Discussion Paper on Novel Formulations of Supplement Compounds Designed to Increase Oral Bioavailability
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Background
1. A second draft statement on the safety of turmeric, considered by the Committee on Toxicity (COT) (TOX/2022/68; COT, 2022a), identified uncertainty regarding novel formulations of turmeric that might enhance the oral bioavailability of associated curcuminoids. In that draft statement, Members highlighted “that some of the… ‘novel’ supplement types such as micellar, nano, and micro formulations should be looked at in further detail, regarding their pharmacokinetics and therefore their impact on the active chemicals.”
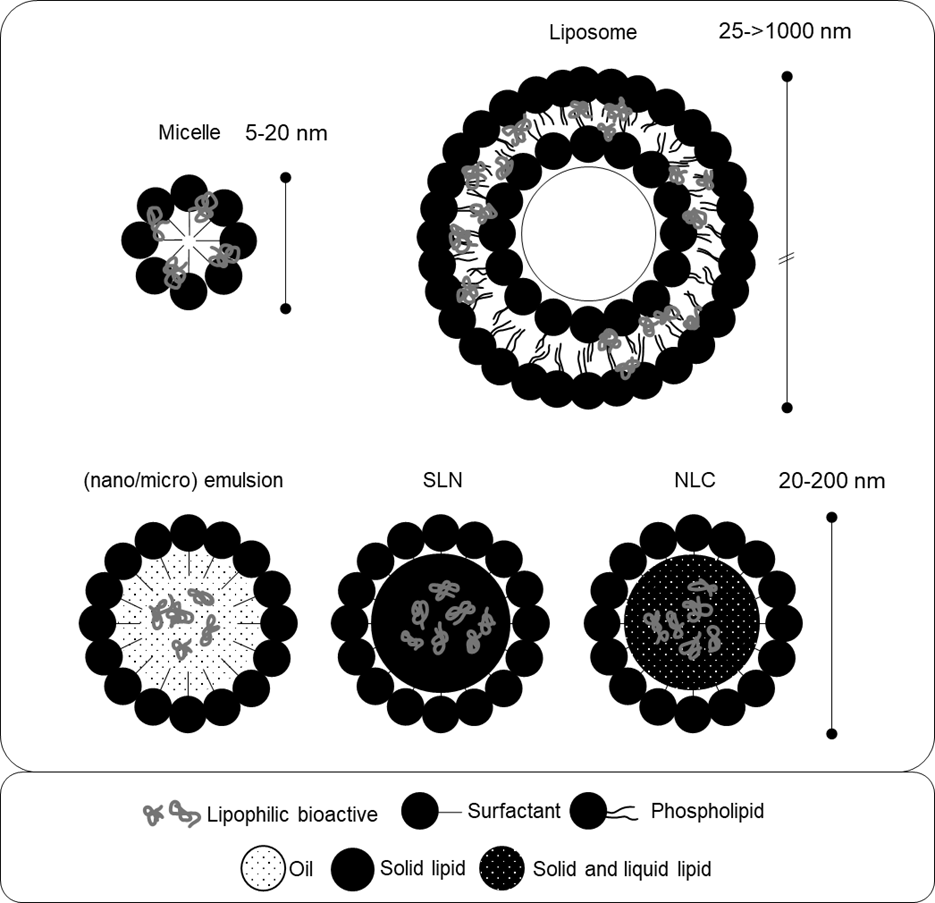
2. A preliminary analysis that had been presented in the discussion paper on turmeric (TOX/2022/35; COT, 2022b) suggested that novel micro and nano formulations of curcumin represented approximately 10% of the curcumin market. The use of phospholipids to solubilise, coat and/or encapsulate curcumin for delivery in colloids (i.e., oil-in-water dispersions, see section “Lipid-based delivery systems” below, paragraphs 5 – 30), micelles, and liposomes was identified as the most prevalent amongst these. Figure 1 (see page 14) provides a schematic representation of these formulations. Many of these products claim that this increases the bioavailability of associated curcuminoids and leads to higher uptake by the consumer. Bioavailability refers to “the proportion of a substance which reaches the systemic circulation unchanged after a particular route of administration” and which is therefore available to biologically interact with target sites (TOX/2022/07; COT, 2023; Annex 5). Owing to its physicochemical properties curcumin has inherently low oral bioavailability which has led to manufacturers’ attempts to produce more bioavailable formulations.
