort 1A.
Female fertility:
167. An overview of results for female fertility parameters is reported in Annex A. No effects on mean oestrus cycle duration were noted in F0 and F1 (cohort 1B) parental generations and all F0 females in the control, 100, 300 and 1,000 mg/kg bw per day groups mated. In the F1 generation 2 and 3 animals from the mid- and the high-dose groups, respectively, were erroneously removed from the study, before mating had been unequivocally confirmed. All other females mated, except one F1 female in the 100 mg/kg bw per day group. With few exceptions, mating occurred at the first oestrus after the females were housed with males. No effects of treatment were observed. The pregnancy rate was slightly lower in the F0 generation at 300 and 1,000 mg/kg bw per day (100, 96, 92 and 92%). As this finding was not confirmed in the F1 generation (100, 95, 94 and100%) the Panel considered it as incidental and not treatment-related.
168. No effects were noted on pregnancy duration, number of implantation sites and post-implantation loss. Although they occurred in the mid-and high-dose groups, three single total litter losses, either from total resorption of all embryos or from death of the litter during or shortly before birth, were not considered to be due to treatment. This is because the two F0 dams had unusually small litters of two pups each, which were stillborn, and the F1 dam showed total resorptions of eight implants at necropsy after failing to litter. Live litter sizes and litter weights were comparable to control values in all dose groups in the F0 and the F1 generation.
169. The EFSA Panel concluded that there were no indications of effects on general toxicity, thyroid or sex hormone levels, reproductive function and fertility in either male or female rats, no effects on pre- and postnatal development or on neurofunctional endpoints in F1 offspring.
170. The EOGRT study with E 171 did not indicate adverse effects up to a dose of 1,000 mg/kg bw per day. Also, no effects were seen in studies retrieved from the literature with TiO2NP < 30 nm up to the highest dose tested of 100 mg/kg bw per day.
Developmental Toxicity
171. Pre-and postnatal lethality and structural abnormalities: No treatment-related pre- or postnatal loss was observed in the F0 and F1 generations. The average litter size at birth in all dose groups was comparable or higher than in the control group and the sex ratio was unaffected. No external or internal abnormalities were detected in F1 and F2 pups at termination.
172. Growth and sexual development: An overview of the results related to growth and sexual development for the F1 and F2 generations is included in Annex A. No treatment-related effects were observed in birth weights and growth of the pups. There were no indications for any androgenic and/or oestrogenic effects on the male and female anogenital distance (AGD) and the retention of nipples in males.
173. The mean age at vaginal opening was comparable between control and treated groups. The statistically significant lower body weight on the day of vaginal opening in cohort 1A at 300 mg/kg bw per day was not considered to be biologically relevant due to the slightly higher litter sizes in all treated groups. A divergence from the required method was examination of balanopreputial gland cleavage instead of examining balanopreputial separation which does not comply with the OECD TG 443 and therefore cannot be considered a measure of puberty in males.
174. Neurofunctional screening: Male and female F1 cohort 2A offspring were tested for auditory startle response between PND 23 and 25, and for a functional observation battery including grip strength evaluation and for quantitative locomotor activity between PND 58 and 64. No differences in the response to an auditory startle stimulus were observed between the control and all the tested doses. Compared to controls, an increase in hindlimb splay was observed in females, reaching statistical significance at 100 and 1,000 mg/kg bw per day. A statistically significant increase in mean forelimb grip strength was noted at 300 mg/kg bw per day in both males and females.
175. To check whether the significant differences in grip strength and hindlimb splay could be due to systematic bias in group testing order, the testing order was checked The Panel considered that there was no systematic bias in group testing order and that this was therefore not a plausible explanation for the observed group differences. Grip strength and hindlimb splay belong to the same domain of neurological function, i.e. motor function and/or sensory–motor coordination. However, the effects observed (i.e. increase in hindlimbs play and increase in mean forelimb grip strength) seem to point in opposite directions when it comes to muscle strength. In particular, an increase in hindlimb splay can be interpreted as muscular weakness whereas an increase in mean forelimb grip strength could be indicative of myotonia.
176. The Panel noted that the effects observed were not correlated to any other changes (e.g. alterations in muscle tone, righting reflex, gait, wire manoeuvre, posture). No dose response was observed for any of these endpoints or for the two functional measurements, indicating that the likelihood of an association with test substance is low. No other changes in the functional observation battery measurements or locomotor activity were noted.
177. Furthermore, there were no notable histopathological findings in brain or in peripheral nerve (sciatic). Based on all the above considerations, the Panel considered that the effects on grip strength and hindlimb splay were not treatment-related. However, the Panel noted that quantitative information on peripheral nerves was not available. Overall, the Panel considered that E 171 had no adverse effects on neurofunctional endpoints in F1 cohort 2A offspring at the doses used.
178. EFSA conclusions on developmental toxicity results of the EOGRT study: No effects of E 171 on pre- and postnatal development were observed. Data on the attainment of puberty in males (i.e. an appropriate assessment of the timing of the balanopreputial separation) were missing. The Panel did not consider this to be critical in this case.
Developmental Immunotoxicity
179. Effects on developmental immunotoxicity were determined in the F1 cohort 3 animals through an examination of their ability to raise an antibody response to a foreign antigen. Animals are sensitised and the primary IgM antibody response to the sensitising antigen, in this case to keyhole limpethaemocyanin (KLH) antigen, is measured. The ability of the test compound to modulate serum anti-KLH antibody titre is taken as indicative of a developmental immunotoxic effect. A KLH-immunised control group also exposed to a known immunosuppressant (i.e. cyclophosphamide (CY), resulting in at least 50% inhibition in serum IgM anti-KLH titre, is considered crucial for the verification of assay performance.
180. These data can be considered in combination with additional data related to potential immunotoxic effects. In the F1 cohort 1A animals, the following may contribute to the general assessment for immunotoxicity: weight and histopathology of the spleen, thymus and lymph nodes, as well as bone marrow histopathology, total and differential peripheral WBC count and splenic lymphocyte subpopulation distribution. T-cell-dependent anti-KLH response (KLH assay). Determination of serum anti KLH-IgM antibodies was performed in F1 cohort 3 (10/sex per group, PND 53–61) using an enzyme-linked immunosorbent assay (ELISA).
181. The animals were sacrificed 5 days after intravenous bolus injection (tail vein) of KLH, blood was withdrawn and the level of anti-KLH IgM was measured in serum. In addition, satellite animals of F1 (10/sex, PND 55) were immunised with KLH and treated with CY (single administration of 40 mg/kg bw by gavage on the same day of KLH treatment) to provide a positive control (for an inhibition of immune response).
182. A slight, but statistically significant decrease in the antigen specific IgM level was measured at the highest dose tested (1,000 mg/kg bw per day) in males only (–9%) and without an apparent dose response. In addition, the Panel noted that treatment with CY was not performed at the same time as the rest of F1 cohort 3, without a separate control for the CY response, conducted at the same time.
