Postdoctoral Fellow Update
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.This is a paper for discussion. It does not represent the views of the Committee and should not be cited.
Advancing in silico methods of assessing toxicological risk
Why and how are you associated with the FSA?
6. The FSA and COT have been reviewing new approach methodologies (NAMs) and developing a UK NAMs roadmap towards the integration and acceptance of NAMs for chemical risk assessment. One of the activities defined in this roadmap was to actively work on advancing in silico methods for assessing toxicological risk, specifically focused on food-related chemicals, but remaining open to work on other classes of chemicals relevant to the FSA’s risk assessments. In this context, I was recruited as a computational toxicology fellow and awarded a 4-year fellowship funded by the FSA, whilst supervised by a team of academic and applied NAM experts. The supervisory team is composed of Prof. Mark Viant and Prof. John Colbourne (University of Birmingham), Dr. George Loizou (former Head of Computational Toxicology at HSE Science and Research Centre), and Dr. Olivia Osborne, Ms. Claire Potter, and Dr. David Gott (FSA).
Broad overview of the FSA fellowship and its aims
7. The programme of work of the fellowship consists of (i) scoping the FSA’s problem space in chemical risk assessment and mapping this to our computational NAMs solution space, thereby aiding the FSA to develop a strategy for the utilisation of NAMs (months 1-24); (ii) ensuring that the FSA is trained in the use of computational NAMs by delivering training courses, including an introduction to existing and emerging NAM technologies, and topics selected from the FSA’s NAM strategy (months 1-48); (iii) developing and evaluating confidence in a new hazard assessment workflow that integrates in vitro omics toxicity data, benchmark dose modelling and PBPK modelling to serve as the basis for quantitative risk assessment for human health, i.e. towards generating human health-based safety thresholds for the FSA and other regulators (months 1-36); and (iv) developing and delivering a second case study that fortifies the community-wide acceptance of 21st century methods in risk assessments, to accelerate the successful application of NAMs within the FSA (months 25-48).
Progress with case studies
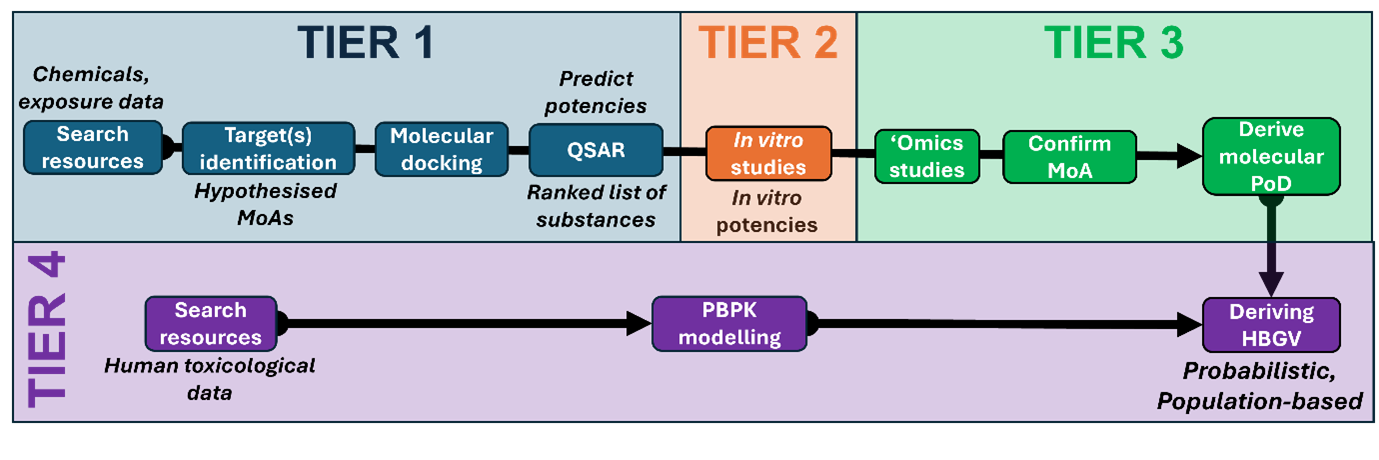
8. The latest case study launched is focusing on plant alkaloids of three large classes: tropane alkaloids (TAs), pyrrolizidine alkaloids (PAs), and glycoalkaloids (GAs). The supervisory team decided to start with TAs. In terms of food safety, the first objective of this case study is to support the UK FSA’s policy need to determine which TAs are the most potent (neuro)toxicants to prioritise specific substances and inform decisions on the UK’s monitoring of these alkaloids in foods. An integral part of this aim is to confirm that neurotoxicity is the primary mode of action of these alkaloids. This aim will be achieved using a tiered-testing strategy of in silico, in vitro and ‘omics NAMs. The second objective of this case study is to derive a HBGV for human exposure for the top priority, i.e. most potent substance within the class of TAs. This will utilise physiologically-based pharmacokinetics (PBPK) modelling and quantitative in vitro to in vivo extrapolation (QIVIVE). From a methodological perspective, a broader third objective of the case study is to evaluate and attempt to build confidence within the FSA in the application of a series of relevant NAMs that have been integrated in a manner to address policy needs. These NAMs are tiered and incorporate existing human in vivo data as well as new testing on human in vitro cell lines, to maximise the relevance and accuracy to human food safety. A tiered approach was proposed to achieve the objectives of this case study and is depicted in Figure 1.
Figure 1. Master workflow describing the proposed tiered approach for the plant toxins case study.
9. The collaborative nature of this case study has been accepted at Accelerating the Pace of Chemical Risk Assessment (APCRA) meeting (Ottawa, CA, 2024) as an international case study; where several potential regulatory partners demonstrated interest and willingness to collaborate.
Progress with papers and conferences
10. Our recent work on PFOA is published (1) and was presented on several occasions. To list a few, PARC Science Day (poster presentation), NURA Dynamic Discussions (oral presentation, online), HSE’s workshop (oral presentation, online), EFSA’s workshop (oral presentation, online), EUROTOX 2023 (poster presentation), ASPIS Open Symposium 2023 (poster presentation), BTS Annual Meeting (2024, oral presentation). PFOA case study was submitted as a nomination to the Lush Prize under the Young Researcher category and has been one of the five projects awarded in 2022. Furthermore, PFOA case study was used in training sessions delivered to the UK HSA (2024) and to the students of the new MSc course (Human and Environmental Toxicology with Law) at the University of Birmingham (2024).
11. The plant alkaloids case study was presented at the APCRA meeting (Ottawa, CA, 2024) and formally accepted as a case study by its internal committee in the beginning of 2025; tier 1 results will be presented at the upcoming APCRA online meeting (2025) and submitted to EUROTOX 2025.