3. As a result of the issues raised in the discussion paper, it was decided that novel supplement formulations would form the basis of a general discussion paper. This current paper aims to define and review some of these novel formulations. It includes a discussion of the physicochemical parameters of several novel formulations, including coatings and/or lipid-based colloidal, micellar, liposomal, and lipid nanoparticles, as well as non-lipid-based delivery systems, and their associated physiological mechanisms. The paper also provides three case-studies assessing the impact of these delivery formulations on the oral bioavailability and pharmaco/toxicokinetic parameters of vitamin C, curcuminoids, and cannabidiol (CBD).
Novel formulations of supplement compounds
4. Paragraphs 5 – 54 outline the key physicochemical properties of several novel supplement formulations previously identified as being potentially relevant with respect to effects on bioavailability and pharmaco/toxicokinetics. The potential mechanisms underlying their ability to increase bioavailability are also discussed. In this paper, the term ‘novel formulations’ refers to formulations of supplement compounds that have been specifically designed to increase oral bioavailability. This generally includes formulation of active compounds with other ingredients intended to solubilise, encapsulate, and/or physiochemically stabilise/protect them. For explanation of the terms used, a technical glossary is provided at the end of the current paper.
Lipid-based delivery systems
Background and overview
5. The oral absorption and bioavailability of certain supplement compounds, many of which are often referred to as ‘nutraceuticals’ in the wider literature, is often limited by their highly lipophilic nature. The absorption of compounds administered by the oral route is related to their relative solubility in polar (i.e., water) and nonpolar solvents (i.e., oil, fat, lipids). For absorption to be effective, compounds need to be solubilised within the aqueous polar environment of the gastrointestinal (GI) tract and to pass readily through the nonpolar phospholipid membranes of the gut wall. Highly hydrophilic or highly lipophilic compounds, therefore, are more poorly absorbed than those of intermediate solubility. Curcumin is an example of one supplement compound with low water solubility and hence poor oral bioavailability. This has led to efforts to develop alternative preparation and delivery methods.
6. The critical factors affecting oral bioavailability of supplement compounds include the proportion of the administered dose available for absorption, the proportion absorbed, and the rate and extent to which the compound is metabolised, which are referred to as bioaccessibility, absorption, and biotransformation, respectively. Formulations designed to enhance the bioavailability of lipophilic and/or poorly absorbed supplements act mechanistically through modifying these factors (McClements et al., 2015). Many of these formulations have directly followed from earlier developments in the pharmaceutical industry targeted at modifying drug pharmacokinetics and reducing toxicity profiles.
7. The most widely investigated strategy to improve oral bioavailability of lipophilic compounds is through their co-formulation in systems composed of amphipathic surfactants and/or lipophilic solvents which are then dispersed in aqueous solutions (Pouton and Porter, 2008). This approach facilitates the aqueous solubility of lipophilic molecules, leading to increased bioaccessibility and absorption when administered by the oral route.
8. There are a range of different formulations, structural characteristics, and physicochemical parameters of these surfactant and/or lipid-based formulations that can be achieved by varying the concentrations and molecular identities of their constituents. As shown in figure 1 (p14), examples of some structures that have been studied for oral bioactive delivery and bioavailability enhancement include: micelles, oil-in-water micro- and nanoemulsions, self-emulsifying drug delivery systems (SEDDS), vesicles/liposomes, and lipid nanoparticles (Yao et al., 2014). Micelles, for instance, are self-assembled spherical structures of amphipathic surfactant molecules. The surfactant molecules in micelles are arranged so that the hydrophilic heads of the molecules face outwards towards the aqueous solvent forming the shell. The hydrophobic tails face inwards forming the hydrophobic core of the micelle (Haley and Frenkel, 2008).