183. The sensitivity of the test was not demonstrated due to invalid CY positive control results. It was noted that the assay conditions may have not been optimal resulting in an apparent low antibody response to KLH when compared to literature (Gore et al., 2004).
184. It was considered that all tested animals in the study had a weak immunogenic response to KLH that was insufficient to identify a T-cell-dependent immunotoxic effect of E 171 therefore no conclusion can be drawn on the effect of E 171 on the developing immune system. The Panel agreed with the conclusion of the study authors.
185. Assessment of pathology, haematology and splenic lymphocyte subpopulations At necropsy, pathology of lymphoid organs, haematology and lymphocyte subpopulations in the spleen were investigated. The following lymphocyte subpopulations were determined via flow cytometry analysis (FACS): T cells, T helper cells, T suppressor/cytotoxic cells, NK cells and B cells. The Panel noted that haematology, spleen weight and histopathology of lymphoid organs in animals from F1 cohort 1A did not indicate any dose-related effects.
186. For the splenic lymphocyte subpopulation analysis, no statistically significant differences were observed in the percentage of T cells, T helper cells, T suppressor/cytotoxic cells, NK cells and B cells of any of the treated groups compared to control in both sexes. The study authors concluded that no test substance-related effect was observed on the proportion of the examined lymphocyte subtypes. The Panel agreed with the study author conclusion that the splenic lymphocyte subpopulations in this cohort were not affected. However, the Panel considered that an isolated observation in F1 cohort 1A is not sufficient to conclude on immunotoxicity.
187. Compared to the animals of F1 cohort 1A, F1 cohort, 3 animals showed a shift in the lymphocyte subpopulation that indicated activation of the immune system by injection of KLH and concluded that increased B-cell proliferation may have led to the production of antigen-specific antibodies. In the F1 cohort 3 animals, no differences in the relative size of the lymphocyte subpopulations were observed between the control group and the E 171-treated groups, after immunisation of the animals with KLH.
188. The proposed reason was that the B-cell shift in F1 cohort 3 was caused by KLH immunisation, supported by the fact that there was no such shift found for the positive control animals that were sensitised to KLH and treated with CY. It was also considered that KLH induced an immune reaction, and that this response was influenced by CY as expected; KLH would increase the percentage of splenic B cells and decrease the percentage of T cells.
189. The conclusion was that the immune response was affected by CY but was not adversely affected by the TiO2 test substance. The Panel did not agree with the conclusion of the study authors that a shift to B cells by KLH was substantiated. The Panel considered that it is incorrect to compare the groups of F1 cohort 1A and of F1 cohort 3 because the groups of animals of F1 cohort 3 had a different age than that of the animals in F1 cohort 1A at the time of sacrifice (PND 87–96 vs. PND 53–61, respectively). In addition, the FACS analyses on the splenic cell suspensions were not all performed in the same round of analysis but were performed separately, while it is known that this may have influenced staining and subsequent quantification. The authors suggested that even if the positive CY control did not perform as expected, the data still indicate there is no effect of E 171 on sensitisation to KLH.
190. It is worth noting that the EFSA Panel did not agree with this conclusion and overall considered that the data did not allow to conclude on developmental immunotoxicity with respect to E 171.
Immunotoxicity Summary
191. A marginal but statistically significant decrease in antigen-induced IgM levels (9%) in males of the F1 Cohort 3 only was noted, with no apparent dose-response.
192. The Panel noted that there were methodological shortcomings in the design of this part of the EOGRT study. Therefore, the Panel could not conclude on immunotoxicity.
193. Some findings regarding immunotoxicity and inflammation with E 171 as well as neurotoxicity with TiO2 Nanoparticles may be indicative of adverse effects including indications of an induction of ACF with E 171.
Neurotoxicity
194. EOGRT Study - Male and female offspring were tested for auditory startle response between PND 23and 25, including grip strength evaluation and for quantitative locomotor activity between PND 58 and 64. No differences in the response to an auditory startle stimulus were observed and an increase in hindlimb splay was observed in females, reaching statistical significance at 100 and 1,000 mg/kg bw per day. A statistically significant increase in mean forelimb grip strength was noted at 300 mg/kg bw per day in both males and females.
195. Grip strength and hindlimb splay belong to the same domain of neurological function, however, the increase in hindlimb splay and increase in mean forelimb grip strength are opposed in this case - increases in hindlimb splay indicate muscular weakness but an increase in mean forelimb grip strength may indicate myotonia. No dose response was observed for any of these endpoints or for the two functional measurements, indicating that the likelihood of an association with test substance is low. No other changes in observed including in histopathological findings in brain or in peripheral nerve tissue.
196. The Panel considered that the effects on grip strength and hindlimb splay were not treatment-related but that quantitative information on peripheral nerves was not available. Overall, the Panel considered that E 171 had no adverse effects on neurofunctional endpoints at the doses used.
197. No neurotoxicity studies performed with E 171 were identified from the published literature that were considered sufficiently reliable. Some papers were identified noting effects of TiO2 NP <30 nm but these are not discussed further in this paper.
Aberrant Crypt Foci
198. A satellite group of the EOGRT study used doses up to 1,000 mg/kg bw per day and up to this dose did not induce ACF in the colon.
Aberrant Crypt Foci Examination in Satellite F0 Animals (EOGRT Study).
Method:
199. Evaluation of ACF in the colon of a satellite group of F0 animals (10/sex per group) treated with 0, 100, 300 and 1,000 mg E 171/kg bw per day and terminated after weaning was undertaken. The colon was excised, opened longitudinally and the contents removed by rinsing with a 0.9% NaCl solution. Thereafter, the tissue was divided in parts of a suitable size for fixation by immersion in 5% buffered formalin. A blind examination of these samples stained with 0.5% (w/v) methylene blue in water was performed under a stereomicroscope at 50x magnification for presence of ACF. The Panel noted that the design of the study did not include a positive control group (e.g. treatment with a known gastrointestinal tract tumour initiator such as dimethyl hydrazine (DMH) for the development of ACF.
Results:
200. The definition of ACF used was ‘foci containing more than 2 ABCs’, taken from Shwter et al. (2016). No ACF were found in the colons of the control and the treated groups. A mildly increased morphological variability (increased size and intensity of the staining of a small portion) of the crypts in the two caudal parts of colon was observed in seven animals (See tables 1 & 2 below). These changes were assessed as inconsistent with the appearance and definition of ACF discussed above. Incidence of these single crypts observed in the mid and high doses was not significantly different from the control. The EFSA Panel agreed with this conclusion.
