Discussion paper on the EFSA Draft Opinion for Public Consultation on Update of the risk assessment of mineral oil hydrocarbons (MOH) in food
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Introduction
1. Mineral oil hydrocarbons (MOH) are a wide range round of chemical compounds, largely derived from petrochemicals. There are two main groups- mineral oil saturated hydrocarbons (MOSH) and mineral oil aromatic hydrocarbons (MOAH). MOH may enter the food chain through a variety of routes - environmental contamination, use of lubricants for machinery, release agents, processing aids, food or feed additives and migration from food contact materials (EFSA, 2023). MOH have been found in a variety of foods and the levels of MOSH are generally higher than the levels of MOAH. The highest levels of MOH have been found in vegetable oils. The European Food Safety Authority (EFSA) have noted that MOSH may accumulate in the liver and the lymphoid system while MOAH may act as a genotoxic carcinogen.
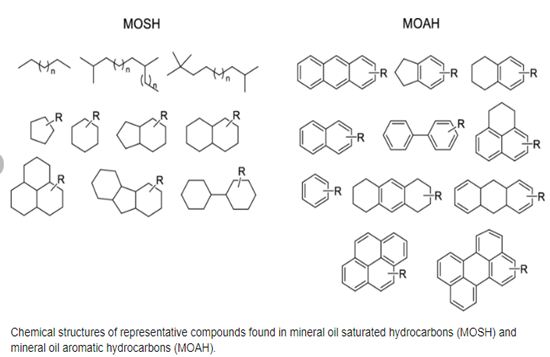
2. Structures of representative compounds are given below (Weber et al, 2018).
3. EFSA were asked by the European Commission (EC) to assess any toxicity studies on MOH, that have become available since EFSA’s last evaluation in 2012 and to update their scientific opinion, if necessary. EFSA was also asked to update the exposure assessment and to update the risk characterisation, if necessary.
4. EFSA launched a public consultation on the “Update of the risk assessment of mineral oil hydrocarbons (MOH) in food” on 15th March 2023. This following paper provides a short overview of the previous EFSA evaluation in 2012, as well as the key points of the 2023 assessment.
5. The deadline for submission of comments to EFSA is the 30th April 2023.
6. The deadline for submission of comments to the Secretariat is the 16th April 2023.
7. If Members who wish to comment on the EFSA assessment could please link their comments to the respective sections/paragraphs.
8. Members will wish to be aware that further assessment of MOH may be conducted later in the year.
Previous evaluations by EFSA
9. EFSA previously assessed the risk related to the presence of MOH in food in 2012, estimating a chronic exposure ranging from 0.03 to 0.3 mg/kg bw per day for MOSH. Insufficient data were available to calculate the exposures to MOAH, however at that time, EFSA estimated it to be 20% of that of MOSH.
10. For MOSH, EFSA identified hepatic microgranulomas associated with inflammation in F334 rats as the critical adverse effect and selected a no observed adverse effect level (NOAEL) of 19 mg/kg bw per day from a sub-chronic study on low-melting point waxes (LMPW) as the reference point (RP). EFSA did not consider it appropriate to derive a health based guidance value (HBGV) but applied the margin of exposure (MOE) approach instead.
11. MOEs for MOSH ranged from 59 to 680 across the different age groups, indicating a potential concern for health.
12. EFSA identified 3-7 ring MOAH with no- or low degree alkylation as the components of main concern regarding their genotoxic and carcinogenic nature. MOAH with a high degree of alkylation could act as tumour promoters and some with less than three rings, like naphthalene could act as carcinogenic via a non-genotoxic mode of action (MoA).
13. However, due to the lack of relevant dose-response data on carcinogenicity, EFSA was unable to conclude on the risk related to the presence of MOAH but considered the dietary exposure to MOAH of potential concern due to the presence of genotoxic and carcinogenic substances.
14. In 2019, EFSA published a rapid risk assessment following the detection of MOAH in batches of infant and follow-on formula. As no information was available on 3-7 MOAH, EFSA concluded, in line with their 2012 assessment, that the estimated exposure levels were of concern due to the possible presence of genotoxic and carcinogenic compounds.
Summary of the 2023 EFSA evaluation
15. The mineral oil hydrocarbons considered in EFSA’s 2023 opinion contain 10 to about 50 carbon atoms and are separated into mineral oil saturated hydrocarbons (MOSH) and mineral oil aromatic hydrocarbons (MOAH).