9. The bioactive molecules formulated in these systems (i.e., the supplements or ‘nutraceuticals’ such as curcumin) are often encapsulated within the lipidic structures and are referred to as the ‘payload’ or ‘cargo molecules.’ The term ‘cargo molecules’ is used in the current paper to indicate the bioactive molecules formulated in these systems. A variety of these surfactant and/or lipid-based systems can be manufactured from food-grade ingredients and are described in paragraphs 14 - 30. Table 1 provides examples of food-grade ingredients used to produce lipid-based carrier systems, with an emphasis on commonly used lipophilic solvents and surface-active substances.
Table 1. Examples of food-grade ingredients used in the formulation of lipid-based delivery systems for supplements, adapted from Yao et al. (2014).
|
Structural component |
Example ingredients |
|
Lipophilic substances |
Triacylglycerol oils (e.g., canola, corn, fish, medium-chain triglycerides, palm, peanut, soybean, sunflower oils), Essential oils (e.g., carvacrol, lemon grass, oregano, thyme, thymol oils), Flavour oils (e.g., lemon, lime, orange, peppermint oils), Indigestible oils (e.g., waxes, hydrocarbon, paraffin, mineral oils). |
|
Surface-active substances |
Small-molecule surfactants (e.g., monoglycerides, diglycerides, polysorbates, sorbitan esters, sugar esters), Phospholipids (e.g., lecithin and lysolecithin), Proteins (e.g., casein, gelatine, soy, and whey), Polysaccharides (e.g., gum arabic and modified starch), Solid particles (e.g., silica or titanium). |
10. The structural characteristics of these systems can be engineered to formulate particles existing at the nanometre scale and are often discussed within the area of ‘nanotechnology’ (Jones et al., 2019) or described as ‘engineered lipid nanoparticles’ (Yao et al., 2014). This small size (see Table 2) and structures of these systems may significantly modulate the pharmacokinetics of associated bioactive cargo molecules through their physicochemical protection (Jones et al., 2019) and engagement of transcellular absorption pathways. These formulations also provide large surface area to volume ratios thus increasing their solubility and providing increased access for biological constituents, thereby modifying the bioavailability of the associated bioactive materials (Tamjidi et al., 2013).
11. In these lipid-based systems, lipophilic compounds preferentially dissolve in the hydrophobic compartment of surfactants composing micellar or vesicular structures and/or within the oil compartment of emulsions and lipid nanoparticle systems, thereby increasing their solubility in aqueous solution. The pathways by which lipid-based formulations alter the pharmacokinetics and bioavailability of associated cargo molecules may occur through physical, chemical, and/or biological mechanisms (Yao et al., 2014). These mechanisms include providing physical protection of cargo molecules, increasing their solubility and bioaccessibility, increasing intestinal permeability and absorption, altering the physiology of intestinal absorptive epithelial cells (enterocytes), promoting lymphatic transport, and facilitating the direct uptake of nanoparticles in the gastrointestinal (GI) tract (see paragraphs 31 – 48).
12. Fundamental to the efficacy of lipid-based delivery systems is their ability to maintain cargo molecules within a soluble and hence bioaccessible form within the GI tract. During their transit through the GI tract, lipid-based formulations are disassembled (partially digested) and reassembled into various kinds of micellar structures - mixed micelles - which facilitates their absorption into enterocytes (Yao et al., 2014). The precise structure and physicochemical properties of the formulation will dictate its fate. Delivery systems which are not digested or only partially digested may bypass first-pass metabolism through intracellular lymphatic trafficking via enterocytes, M-cells, and/or paracellular transport, thereby delivering their cargo molecules to the systemic circulation (Trevaskis et al., 2008).
13. Several common lipid-based formulation delivery systems currently under development and/or available on the market for dietary supplements are reviewed in this discussion paper. The following paragraphs give a general overview of their structures and physicochemical properties. This is followed by an overview of their modes of action and influence on the absorption and pharmacokinetics of associated bioactive cargo molecules. Several of these mechanisms are shared and overlap between the various formulations, and the pharmacokinetic parameters of a specific formulation will depend on its precise physicochemical properties and on that of the associated cargo. This is explored in detail with respect to vitamin C, curcuminoids, and CBD, in later sections of the current paper.