Table 1: Aberrant Crypt Foci Presence in Satellite F0 Animals.
|
Dosage Group |
Control, Aberrant Crypt Foci Present |
Low-Dose |
Mid-Dose |
High-Dose |
Total |
|
Females |
1/10 |
0/10 |
1/10 |
2/10 |
4 |
|
Males |
1/10 |
0/10 |
1/10 |
1/10 |
3 |
201. An additional submission of data included photomicrographs of mildly increased variability in crypt morphology from all seven animals. A re-examination was extended to an additional randomly selected nine control animals (4 males and 5 females) and eight high-dose group animals (3 males and 5 females). A mild increased variability in crypt morphology was observed in eight of the nine controls and six of the eight high-dose animals (see Table x). The Panel considered that oral exposure to E 171 at doses up to 1,000 mg/kg bw per day did not induce ACF in the colon.
Table 2: Aberrant Crypt Foci Presence in the Re-Examination of Satellite F0 Animals.
|
Dosage Group |
Control, Aberrant Crypt Foci Present |
High-Dose |
Total |
|
Females |
4/5 |
3/3 |
7 |
|
Males |
4/4 |
3/5 |
7 |
202. With regards to the induction of aberrant crypt foci, EFSA considered additional studies available in the literature in order to form their conclusions. These studies are discussed in detail below.
Bettini et al 2017
Test materials:
203. The test materials used in this study were:
I. E 171, anatase, 20–340 nm (118 nm) (TEM); 44.7% particles<100 nm;
II. TiO2 Nanoparticles (NM-105), anatase/rutile, 15–24 nm. Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure) of 1.
Internal exposure:
204. Qualitative measurement in tissues, methodology reliable with some limitations. Adult Wistar rats were administered by gavage 10 mg E 171/kg bw per day for one week or 100 days. In addition, a group of animals were exposed to TiO2 Nanoparticles (NM-105) 10 mg/kg bw per day for one week.
Method:
205. First experiment: 12 male rats were pre-treated with a single injection (180 mg/kg intraperitoneal in isotonic saline) of DMH. The aberrant crypts average per colon was 190 ACF and 30 large ACF after 100 days. In DMH pre-treated rats also subsequently (7 days later) exposed to either 0.2 or 10 mg/kg bw per day E 171 in drinking water (12 rats/group), there was a statistically significant increase per colon in number of aberrant crypts and large ACF and a statistically non-significant increase in total number of ACF in the high-dose group compared to DMN only controls. No statistically significant differences were observed between the low-dose and control groups. The incidence of ACF was not reported.
206. Second experiment: Male rats received either drinking water (12 controls) or 10 mg E 171/kg bw per day in drinking water (n=11) for 100 days. No ACF were observed in the colons of controls. Four rats in the treated group developed one to three ACF per colon (which in three rats consisted of 1–3 aberrant crypts/ACF with the remaining rat having 12 aberrant crypts in an ACF). The increase in the incidence of rats with ACF (4/11 vs. 0/12 in the control group) was statistically significant.
207. The Panel considered that these data indicate that E 171 has pro-inflammatory potential at the systemic level, paralleled by the development of an inflammatory microenvironment in the intestinal mucosa.
208. The Panel considered that E 171 alone at a dose of 10 mg/kg bw per day may induce development of ACF in male rats. The Panel also noted that E 171 at a dose of 10 mg/kg bw per day increased the number of ACF initiated by a genotoxic carcinogen (not considered further in this paper).
Results:
209. Titanium was detected in the immune cells of Peyer’s patches in which patches dendritic cell percentage were increased. Effects were not noted in the spleen. It was noted that this effect was transient (observed at 7 days but not at 100 days). The percentage of regulatory T cells and T-helper (Th) cells were significantly decreased at both time points in E 171 exposed animals. Stimulation of immune cells isolated from Peyer’s patches showed a decrease in T-helper (Th)-1 IFN-c secretion, while splenic Th1/Th17 inflammatory responses sharply increased, as measured in cells taken from exposed rats, stimulated in-vitro with anti CD3/CD28 antibodies.
Intestinal mucosal inflammation:
E 171 treatment:
210. One week = no intestinal inflammation, 100-day = colon microinflammation evidenced by significantly increased IL-1b, IL-8 and TNF-a expression in the colon in addition to increased IL-10. Aberrant crypts were examined in the colon after staining with methylene blue. Data on the effects of TiO2 Nanoparticles on intestinal mucosa were not presented.
211. ACF Definition: The authors did not explicitly give their definition of an aberrant crypt foci (ACF) but the Panel presumed it was 1-or more aberrant crypts/ACF. The authors defined a ‘large ACF’ as consisting of more than three aberrant crypts per ACF.
Blevins et al. 2019
212. The test material used was E 171, anatase, 110–115 nm (SEM), 36% particles <100 nm. Scoring for nanoscale considerations (dispersion and/or confirmation of internal exposure): 3.
213. Internal exposure was not examined.
Method:
214. Six-week-old male Wistar Han IGS rats were exposed to E 171 in a standard diet at 4 concentrations between 0-5,000 mg/kg diet concentration in two studies of 7 days (n=5/group) and 100 days (n=15/group) (equal to 1.8, 4.8, 31.4, 374 mg/kg bw per day) for 7 and (equal to 1.3, 3.5, 22.4 or 267 mg/kg bw per day) for 100 days. The two studies were performed at different Institutions, with the 7-day study performed twice whereas the 100 day study was performed once.
215. The objectives of the study were to evaluate the acute (7 days) and sub-chronic (100 days) effects of dietary E 171 exposure on the immune system of the GI tract and periphery as well as to evaluate effects of the sub-chronic exposure either alone or after pre-administration of a known intestinal genotoxic carcinogen, DMH and an examination of colon for presence of aberrant crypt foci and of aberrant crypt was included.
216. Immune system metrics: Phenotyping of immune cells (i.e. CD103+DC, total and activated T helper cells, total and activated Treg cells) and inflammatory cytokines [(IL-1a,IL-1b, IL-6, interferonc (IFNc), IL-12p70, IL-17A, IL-18, IL-33. CCL2/MCP-1, CXCL1/KC (IL-8), GM-CSF and tumour necrosis factor a(TNF-a).
217. 7-Day Studies: Rats were randomised into 4 groups of 5 animals and the data from the two studies were pooled. Total food and water consumption were determined at the end of each study. Body weights were determined at the start and end of the 7-day. As calculated by the Panel and taking the mean of the two periods given in the paper: 1.5, 3.9, 25.5, 294 or 1.1, 3, 19, 236 mg/kg bw per day for weeks 1-10 or 11-15, respectively (groups 1–4) and 1.5, 4.1, 25.7, 300 or 1.1, 3.1, 19.2, 237 mg/kg/bw per day for weeks 1–10 or 11–15, respectively (groups 5–8) exposure period at the time of euthanasia.
218. 100-day Study: Animals were randomised into 8 groups of 15 animals each. At the start of the study, animals in groups 5–8 were treated with a single intraperitoneal injection of a sterile dose of 180 mg/kg bw dimethylhydrazine (DMH) dihydrochloride while groups 1–4 were treated with vehicle only. Seven days after intraperitoneal injection, dietary administration of 0, 40, 400 or 5,000 mg/kg diet E 171 was started and continued for 100 days. Bodyweights were determined weekly beginning on day 0 of the study and just prior to euthanasia. Food consumption was determined weekly beginning with administration of the E 171 supplemented diets. Water consumption was determined during weeks 3, 8 and 13 of the study.