16. As noted above, MOH can enter food from numerous sources, such as environmental contamination (air, soil, aquatic), use of lubricants for machinery, release agents, processing aids, food or feed additives and migration from food contact materials such as printing inks and adhesives used in food packaging.
17. MOH found in food and considered as contaminants often have similar or identical compositions as MOHs authorised for use. Discrimination between them is difficult and often unclear. The toxicological evaluation of mineral oil products is made difficult by the often-ill-defined composition. Most oils however are treated with the intention to remove the carcinogenic MOAH.
MOSH
Toxicity
18. Since 2012, a number of studies have become available on the retention of MOSH in the liver, spleen, adipose tissue and carcass of female F344 rats. Accumulation in the liver and spleen ranged from n-C20 to about n-C45, in adipose tissue it was below n-C16 to about n-C35. In the spleen there was no indication that it would reach a steady state, while a plateau between 90 and 120 days seemed to have been reached in the liver. At all doses tested, the concentrations were a magnitude higher in the liver than the spleen, adipose tissue or carcass, with a retention of MOASH in all studied tissues. The concentrations in the liver, spleen and carcass were reduced, with a reduction of up to 53% in liver after 30 days of depuration. Little change was seen in the adipose tissue.
19. Based on the composition of MOSH in the different tissues, it appeared that n-alkanes and probably other wax components are poorly bio transformed and eliminated in F344 rats. Retention in the liver and spleen was dominated by substituted naphthenes and highly branched iso-alkanes following exposure with L-C25. Experiments in female Sprague Dawley (SD) rats indicated that wax components are poorly retained in the liver.
20. New data on the presence and composition of MOSH in human tissue taken at autopsy was available, with concentrations varying greatly between individuals and tissues. Levels were highest in spleen, mesenteric lymph node (MLN), liver and adipose tissue, but substantially lower in the heart and kidney and below the limit of detection (LOD) in the brain. The composition was similar in liver and spleen, with unresolved highly isomerized iso-alkanes and largely alkylates naphthenes (n-C20 to n-C40), however, hardly any n-alkanes (including those naturally occurring in food) or terpenes were detected. The composition in adipose tissue and MLN were similar, but different from those in liver and spleen, with more n-alkenes (including naturally occurring and terpenes) and less isomerized hydrocarbons (n-C16 to n-C36).
21. In line with their 2012 assessment, EFSA concluded that MOASH had minimal acute toxicity. Studies on MOSH in F344 rats confirmed granuloma formation in liver and MLN, associated signs of inflammation and increases in liver, spleen and MLN weight. However, EFSA considered the effect in liver and spleen to be F344 specific due to the higher tendency to retain n-alkanes compared to SD rats and other test animals. Treatment of F344 rats with a deparaffinated MOSH product, treated to minimise the presence of wax components, did not induce organ weight changes or liver granuloma up to the highest concentration tested of 236 mg/kg bw per day.
22. The limited data on MOSH and synthetic gas to liquid oils (GTL) did not show the same effects in SD, Wistar and Long Evans rats and Beagle dogs as associated with F344 rats. In SD rats minimal signs of inflammation were reported in the liver at concentrations of 1624 mg paraffinic oil per kg bw per day (subchronic study). GTL were considered due to their similar composition as mineral oil-derived products, and exposure to GTL oils in SD rats resulted in mild to moderate apoptosis and necrosis in the intestinal mucosa at 1267 g/kg bw per day. In a subchronic study with paraffin wax, no adverse effects were observed at concentrations up to 9 g/kg bw per day.
23. EFSA further confirmed that MOSH is neither genotoxic nor carcinogenic by oral exposure.
24. New studies on GTL oils confirmed EFSAs previous conclusion that MOSH does not induce developmental or reproductive effects. Further studies also confirmed that there is no evidence of dietary MOSH to induce autoimmunity.
25. EFSA noted that the lipogranulomas observed in humans with MOSH exposure differ from the epithelioid granulomas observed in F344 rats. Hence, EFSA confirmed their previous conclusion, in the absence of new data, that lipogranulomas in human liver, spleen, lymph nodes and other organs were not associated with adverse effects of MOSH.