Emulsions
14. Emulsions are colloidal mixtures of hydrophobic and hydrophilic liquids, surfactants and co-surfactants, in which one phase (the dispersed phase) is dispersed as droplets within the other (the continuous phase). These droplets are stabilised by an interfacial film of surfactants which prevents phase separation and contributes to thermodynamic and kinetic stability (Singh et al., 2017). Emulsions can either be oil-in-water or water-in-oil, in which the dispersed phase is oil or water, respectively, with the corresponding substance composing the continuous phase. Oil-in-water emulsions are used to encapsulate lipophilic target compounds, whereas water-in-oil emulsions encapsulate hydrophilic active compounds. The structural characteristics of the dispersed phase can be varied, giving rise to emulsions containing spheroid/micelle/reversed micelle, cylindrical, and plane-like droplets.
15. Emulsions are categorised by their droplet size and thermodynamic stability. Conventional emulsions have droplet sizes >200 nm (Choi and McClements, 2020), whilst micro and nanoemulsions have droplet sizes <200 nm. Despite their prefixes, microemulsions tend to have smaller average droplet sizes than nanoemulsions, and the key distinction between these two systems is thermodynamic stability (McClements, 2012). Microemulsions are thermodynamically stable, and nanoemulsions are thermodynamically unstable systems. The size range defined for nanoemulsions differs between publications, with upper limits set at 100 nm, 200 nm, and 500 mm (Mason et al., 2006; McClements, 2012; Choi and McClements, 2020). McClements (2012) states “there is no distinct change in the physicochemical or thermodynamic properties of an oil-in-water emulsion when one reduces the droplet size from the micrometre range to the nanometre range.” There may, however, be differences in bioavailability, as discussed below.
16. Emulsification has been used to promote the solubilisation of poorly soluble lipophilic compounds. This process takes advantage of the hydrophobic core of emulsion droplets to allow dispersion of the target compound(s) (i.e., cargo molecules) in an aqueous solution (Yao et al., 2014). In this way, emulsions enhance the physicochemical stability of target compounds, increase their solubility, and promote intestinal permeability, thereby facilitating biological uptake (Ting et al. 2014). Therefore, emulsions have been used as delivery systems for poorly soluble bioactive drugs and supplement compounds.
17. SEDDS are isotropic mixtures (meaning their physical properties remain the same when tested in different directions) of oils (lipids), surfactants, and/or co-surfactants that are incorporated into capsules. Upon physical agitation within aqueous media (i.e., in the conditions of the GIT), these systems self-emulsify. SEDDS may be designed to form mixtures that have the physical properties of micro- or nanoemulsions upon solubilisation (self-microemulsifying and self-nanoemulsifying drugs delivery systems, respectively SMEDDS and SNEDDS). (Gursoy and Benita, 2004; Tanya and Hari, 2017).