Results:
219. No significant changes in food intakes or body weights or liver and spleen weights were found and no mortality was observed. A trend towards increased food consumption in rats of the high E 171 group was observed. Dietary E 171 produced no general signs of overt toxicity at the highest dose tested, over 100 days.
220. Following the 7- and 100-day feeding periods, rats were euthanised and measurements of inflammatory cytokines (using the LEGEND plex rat inflammation Panel) and phenotyping of immune cells (by-flow cytometry) in the periphery and GI tract were performed. Peyer’s patches, peripheral blood mononuclear cells (PBMC) and spleen cells were analysed for inflammatory and regulatory T-cell responses directly ex-vivo or after in-vitro stimulation with anti-ratCD3 (5lg/ml) and anti-rat CD28 (5lg/ml) for 4 days. Histopathology, ACF, ABC and goblet cell evaluations were performed on rats in the 100-day study.
221. All tissues were collected from well-defined areas, and measurements, procedures and evaluations were performed in a standardised and blinded manner. CD103+dendritic cells (DC) were evaluated in the gut, Peyer’s patches, spleen and in peripheral blood over time period. No change in the percentage of CD103+DC in peripheral blood, spleen or Peyer’s patches due to acute or sub-chronic dietary E 171 consumption alone was observed. The total percentage of CD4+T helper cells, the percentage of T helper cells expressing CD25, an indicator of T helper activation, and the percentage of Treg cells (CD4+FoxP3+) and activated Treg (CD4+CD25+FoxP3+) which could lead to a low level inflammatory response in the absence of increased inflammatory cells, were quantified in peripheral blood, spleen and Peyer’s patches. Dietary E 171 exposure did not change the frequency of CD4+T helper cells systemically or in intestinal Peyer’s patches. In addition, there was no detectable impact on the percentage of activated CD4+T helper cells or on the percentage of Treg cells either peripherally or locally in the Peyer’s patches of treated rats fed for 7 or 100 days. Collectively, these results suggest that E 171 consumption does not alter T-cell-mediated mechanisms of immune control, either promoting inflammatory CD4+T helper cell activation or in reducing the percentage of anti-inflammatory Treg cells.
222. Regarding the effects on cytokines, the data presented suggest that dietary E 171 does not induce inflammation peripherally or in the GI tract at both time points. In addition, studies were conducted to explore the possibility that E 171 might alter the effector cytokine profile of T helper cells in lymphoid tissue or circulation, which may not be manifest without T cell-specific stimuli. Lymphocytes were isolated from peripheral blood, spleen and Peyer’s patches and activated ex-vivo with anti-CD3/anti-CD28 for 4 days to induce T helper cell cytokine production. No effects of E 171 exposure on any of the induced cytokines produced from ex-vivo stimulated T helper cells were observed.
223. In the 100-day study, all animals were treated with E 171, some groups were initiated with 180mg/kg bw DMH before the start of the dietary exposure to E 171 and an additional control initiated with DMH was also included. The same parameters as described above were evaluated, with some differences observed. A modest increase in the relative spleen weight in 22.4 mg E 171/kg bw per day + DMH compared to not initiated animals, an increase in IL-17A in colon (22.4 mg E 171/kg bw per day + DMH) and IL-12p70 in plasma (3.5 mg E 171/kg bw per day + DMH), with no dose-related effects, were observed. There were no changes in spleen cellularity across any of the treated groups. No changes were observed in the percentage of CD103+DC, CD4+T helper cells or total or activated.
224. Safety assessment of the food additive titanium dioxide in peripheral blood, spleen or Peyer’s patches in animals exposed to E 171+DMH compared to animals treated with only DMH. No treatment related histopathological changes in the duodenum, jejunum, ileum, spleen, liver, lung and testes in animals exposed only to E 171 were found. Rats that were initiated with DMH only and those which received 171 in the diet after the initiation displayed several histopathological abnormalities. There were two invasive adenocarcinomas in one animal in the 1.3 mg E 171/kg bw per day + DMH group, and single adenomas in one animal in the 3.5 mg E 171/kg bw per day E 171+DMH group and in one animal in the 22.4 mg E 171/kg bw per day + DMH group.
225. There were no other histopathological changes in the large intestines of the other animals treated with DMH. One rat in the 1.3 mg E 171/kg bw per day +DMH group and one rat in the 22.4mg E 171/kg bw per day +DMH group had subpleural lymphocytes in the lung, but without any evidence of acute inflammatory changes or hyperplasia.
226. Limitations: A significant amount of the epithelial surface of the sampled colon (proximal, middle and distal) was obscured when observed by light microscopy and so were unable to examine the entire surface of the colon samples. The results for the areas of epithelium that were examined indicated an increase in ACF/cm2 and ABC/cm2 in groups initiated with DMH compared to the groups that were not initiated with DMH. E 171 treatment administered after DMH did not result in statistically significant increases in aberrant crypt foci or aberrant crypts or any change in the number of aberrant crypt foci or aberrant crypt were observed due to E 171 exposure alone.
227. The Panel noted a considerable variability in the results, which may mask possible effects. Furthermore, the Panel noted that the examination for presence of ACF and ABC was not performed on the whole colon but was limited to three 2 cm long samples (one from the proximal, mid-portion and the distal parts).
228. Dietary E 171, with or without treatment with DMH, had no effect on the length of the colonic glands examined or the number of goblet cells/unit. The Panel considered that this study indicates that acute and sub-chronic dietary intake of E 171 resulted in no significant effects on either peripheral or GI tract immune homeostasis as evidenced by immune cell phenotyping or inflammatory cytokine analysis. Limitations in the pathological examination for ABC and ACF preclude a conclusion on potential for ABC and ACF formation.
Overall EFSA conclusion on ACF
229. The Panel considered that there was uncertainty regarding the extent of the internal exposure to E171 TiO2 nanoparticles across the range of tested doses. The Panel considered that the effect of E 171 in producing ACF reported by Bettini et al. (2017) was not replicated in later investigations (EOGRT study and Blevins et al., 2019).
230. One source of uncertainty was that it was noted that there were methodological limitations in Blevins et al. A further source of uncertainty is being unclear to what extent animals were exposed to TiO2 Nanoparticles in both the EOGRT study and Blevins et al. The Panel concluded that E 171 may induce ACF in male rats at a dose of 10 mg/kg bw per day when the test substance is pre-dispersed and stabilised in a liquid medium preventing agglomeration of nanoparticles prior to administration by gavage.
EFSA’s Concluding remarks
231. No reproductive or developmental toxicity studies performed with E 171 and considered sufficiently reliable with respect to their internal validity were identified from the published literature.