26. New clinical trials with pharmaceutical grade mineral oil products (used as placebo at 1-4 g/day) might have caused adverse effects. However, while some long-term studies showed increases in atherogenic lipoproteins and inflammation biomarkers, other studies, small and short in duration, did not. EFSA considered these studies observational and associated with a large uncertainty.
Critical endpoint and derivation of a reference point (RP)
27. EFSA concluded that the formation of hepatic epithelioid granuloma and associated effects in F344 rats exposed to MOSH were not relevant to humans. These effects, as well as the increased liver weights and inflammatory response, were related to hepatic accumulation of n-alkanes > C25 and other wax components and new evidence indicated that n-alkanes are not accumulated in human liver.
28. EFSA considered the L-C25 composition to best represent findings in human tissues regarding mass range and low occurrence or absence of n-alkenes. Hence, in the absence of a clear critical effect for MOSH, EFSA selected the Nygaard et al. (2019) study and a NOAEL of 236 mg/kg bw per day in F344 rats, corresponding to the highest tested dose of L-C25, as the relevant reference point (RP) for MOSH.
29. In addition, no adverse effects were observed following exposure to MOSH at or below this level in other experimental animals.
30. Due to the limitations in the data set, EFSA did not consider it appropriate to derive a HBGV but applied the MOE approach instead. EFSA considered an MOE of 1200 to be of low concern to human health. This took into consideration the default uncertainty factors (UF) for interspecies differences (10), interspecies differences (10), and the shorter duration of the key study (120 days) compared to a life time exposure (2). EFSA also considered an additional factor of 6 due to the uncertainties in the hazard identification and characterisation of MOSH.
Conclusions on MOSH
31. Based on the occurrence data available to EFSA, all MOEs were above 1200, for all age groups. The only exception being infants exclusively feed with infant formula, which could have exposures with MOEs ranging from 790 to 1070 and 680 to 870 for mean and high level consumption, respectively. However, as exposure via infant formula is of short duration, EFSA concluded that these MOEs did not raise concern. Overall, EFSA concluded that the current dietary exposure to MOSH for all age groups raised no concern for human health.
32. However, the possible consequences for human health regarding consumption of certain foods (products of animal origin) potentially resulting in selective exposure to accumulated MOSH components were not investigated and are therefore uncertain.
MOAH
Toxicity
33. No new studies on acute toxicity, repeat dose toxicity or carcinogenicity of MOAH were identified since EFSA’s assessment in 2012.
34. Dermal toxicity studies on petroleum extracts (containing 3- or more ring MOAH) resulted in fetotoxicity and developmental effects. These studies also showed a correlation between the developmental toxicity potency and the presence of 3- or more ring MOAH and the extent of trans-activation of Ah receptors. No adverse effects were observed in an oral screening reproductive and developmental study with lubricating base oil treated to reduce the concentration of 3- or more ring MOAH.
35. EFSA had previously concluded that there was evidence of non-genotoxic components acting as tumour promotors, such as certain aromatic hydrocarbons like naphthalene; no new evidence was available. Overall, the lack of robust data on the oral toxicity of MOAH makes it difficult to identify a critical effect related to the non-genotoxic and carcinogenic fraction of MOAH.
36. New studies on the genotoxicity of MOAH however confirmed EFSAs previous conclusion that the genotoxic effects were associated with the presence of some MOAH with 3- or more aromatic rings
37. In 2012, EFSA expressed concern about the presence of a genotoxic and carcinogenic fraction, constituted by substances with 3-7 aromatic rings and various degrees of side chain alkyl moieties. The genotoxic and carcinogenic effects of these components are likely due to bioactivation of the aromatic ring system, which has been well established for (unsubstituted) polycyclic aromatic hydrocarbons (PAH). However, their potency would be expected to be modulated by the number, size and position of the alkyl side chain.
38. New in vitro metabolism data on methylated phenanthrene and naphthalene showed a preferential metabolic oxidation of the alkyl side chains, or an overall reduced metabolism in the presence of long side chains with higher steric hindrance (Wang et al., 2020; 2022). EFSA considered that the residence time in the tissue might influence the occurrence of side-chain and aromatic ring oxidation. However, EFSA agreed with the conclusions by Pirow et al. (2018) that it is not possible to predict how alkylation effects the carcinogenic potential of MOAH compared to non-alkylated PAHs.