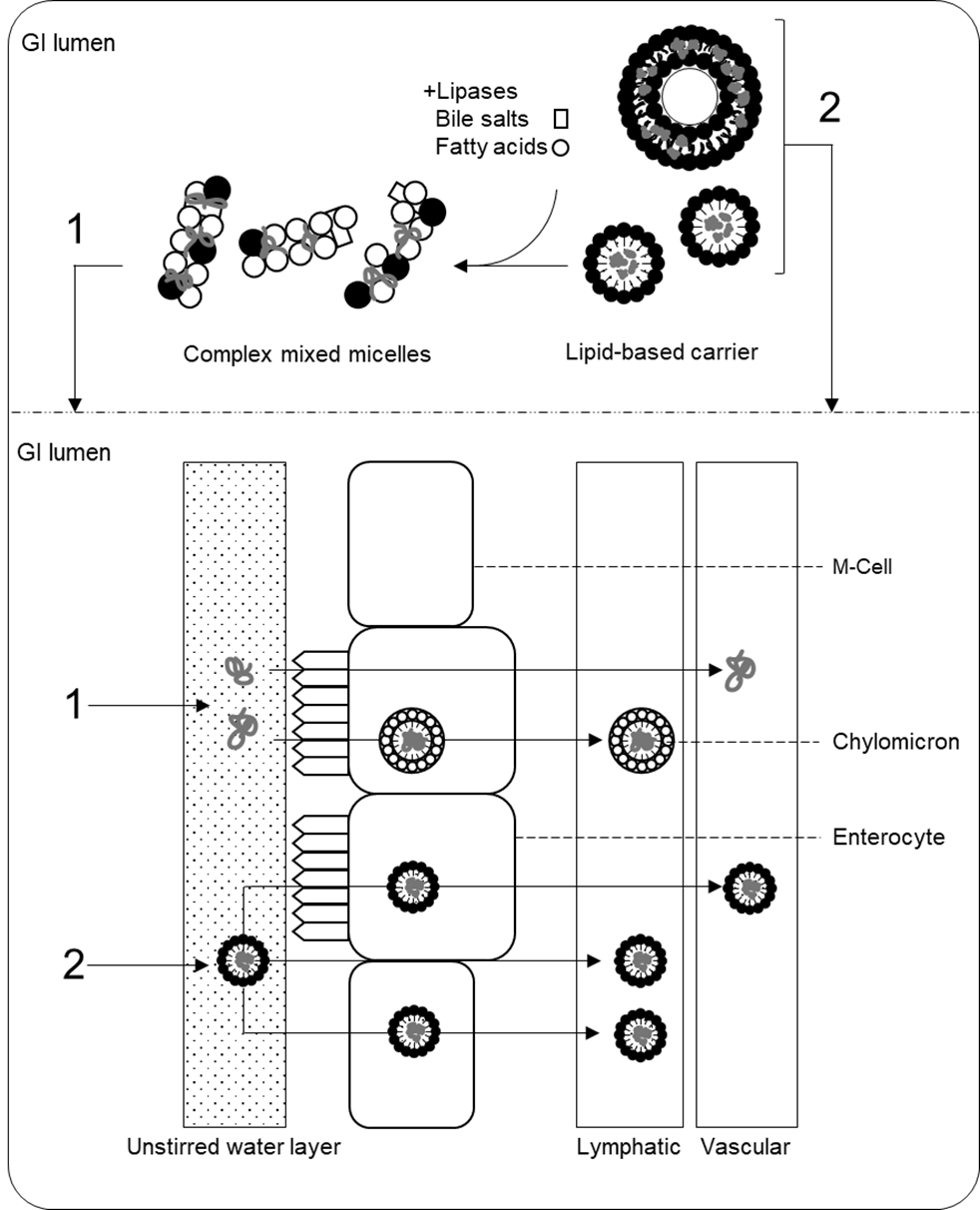
Micelles
18. Micelles are similar in composition to emulsions in that they are colloidal dispersions of spherical surfactant droplets dispersed within a continuous phase. When dispersed above a specific concentration in aqueous media – known as the critical micelle concentration – amphiphilic surfactants such as phospholipids spontaneously self-assemble into spherical structures. Micellar structures can be altered or ‘tuned’ and “combinations of surfactants with different molecular characteristics are often used to improve the formation, stability, or performance of micelles.” (Yao et al., 2014).
19. Micelles are generally considered to be primarily two-phase (rather than three-phase) systems composed of surfactant molecules and water with only low to no quantities of lipophilic solvent (Siano, 1982). As such, and as opposed to emulsions, micelles do not enclose a core of oil-based solvent. Although various size ranges have been reported for micelles, depending on the curvature and tail length of the surfactant, they are nano-sized structures existing at up to approximately 100 nm (Huang et al., 2010) and often smaller (e.g., 15-30 nm) (Ali et al., 2019). Micelles co-formulated with cargo molecules, such as supplements or drugs, have been referred to as ‘swollen micelles’ (Yao et al., 2014).
20. Micelles can also be formed through the spontaneous self-assembly of block copolymers, molecules composed of covalently linked hydrophilic and hydrophobic segments, to form polymeric micelles. These are of significant interest as drug delivery vehicles (Kulthe et al., 2012).
Liposomes
21. Liposomes are spherical vesicular structures composed of one or more phospholipid bilayers (Liu et al., 2020). They range in size from small vesicles (30 - 100 nm), to large (100 – 300 nm) and giant vesicles (1 – 100 µm), enclose an aqueous core, and may have complex internal structures including concentric bilayers (Sharma and Shamra, 1997; Liu et al., 2020). Single membrane liposomes are termed unilamellar vesicles, liposomes with one or more bilayers are termed multilamellar vesicles, whilst liposomes enclosing smaller liposomes are termed multivesicular liposomes (Akbarzadeh et al., 2013). Cholesterol and other modifiers such as polysaccharides (hyaluronic acid, mannans, dextrans, etc.) (Turánek et al., 2019) and polymers (such as polyethylene glycol) (Shen et al., 2018) may be incorporated into liposomes to increase their stability and modulate their biological activity.