232. No maternal and developmental effects were observed up to 1,000 mg/kg bw per day, the highest dose tested, in a single rat developmental toxicity study with five different TiO2 materials, TiO2 Nanoparticles or TiO2 containing a fraction of nanoparticles (Warheit et al., 2015a) (Score: 4 for NSC).
233. In mice, the effects of TiO2 nanoparticles <30 nm on the testis (decreased weight, decreased seminiferous tubule diameter, germ cell apoptosis) and sperm (decreased sperm counts and motility, increased percentage of abnormal spermatozoa) were observed in three studies (Khorsandi et al., 2016, 2017; Karimi et al., 2019) at doses ranging from 50 to 300 TiO2 Nanoparticles /kg bw per day. The lowest dose at which the effects were observed was 50 mg TiO2 Nanoparticles /kg bw per day (Karimi et al., 2019). In a mouse study by Lu et al. (2020), no effects were observed at the lowest dose tested, 10 mg/kg bw per day (Score: 4 for NSC). In rats, administration of TiO2 Nanoparticles (21 nm) did not show effects at any dose level in a developmental toxicity study up to 1,000 mg/kg bw per day (Lee et al., 2019, Score: 3 for NSC).
Literature Search
234. A number of studies available in the literature were assessed by the Panel in addition to the extended one-generation reproduction toxicity (EOGRT) study. No E171 studies were identified in the literature search with reliability scores of 1 or 2. No effects were reported up to a dose of 1,000 mg/kg bw per day for titanium dioxide containing a fraction of nanoparticles, the highest dose tested in the EOGRT study. Several studies using TiO2 Nanoparticles <30 nm were reported. These are detailed below and summarised in Annex B. These were included for additional information and may be relevant with respect to whether a minimum limit for particle size should be included in the EU specifications for E 171 however EFSA considered these of limited relevance to the safety of E171.
Studies on TiO2 Nanoparticles - Toxicity of Titanium Dioxide as a Food Additive
In this guide
In this guideThis is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
235. The additional toxicity studies used to assess the toxicity of titanium dioxide were scored from 1-4 and specified nanoparticle size where available, with studies ranked 1 and 2 and using nanoparticles > 30nm being the optimal studies for consideration, however, some studies ranked 3 and 4 and with nanoparticles < 30nm were used in the absence of studies ranked 1 and 2.
Studies in mice: None available
Studies in rats:
Reproductive Toxicity Studies with TiO2 fraction Nanoparticles
236. Warheit et al. (2015b) (Score: 4 for NSC) - Oral prenatal developmental toxicity study in rats using five different TiO2 materials, TiO2 Nanoparticles or TiO2 containing a fraction of nanoparticles up to a dose of 1,000 mg TiO2 Nanoparticles/kg bw. No maternal and developmental effects were observed up to the highest dose tested, when administered from gestation days (GDs) 6 to 15.
237. In three studies, time-mated pregnant Sprague–Dawley, Crl:CD(SD), rats (n=22/group) were daily exposed to TiO2 (uf-1, uf-3 and pg-1) by gavage on GDs 6–20. In three additional studies, pregnant Wistar rats (n=22–23/group) were daily exposed to TiO2 (uf-2 and pg-2) by gavage from GDs 5 to 19. The dose levels used in the studies were 0, 100, 300 or 1,000 mg/kg bw per day. The dose volume was 5 mL/kg bw per day.
I. anatase/rutile (89/11%)(uf-1), d50 = 43 nm (XSDC), d50 = 23 nm (TEM), irregular;
II. anatase (100% nano) (uf-2), d50=42 nm (XSDC), d50=19 nm (TEM), irregular;
III. rutile (100% nano) (uf-3), d50=47 nm (XSDC),d50=22 nm (TEM), rod-like;
IV. anatase (27% nano) (pg-1), d50=153 nm (XSDC), d50=120 nm(TEM), irregular;
V. rutile (11% nano) (pg-2), d50=195 nm (XSDC), d50=165 nm (TEM), irregular.
238. Gross necropsy included gross examination of the dam, counting of the number of corpora lutea, implantation sites, resorptions, live and dead fetuses, fetal sex and weight. Fetal pathological external, visceral and skeletal examinations were performed in order to identify any abnormalities. At 1,000 mg uf-1/kg per day, mean fetal sex ratio and the means for male and female fetuses per litter were statistically significantly different from the control group means. The mean number of male fetuses was 7.2 compared with 5.5 male fetuses for the concurrent control group; the test facility historical control group data ranged at that time from 5.2 to 7.4. The mean number of female fetuses was 4.8 compared with 6.7 for the concurrent control group; the test facility historical control group data ranged at that time from 5.8 to 8.3. Mean fetal sex ratio of the 1,000 mguf-1/kg bw per day group was 60% (males/females) compared with a sex ratio of 46% in the concurrent control group; the test facility historical control group data ranged at that time from 43% to 53%.
239. Apart from some incidental changes in body weight and feed intake, no other changes were observed in the dams or the fetuses in these studies. The authors concluded that there were no significant toxicological or developmental effects in females or fetuses at any of the dose levels or compounds tested and considered the NOAEL for each compound to be 1,000 mg/kg bw per day, the highest dose tested. The Panel agreed with both the author and the ANS Panel conclusions (2016 ANS Panel - Overall, the Panel noted that prenatal developmental studies with three pigment-grade (pg-1, pg-2 and pg-3) and three ultrafine (uf-1, uf-2 and uf-3)/nanoscale (anatase and/or rutile) TiO2 particulates performed according to the OECD guidelines (TG 414) did not give concern for maternal or developmental toxicity up to the highest dose tested (1,000 mg/kg bw per day).
TiO2 Nanoparticles < 30 nm (See Annex B for data summaries)
Studies in mice:
Karimipour et al. 2018 (Score: 2 for NSC) –
240. The effects of oral administration of TiO2 Nanoparticles (10–25 nm) were tested at 100 mg/kg bw per day for 5 weeks on the histology of ovaries, oestrogen and malondialdehyde (MDA) serum levels (7 animals/group), fertility (10 animals/group) and IVF rates (10 animals/group) in female mice.
241. A significantly decreased pregnancy rate was observed (70% vs. 100% in the control group), along with a 20% decrease in litter size and increases in circulating oestrogen (20%) as well as MDA (25%). Degeneration and reduction of follicles, cyst formation and impairment of follicular development in the ovaries of the TiO2 Nanoparticles group (no quantitative data). A lower number of oocytes were isolated from the exposed group and a higher percentage of developmental arrest before the blastocyst stage after in vitro fertilisation. It was suggested that the observed effects could be the consequence of an indirect effect of TiO2 Nanoparticles through the generation of increased ROS levels.
242. Impairment of female fertility at the only dose tested was observed, therefore the Panel considered that the study showed an impairment of female fertility at a dose of 100 mg TiO2 Nanoparticles (10–25 nm)/kg bw per day.