39. No additional studies were identified to reduce the uncertainties highlighted in the previous assessment. Little is known on the toxicity of MOAH, other than genotoxicity of some 3- or more ring MOAH.
Critical endpoint and derivation of a reference point (RP)
40. Due to the potential presence of genotoxic and carcinogenic components within MOAH, EFSA did not consider it appropriate to derive a HBGV but applied the MOE approach instead.
41. As no studies were available to define a RP for 3- or more ring MOAH, EFSA instead applied a conservative approach, making use of the structural similarities and the plausible common MoA for genotoxicity and carcinogenicity of 3- or more ring MOAH and PAHs (EFSA, 2008).
42. EFSA considered it most appropriate to select the BMDL10 of 0.49 mg/kg bw per day for increased incidence of total tumour bearing animals, calculated from a 2-year carcinogenicity study by Culp et al (1998) on non-alkylated polycyclic aromatic hydrocarbons (PAH, using the sum of eight PAH (PAH8)). The substantial part of MOAH in food consists of alkyl substitutes, further supporting the conservative nature of the surrogate selection.
43. The lack of robust data for oral toxicity makes the identification of potential critical effects and a RP associated with the non-genotoxic and non-carcinogenic fraction of MOAH difficult.
Conclusions on MOAH
44. The highest dietary exposure to MOAH was estimated in the young population, especially infants. However, no data are available on the concentration of 3- or more ring MOAH in food.
45. EFSA acknowledged that MOH products treated to reduce 3- or more ring MOAH are likely the main contributors to the presence in food. However, certain products containing a higher level of 3- or more ring MOAH, such as batching oils, fuels and bitumen, are also expected to contribute to exposure via food.
46. An estimation of the presence of 3- or more ring MOAH is highly uncertain as the contribution of different sources of MOAH will differ in different food groups. EFSA considered therefore two exposure scenarios in their assessment, using the selected BMDL10 and average content of 10% (Scenario 1) or 1% (Scenario 2) of 3- or more ring MOAH in the MOAH fraction in the different foods.
47. Scenario 1 resulted in MOEs consistently lower than 10,000 for mean (158 to 12,250) and high (83 to 4,900) consumers and therefore would result in a risk to human health related to the presence of 3- or more ring MOAH.
48. Scenario 2 resulted in MOEs below 10,000 for upper bound (UB) estimates, for most at mean exposures, and all at high exposures but in MOEs > 10,000 for lower bound (LB) estimates, for all mean and most high exposures. The only exception to the latter being young age groups showing high exposure LB MOEs of 4,000 to 8,000. This scenario would raise concern for human health, in particular for high consumers in the younger age groups.
49. EFSA considered the above conclusion conservative as the risk characterisation was based on a surrogate RP.
50. Overall, taken all uncertainties into consideration EFSA concluded that the certainty of MOEs being below 10,000 is extremely likely (99 to 100% certain) for mean and high consuming toddlers and likely 96 to 65% certain) for all other age groups.
51. EFSA was unbale to establish a RP for MOAH other than 3- or more ring MOAH and therefore concluded that the absence of reliable toxicity data may raise concern related to dietary exposure to 1-2 ring MOAH in food.
Conclusions
52. In contrast to the 2012 assessment, EFSA concluded in 2023, that the formation of hepatic epithelioid granuloma and associated effects in F344 rats exposed to MOSH were not relevant to humans. Hence, the L-C25 composition was considered to best represent findings in human tissues and a NOAEL of 236 mg/kg bw per day in F344 rats, corresponding to the highest tested dose of L-C25, was selected at the relevant reference point (RP) for MOSH. Due to the limitations in the data set, EFSA did not consider it appropriate to derive a HBGVs but applied the MOE approach instead.
53. In line with the previous assessment, EFSA still considered the risk related to the presence of MOAH to be based on the potential genotoxic and carcinogenic effects of certain MOAH. Due to structural similarities and the plausible common MoA for genotoxicity and carcinogenicity of 3- or more ring MOAH and PAHs, EFSA applied the BMDL10 for increased incidence of total tumour bearing animals for non-alkylated polycyclic aromatic hydrocarbons (PAH, using the sum of eight PAH (PAH8)) to the MOE approach for MOAH.
54. Overall, EFSA concluded that the current dietary exposure to MOSH for all age groups raised no concern for human health. However, the dietary exposure to MOAH, raised concerns for human health, especially in younger age groups.