22. Conjugation of liposomal phospholipids to polyethylene glycol, for instance, has been frequently used to increase liposome circulation time within pharmaceutical research and development. Within the food and feed industry, however, liposomes with surface modifications are rarely formulated (Liu et al., 2019). The major phospholipid used in liposomes for food-based applications is phosphatidylcholine derived from soy lecithin, egg lecithin (Akbarzadeh et al., 2013), marine lecithin (Imran et al., 2015), and milk phospholipids (Thompson et al., 2007).
23. Liposomes form spontaneously after phospholipid precipitated from organic solvent evaporation are dispersed in aqueous solution, although more recent methods for their production include the use of supercritical fluids and microfluidisation (Liu et al., 2020; Ajeeshkumar et al., 2021). The resulting preparation may be further modified to refine particle size, structure, and distribution, and loaded with bioactive cargo (e.g., curcumin) through active or passive loading methods (Akbarzadeh et al., 2013).
24. Due to the amphipathic nature of the phospholipid building blocks, liposomes can serve as carrier vehicles for lipophilic and/or hydrophilic bioactive cargo molecules; whilst hydrophilic molecules may be encapsulated within the core of liposomes, lipophilic molecules are embedded in the lipid bilayer, with molecules of intermediate polarity distributing between the components dependent upon their preferential solubility in polar and nonpolar substances (referred to as the ‘LogP’, i.e., the partition coefficient as derived from the experimental solubilisation of a solute in water and octanol).
25. The liposomal encapsulation of drug molecules was initially developed by the pharmaceutical industry to enhance the pharmacokinetics of therapeutic compounds, including oral bioavailability, tissue distribution, and toxicity profile, although they have also been used in the cosmetic industry (Müller et al., 2000). A key example is the liposomal formulation of the chemotherapeutic agent doxorubicin that was approved by the FDA in 1995 (Liu et al., 2022). Liposomal encapsulation reduces the inherent cardiotoxicity associated with oral doxorubicin administration and improves the overall response rate by modulating its tissue distribution profile (Xing et al., 2015). Use of liposomes in the food and feed sector follows these earlier developments in the pharmaceutical industries. Their use in supplements is primarily tied to attempts to enhance the oral bioavailability of poorly absorbed compounds and for the physicochemical protection of target molecules that are sensitive to environmental and physiological conditions (e.g., UV, pH, temperature).
26. As they interact with the biomolecular milieu of the blood stream, liposomes and other (lipid) nanoparticles become coated with a “protein corona”. The protein corona imparts a “new biological identity” to circulating nanoparticles, thereby modulating their fate and which may have important effects on the bioavailability of associated bioactive molecules (Giulimondi et al., 2019).
Lipid nanoparticles
Solid lipid nanoparticles
27. Solid lipid nanoparticles (SLNs) also have a similar structure to nanoemulsions in that they are colloidal dispersions of (nano-sized) lipid droplets (20-200 nm) within aqueous media stabilised by a surfactant interfacial region. However, in SLNs the core of these particles is either fully or partially crystalline at body temperature, as opposed to a liquid (Yao et al., 2014). The lipidic component of SLNs can consist of triglycerides, mono-, di-, and triglyceride mixtures, waxes, and hard fats. Lipophilic bioactive molecules may be incorporated in the lipidic phase of SLNs and can either be dispersed, enriched in the core or enriched in the shell of the particle (Müller et al., 2000).
28. The solid state of the lipidic component improves control over the release of encapsulated molecules out of the particle (Weiss et al., 2008). For instance, prednisolone encapsulated in SLNs demonstrated a prolonged and sustained in vitro release for up to 7 weeks that was modulated by particle size, preparation method, and composition of the lipid matrix (Zur Mühlen and Mehnert, 1998). SLNs also provide physicochemical protection to encapsulated molecules, thereby increasing their stability under various environmental and physiological conditions (Müller et al., 2000). For instance, SLN encapsulation may protect labile bioactive molecules from degradation with the stomach thus enhancing transit of intact molecules to the small intestine for absorption. Coating of SLNs with chitosan and other modifications have been proposed that might further enhance the stability of SLNs within the GI tract and prevent burst-release of bioactive cargo molecules in the stomach (Ganesan et al., 2018).