Khorsandi et al. 2016 (Score: 2 for NSC) –
243. The effects of oral administration of TiO2 Nanoparticles on testicular parameters in young adult male NMRI mice at 4 doses between 0 and 300 mg/kg bw per day (8 animals/group) for 35 days were investigated. Dose-dependent decreases in testis weight occurred from a dose of 100 mg/kg bw per day. At higher doses, additional testicular parameters were affected. While body weight was unaffected by treatment, the authors reported dose-dependent decreases in testis weight from a dose of 100 mg/kg bw per day. Both the mid- and high-dose groups showed decreases in serum and testicular testosterone levels, the diameter and total volume of seminiferous tubules, the height of the spermatogenic epithelium and total Leydig cell numbers. Contrarily, the total volume of the interstitial tissue was found to be increased. The Panel considered that TiO2 Nanoparticles (size unknown) from 100 mg/kg bw per day had an effect on testis weight.
Khorsandi et al. 2017 (Score: 2 for NSC)
244. TiO2 Nanoparticles (20–30 nm) were administered by oral gavage at 300 mg/kg bw per day to eight young adult male NMRI mice for 35 days. The authors reported significant decreases in testis weight, circulating and testicular testosterone, testicular catalase (CAT) and superoxide dismutase (SOD) concentrations, sperm counts and sperm motility.
245. Significant decreases in testis weight, circulating and testicular testosterone, testicular catalase (CAT) and superoxide dismutase (SOD) concentrations, sperm counts and sperm motility were observed. Significant increases were also found in the percentage of abnormal or degenerative spermatogenic tubules, germ cell apoptosis, testicular MDA concentration and in the percentage of sperm with abnormal morphology. The Panel considered that testicular toxicity was observed with TiO2 Nanoparticles (20–30 nm) at 300 mg/kg bw/d, the only dose tested.
Karimi et al. 2019 (Score: 2 for NSC)
246. Eight 6- to 8-week-old male NMRI mice were treated daily by gavage with 50 mg TiO2 Nanoparticles (<30 nm)/kg bw per day for 35 days.
247. The TiO2 nanoparticles significantly reduced testis weight accompanied by reduced serum testosterone, reduced seminiferous tubule diameter and epithelium height and reduced the maturity of the germinal epithelium. Reduced sperm counts, increased sperm abnormalities and reduced sperm motility. The Panel noted that 50 mg TiO2 Nanoparticles/kg bw per day, the only dose tested, resulted in adverse effects on the testis compared with a control group.
Lu et al. (2020) (Score: 4 for NSC)
248. Four groups of 15 male ICR mice, age 6–8 weeks were treated daily by gavage with TiO2 Nanoparticles (7 nm) at 4 doses between 0 and 100 mg/kg bw per day for 30 days.
249. It was noted that there was tight junction damage in the blood–testis barrier (BTB) at 50 and 100 mg/kg bw. Serum testosterone was 50% decreased at the two highest doses tested.
520. Sperm motility was dose-relatedly reduced, accompanied by increased sperm malformation rates.
251. The Panel noted that the histopathological pictures on BTB were hard to interpret and considered that TiO2 Nanoparticles (7 nm at 50 or 100 mg/kg bw per day) resulted in a dose-related reduction of sperm motility and increased sperm malformations, accompanied by histological observations in the testis, changes in BTB-related protein levels, changes in MAPK-related levels and reduced circulating testosterone concentrations.
Studies in rats:
Lee et al. 2019 (Score: 3 for NSC)
252. Mated female Sprague–Dawley rats (12 females per group) were treated with TiO2 Nanoparticles (21 nm) daily by gavage at dose levels of 0, 100, 300 and 1,000 mg/kg bw per day from GDs 6 to 19.
253. No statistically significant differences were noted in general clinical signs, bodyweight, organ weights (absolute and relative to body weight), macroscopic findings. No significant differences for caesarean section parameters and fetal external and visceral examinations. The Panel considered that no adverse maternal and development al effects were reported with TiO2 Nanoparticles (21 nm) up to 1,000 mg/kg bw per day, the highest dose tested.
Further Considerations- Toxicity of Titanium Dioxide as a Food Additive
In this guide
In this guideThis is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Evaluation of Titanium Dioxide as a Feed Additive
254. In parallel to the re-evaluation of titanium dioxide as a food additive, the EFSA FEEDAP Panel was also evaluating the safety of titanium dioxide in feed for all animal species. This assessment has been put on hold, awaiting submission of the data requested as a follow-up of the re-evaluation of the food additive.
Summary
Reproductive and Developmental Toxicity
255. Developmental toxicity – EOGRT study - No treatment-related pre- or postnatal loss was observed in the F0 and F1 generations. The average litter size at birth in all dose groups was comparable or higher than in the control group. The sex ratio was unaffected. No external or internal abnormalities were detected in F1 and F2 pups at termination. No effects of E 171 on pre- and postnatal development were observed. Uncertainties included missing data on puberty in males (i.e. an appropriate assessment of the timing of the balanopreputial separation) however, given the lack of any other treatment-related effects, the EFSA Panel did not consider this to be critical.
256. Sexual function and fertility - EOGRT study - No statistically significant or dose-related effects on sperm motility, total spermatids/gram testis, percentage of abnormal spermatozoa and male mating index were observed in the F0 generation and no effects on any of the sperm endpoints in F0 or F1 generations in males. It was also noted that there were no effects on mean oestrus cycle in F0 and F1 and all F0 females in the control, 100, 300 and 1,000 mg/kg bw per day groups mated. The pregnancy rate was slightly lower in the F0 generation at 300 and 1,000 mg/kg bw per day but was not confirmed in the F1 generation. The Panel considered it as incidental and not treatment-related. No effects were noted on pregnancy duration, number of implantation sites and post-implantation loss. No effects of E 171 on sexual function and fertility were observed.
Literature Search (See Annex B for data summaries)
257. The EFSA Panel agreed with the author conclusions that there were no significant toxicological or developmental effects in females or fetuses at any of the dose levels or compounds tested and considered the NOAEL for each compound to be 1,000 mg/kg bw per day, the highest dose tested (Warheit et. al , score: 4). An additional study (score 2) showed an impairment of female fertility at a dose of 100 mg TiO2 Nanoparticles (10–25 nm)/kg bw per day.
258. For the literature review papers scored 2, The Panel considered that TiO2 Nanoparticles (size unknown) at 100 mg/kg bw per day had an effect on testis weight, testicular toxicity was observed with TiO2 Nanoparticles (20–30 nm) at a dose of 300 mg/kg bw per day, the only dose tested, and a further two studies showed 50 mg TiO2 Nanoparticles (< 30 nm)/kg bw per day resulted in adverse effects on the testis, and TiO2 Nanoparticles (7 nm), at 50 or 100 mg/kg bw per day, resulted in a dose-related reduction of sperm motility and increased sperm malformations, accompanied by histological observations in the testis, changes in BTB-related protein levels, changes in MAPK-related mRNA levels and reduced circulating testosterone concentrations.