Questions to the Committee
i. Do the Committee agree with EFSAs approach and selection of the NOAEL for MOSH?
ii. Do the Committee agree with using the BMDL10 for PAH8, in the absence of specific toxicity data for MOAH?
iii. Do the Committee have any comments on the EFSA 2023 assessment?
Secretariat
March 2023
List of Abbreviations
|
BMDL |
Benchmark dose limit |
|
EC |
European Commission |
|
EFSA |
European Food Safety Authority |
|
GTL |
Gas to liquid oils |
|
HBGV |
Health based guidance value |
|
LB |
Lower bound |
|
LMPW |
Low-melting point waxes |
|
LOD |
Limit of detection |
|
MLN |
Mesenteric lymph node |
|
MoA |
Mode of action |
|
MOE |
Margin of exposure |
|
MOH |
Mineral oil hydrocarbons |
|
MOAH |
Mineral oil aromatic hydrocarbons |
|
MOSH |
Mineral oil saturated hydrocarbons |
|
NOAEL |
No observed adverse effect level |
|
PAH |
Polycyclic aromatic hydrocarbons |
|
RP |
Reference point |
|
SD |
Sprague Dawley rats |
|
UB |
Upper bound |
|
UF |
Uncertainty factor |
References
Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA (1998). A comparison of the tumours induced by coal tar and benzo[a]pyrene in a 2-year bioassay. Carcinogenesis, 19: 117-124. https://doi.org/10.1093/carcin/19.1.117
EFSA (2008). Polycyclic Aromatic Hydrocarbons in Food - Scientific Opinion of the Panel on Contaminants in the Food Chain. The EFSA Journal, 724, 1-114. Polycyclic Aromatic Hydrocarbons in Food [1] - Scientific Opinion of the Panel on Contaminants in the Food Chain | EFSA (europa.eu)
EFSA (2012). Scientific Opinion on Mineral Oil Hydrocarbons in Food. The EFSA Journal, 10(6):2704. Scientific Opinion on Mineral Oil Hydrocarbons in Food | EFSA (europa.eu)
Nygaard UC, Vege A, Rognum T, Grob K, Cartier C, Cravedi JP, Alexander J (2019). Toxic effects of mineral oil saturated hydrocarbons (MOSH) and relation to accumulation in rat liver. Food and Chemical Toxicology, 123, 431-442. https://doi.org/10.1016/j.fct.2018.11.022
Pirow R, Blume A, Hellwig N, Herzler M, Hihse B, Hutzler C, Pfaff K, Thierse HJ, Tralau T, Vieth B, Luch A (2019). Mineral oil in foods, cosmetic products, and in products regulated by other legislations. Critical Reviews in Toxicology, 49(9). Full article: Mineral oil in food, cosmetic products, and in products regulated by other legislations (tandfonline.com)
(Please note, EFSA did not provide the reference, the Secretariat has found the above)
Wang D, Bruyneel B, Kamelia L, Wesseling S, Rietjens IMCM, Boogaard PJ (2020). In vitro metabolism of naphthalene and its alkylated congeners by human and rat liver microsomes via alkyl side chain or aromatic oxidation. Chemico-Biological Interactions, 315:108905.
https://doi.org/10.1016/j.cbi.2019.108905
Wang D, Schramm V, Pool J, Pardali E, Brandenburg A, Rietjens IMCM, Boogaard, PJ (2022). The effect of alkyl substitution 4098 on the oxidative metabolism and mutagenicity of phenanthrene. Archives of Toxicology, 96(4), 1109–1131.
https://doi.org/10.1007/s00204-022-03239-9
Weber S, Schrag K, Mildau G, Kuballa T, Walch S, Lachenmeier D (2018) Analytical Methods for the Determination of Mineral Oil Saturated Hydrocarbons (MOSH) and Mineral Oil Aromatic Hydrocarbons (MOAH)—A Short Review. Analytical Chemistry Insights, 13,
https://doi.org/10.1177/1177390118777757
Annex
This Annex contains the EFSA Opinion on “Discussion paper on the EFSA Draft Opinion for Public Consultation on “Update of the risk assessment of mineral oil hydrocarbons (MOH) in food””.
This opinion can also be accessed at:
Public consultation: (europe.eu)