259. One study with a score of 3 showed that no adverse effects were reported with TiO2 Nanoparticles (21 nm) up to 1,000 mg/kg bw per day, the highest dose tested.
Developmental Immunotoxicity
260. EOGRT Study - It was noted that the assay conditions may have not been optimal resulting in an apparent low antibody response when compared to the literature. The study authors considered that all tested animals in the study had a weak immunogenic response that was insufficient to identify a T-cell-dependent immunotoxic effects of E 171. The study authors therefore considered that no conclusion can be drawn on the effect of E 171 on the developing immune system and the EFSA Panel agreed.
Literature Search (See Annex B for data summaries)
261. For studies scored 1, The Panel considered that the results of one study suggest that while E 171 (5 mg/kg bw per day) alone administered for 10 weeks had no effect on tumour formation, it can potentiate intestinal tumour formation in mice. A second study analysis, which were limited to few animals, showed some evidence for modest inflammation which cannot be clearly identified as adverse.
262. A further study demonstrated that particles in E 171 administered via the diet are taken up by basal cells of intestinal lymphoid follicles, however, the parameters investigated did not show an effect on the immune system or inflammation.
263. Another study (several test materials containing different percentages of Nanoparticles) indicated that E 171 has pro-inflammatory potential at the systemic level, paralleled by the development of an inflammatory microenvironment in the intestinal mucosa and that E 171 alone at a dose of 10 mg/kg bw per day may induce development of ACF in male rats. The Panel also noted that E 171 at a dose of 10 mg/kg bw per day increased the number of ACF initiated by a genotoxic carcinogen.
264. For NP studies scored 2, the EFSA Panel noted several conclusions including changes for inflammatory markers at doses starting from 100 mg TiO2 Nanoparticles (5–12 nm)/kg bw per day, histopathologically, reduced numbers of goblet cells were found as a result of exposure, as well as inflammatory infiltration, data to indicate an effect of TiO2 Nanoparticles (5–6 nm) exposure at all dose levels tested, as evidenced by histopathological lesions, corroborated by intermediate endpoints indicating disturbance of intracellular ion homeostasis that were adrenergic receptors in the heart.
Questions for the Committee - Toxicity of Titanium Dioxide as a Food Additive
In this guide
In this guideThis is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
i. What is the Committee's conclusion on the absorption of titanium dioxide??
ii.Is the Committee able to conclude on the potential of titanium dioxide to induce aberrant crypt foci?
iii.Is the Committee able to establish a point of departure on the reproductive effects of titanium dioxide?
iv.What are the Committee’s views on the toxicity of nanoparticles and whether a point of departure can be established for the information presented?
v. Regarding the studies that were excluded by the EFSA Panel, does the COT want to review any of these studies as part of their own consideration of titanium dioxide?
vi.Does the Committee have any other comments?
Secretariat
March 2023
References - Toxicity of Titanium Dioxide as a Food Additive
In this guide
In this guideThis is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Ammendolia MG, Iosi F, Maranghi F, Tassinari R, Cubadda F, Aureli F, Raggi A, Superti F, Mantovani A and DeBerardis B, 2017. Short-term oral exposure to low doses of nano-sized TiO2and potential modulatory effectson intestinal cells. Food and Chemical Toxicology, 102, 63–75.
Bettini S, Boutet-Robinet E, Cartier C, Comera C, Gaultier E, Dupuy J, Naud N, Tache S, Grysan P, Reguer S,Thieriet N, Refregiers M, Thiaudiere D, Cravedi JP, Carriere M, Audinot JN, Pierre FH, Guzylack-Piriou L andHoudeau E,, 2017. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Scientific Reports, 7, 40373.
Bischoff NS, de Kok TM, Sijm DTHM, van Breda SG, Briedé JJ, Castenmiller JJM, Opperhuizen A, Chirino YI, Dirven H, Gott D, Houdeau E, Oomen AG, Poulsen M, Rogler G, van Loveren H., 2020. Possible Adverse Effects of Food Additive E171 (Titanium Dioxide) Related to Particle Specific Human Toxicity, Including the Immune System. International Journal of Molecular Science, 22(1):207.
Blevins LK, Crawford RB, Bach A, Rizzo MD, Zhou J, Henriquez JE, Khan D, Sermet S, Arnold LL, Pennington KL,Souza NP, Cohen SM and Kaminski NE, 2019. Evaluation of immunologic and intestinal effects in rats administered an E 171-containing diet, a food grade titanium dioxide (TiO2). Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 133.
Chen Z, Zheng P, Han S, Zhang J, Li Z, Zhou S and Jia G, 2020a. Tissue-specific oxidative stress and elementdistribution after oral exposure to titanium dioxide nanoparticles in rats. Nanoscale, 12, 20033–20046.
Chen Z, Han S, Zheng P, Zhou D, Zhou S and Jia G, 2020b. Effect of oral exposure to titanium dioxidenanoparticles on lipid metabolism in Sprague-Dawley rats. Nanoscale, 12, 5973–5986.
Comera C, Cartier C, Gaultier E, Catrice O, Panouille Q, El Hamdi S, Tirez K, Nelissen I, Theodorou V and Houdeau E, 2020. Jejunal villus absorption and paracellular tight junction permeability are major routes for early intestinal uptake of food-grade TiO2 particles: an in vivo and ex vivo study in mice. Part Fibre Toxicol, 17, 26.
Disdier C, Devoy J, Cosnefroy A, Chalansonnet M, Herlin-Boime N, Brun E, Lund A and Mabondzo A, 2015. Tissue biodistribution of intravenously administrated titanium dioxide nanoparticles revealed blood-brain barrier clearance and brain inflammation in rat. Particle and Fibre Toxicology, 12, 24.
EFSA ANS Panel (EFSA Panel on Food Additives and Nutrients Sources added to Food), 2016. Re-evaluation of titanium dioxide (E 171) as a food additive. EFSA Journal 2016;14(9):4545, 83 pp.
EFSA ANS Panel (EFSA Panel on Food Additives and Nutrients Sources added to Food), 2017. Approach followed for the refined exposure assessment as part of the safety assessment of food additives under re-evaluation. EFSA Journal 2017;15(10):5042, 9 pp.
EFSA FAF Panel (EFSA Panel on Food Additive and Flavourings), 2019. Scientific opinion on the proposed amendment of the EU specification for titanium dioxide (E 171) with respect to the inclusion of additional parameters related to its particle size distribution. EFSA Journal 2019;17(7):5760, 23 pp.
EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) (2021): Safety assessment of titanium dioxide (E171) as a food additive, EFSA Journal 2021; 19(5):6585.
EFSA Scientific Committee, 2009. Guidance of the scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp.
EFSA Scientific Committee, 2011a. Scientific Opinion on Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA Journal 2011;9(5):2140, 36 pp.
EFSA Scientific Committee, 2018a. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: part 1, human and animal health. EFSA Journal 2018;16(7):5327,95 pp.
Geraets L, Oomen AG, Krystek P, Jacobsen NR, Wallin H, Laurentie M, Verharen HW, Brandon EF and de Jong WH, 2014. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part Fibre Toxicol, 11, 30.
Gore ER, Gower J, Kurali E, Sui JL, Bynum J, Ennulat D and Herzyk DJ,, 2004. Primary antibody response to keyhole limpet hemocyanin in rat as a model for immunotoxicity evaluation. Toxicology, 197, 23–35.
Guillard A, Gaultier E, Cartier C, Devoille L, Noireaux J, Chevalier L, Morin M, Grandin F, Lacroix MZ, Comera C,Cazanave A, de Place A, Gayrard V, Bach V, Chardon K, Bekhti N, Adel-Patient K, Vayssiere C, Fisicaro P, FeltinN, de la Farge F, Picard-Hagen N, Lamas B and Houdeau E, 2020. Basal Ti level in the human placenta and meconium and evidence of a materno-foetal transfer of food-grade TiO2 nanoparticles in an ex vivo placental perfusion model. Part Fibre Toxicol, 17, 51.
Hendrickson OD, Pridvorova SM, Zherdev AV, Klochkov SG, Novikova OV, Shevtsova EF, Bachurin SO and DzantievBB,, 2016. Size-dependent differences in biodistribution of titanium dioxide nanoparticles after sub-acute intragastric administrations to rats. Current Nanoscience, 12, 228–236.
Hendrickson OD, Platonova TA, Piidvorova SM, Zherdev AV, Gmoshinsky IV, Vasilevskaya LS, Shumakova AA,Hotimchenko SA and Dzantiev BB, 2020. Electron-microscopic investigation of the distribution of titaniumdioxide (rutile) nanoparticles in the rats’small intestine mucosa. Liver, and Spleen Current Nanoscience, 16,268–279.
Heringa MB, Peters RJB, Bleys R, van der Lee MK, Tromp PC, van Kesteren PCE, van Eijkeren JCH, Undas AK,Oomen AG and Bouwmeester H, 2018. Detection of titanium particles in human liver and spleen and possiblehealth implications. Particle and Fibre Toxicology, 15, 15.
Karimi S, Khorsandi L and Nejaddehbashi F,, 2019. Protective effects of Curcumin on testicular toxicity induced bytitanium dioxide nanoparticles in mice. JBRA Assisted Reproduction, 23, 344–351.
Karimipour M, Zirak JM, Ahmadi A and Jafari A, 2018. Oral administration of titanium dioxide nanoparticle throughovarian tissue alterations impairs mice embryonic development. International Journal of Reproductive Biomedicine (Yazd, Iran), 16, 397–404.
Khorsandi L, Orazizadeh M, Mansouri E, Hemadi M and Moradi-Gharibvand N,, 2016. Morphometric and stereological assessment of the effects of titanium dioxide nanoparticles on the mouse testicular tissue. Bratislavske lekarske listy, 117, 659–664.
Khorsandi L, Orazizadeh M, Moradi-Gharibvand N, Hemadi M and Mansouri E,, 2017. Beneficial effects of quercetinon titanium dioxide nanoparticles induced spermatogenesis defects in mice. Environmental Science and Pollution Research International, 24, 5595–5606.
Kreyling WG, Holzwarth U, Haberl N, Kozempel J, Hirn S, Wenk A, Schleh C, Schaffler M, Lipka J, Semmler-BehnkeM and Gibson N, 2017a. Quantitative biokinetics of titanium dioxide nanoparticles after intravenous injection inrats: part 1. Nanotoxicology., 11, 434–442.
Kreyling WG, Holzwarth U, Schleh C, Kozempel J, Wenk A, Haberl N, Hirn S, Schaffler M, Lipka J, Semmler-BehnkeM and Gibson N,, 2017b. Quantitative biokinetics of titanium dioxide nanoparticles after oral application in rats: part 2. Nanotoxicology, 11, 443–453.
Lee J, Jeong JS, Kim SY, Park MK, Choi SD, Kim UJ, Park K, Jeong EJ, Nam SY and Yu WJ, 2019. Titanium dioxide nanoparticles oral exposure to pregnant rats and its distribution. Particle and Fibre Toxicology, 16, 31.
Lu T, Ling C, Hu M, Meng X, Deng Y, An H, Li L, Hu Y, Wang H, Song G and Guo S, 2020. Effect of nano-titanium dioxide on blood-testis barrier and MAPK signaling pathway in male mice. Biological Trace Element Research.
Pele LC, Thoree V, Bruggraber S, Koller D, Thompson RP, Lomer MC and Powell JJ, 2015. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Particle and Fibre Toxicology, 12, 26.
Peters RJB, Oomen AG, van Bemmel G, van Vliet L, Undas AK, Munniks S, Bleys RLAW, Tromp PC, Brand W andvan der Lee M, 2020. Silicon dioxide and titanium dioxide particles found in human tissues. Nanotoxicology, 14,420–432.
Riedle S, Wills JW, Miniter M, Otter DE, Singh H, Brown AP, Micklethwaite S, Rees P, Jugdaohsingh R, Roy NC,Hewitt RE and Powell JJ, 2020a. A murine oral-exposure model for nano- and micro-particulates: demonstrating human relevance with food-grade titanium dioxide. Nano-Micro Small, 16, 2000486.
Sprong C, Bakker M, Niekerk M and Vennemann F, 2015. Exposure assessment of the food additive titanium dioxide (E 171) based on use levels provided by the industry. RIVM Letter report, 2015-0195.
Talamini L, Gimondi S, Violatto MB, Fiordaliso F, Pedica F, Tran NL, Sitia G, Aureli F, Raggi A, Nelissen I, Cubadda F,Bigini P and Diomede L, 2019. Repeated administration of the food additive E171 to mice results inaccumulation in intestine and liver and promotes an inflammatory status. Nanotoxicology, 13, 1087–1101.
Tassinari R, Cubadda F, Moracci G, Aureli F, D’Amato M, Mauro Valeri M, De Berardis B, Raggi A, Mantovani A,Passeri D, Rossi M and Francesca Maranghi F, 2014. Oral, short-term exposure to titanium dioxidenanoparticles in Spragues-Dawley rat: focus on reproductive and endocrine systems and spleen.Nanotoxicology, 8, 654–662.
Verleysen E, Waegeneers N, Brassinne F, De Vos S, Jimenez IO, Mathioudaki S and Mast J,2020. Physico-chemical characterization of the pristine E171 food additive by standardized and validated methods. Nanomaterials (Basel), 10, 592.
Warheit DB, Boatman R and Brown SC,, 2015b. Developmental toxicity studies with 6 forms of titanium dioxide test materials (3 pigment-different grade & 3 nanoscale) demonstrate an absence of effects in orally-exposed rats. Regulatory Toxicology and Pharmacology, 73, 887–896.
Additional Documents
Annex A: EOGRT Study Data
Annex B: Summary of Papers