Nanostructured lipid carriers
29. Nanostructured lipid carriers (NLCs) are a modified form of SLN in which the lipid phase is composed of both solid and liquid lipids at body temperature. Consequently, NLCs exhibit a more amorphous and less crystalline internal structure than SLNs. NLCs were developed to enhance entrapment of bioactive cargo molecules and to prevent their expulsion by reducing crystalline transitions of the lipidic components and increasing intermolecular spaces (Müller et al., 2002; Kharat and McClements, 2019). Three types of structural characteristics of NLCs have been described which indicate the phase of the lipid interior (Tamjidi et al., 2013): imperfect type, in which the core consists of irregularly spaced solid and liquid lipids; amorphous type, in which the lipidic core congeals as a non-ordered solid; and multiple type, in which liquid nano-compartments exist within a solid matrix.
30. Table 2 summarises the key features of the lipid-based delivery systems introduced above, whilst Figure 1 provides a schematic overview of their structures.
Table 2. Overview of lipid-based formulations of supplement compounds.
|
Formulation type |
Structural features |
Components* |
Size range |
Refs |
|
Microemulsion |
Thermodynamically stable oil in water droplets; composed of lipophilic cores stabilised by polar surfaces. |
Surfactant, (Co-surfactant) Oil, Water. |
Often smaller than nanoemulsions; often <100 nm. |
McClements, 2012; Aswathanarayan and Vittal, 2019. |
|
Nanoemulsion |
Similar to microemulsions but thermodynamically unstable. |
Surfactant, (Co-surfactant) Oil, Water. |
20 – 200 nm. |
Choi and McClements, 2020. |
|
S(N/M)EDDS |
Isotropic mixtures of oils and surfactants that spontaneously emulsify upon agitation in aqueous environments (i.e., GIT). |
Surfactant, (Co-surfactant), Oil. |
100-300 nm <50 nm (SM/NEDDS)
|
Neslihan and Benita, 2004. |
|
Micelles |
Spherical structures: inward facing hydrophobic core and outward facing hydrophilic head; no internal bulk phase (i.e., oil). |
Amphipathic surfactants , Water. |
up to 100 nm. |
Huang et al., 2010. |
|
Solid lipid nanoparticles |
Lipid droplets with solid crystalline interior. |
Surfactant, (Co-surfactant), Oil, Water. |
20-200 nm. |
Yao et al., 2014. |
|
Nanostructured lipid carriers |
Lipid droplets with partially crystalline/two state/amorphous interior. |
Surfactant, (Co-surfactant), Oil, Water. |
20-200 nm. |
Yao et al., 2014. |
|
Liposomes |
One or more concentric shells composed of lipid bilayers. |
Phospholipids, Water. |
25->1000 nm. |
Sharma and Shamra, 1997; Liu et al., 2020. |
*Components will also include the bioactive molecule of interest; S(N/M)EDDS: self (nano/micro) emulsifying drug delivery system.
Figure 1. Schematic diagram of lipid-based formulation systems. SLN = solid lipid nanoparticle. NLC = nanostructured lipid carrier. Figure based on the text and generated by the Secretariat.
Mechanisms of enhanced bioavailability in lipid-based delivery systems
Physicochemical parameters
31. Preparation of lipophilic molecules with amphiphilic and/or oil/lipidic components aids their solubility and dispersion within aqueous media. The solubility of a specific molecule will be formulation dependent and a function of the target molecule, (co)surfactant molecules, and/or oil/lipid phase and their relative concentrations (Choi and McClements, 2020). For instance, the encapsulation efficiency of a lipophilic molecule into lipid carriers/vesicles/particles, its partition coefficient, and its tendency to leach out of the lipid phase into the aqueous phase, dynamically affects solubility in these formulations. The structural characteristics of the preparation itself may exhibit a degree of instability, for example through the tendency to aggregate, swell or burst, which will also affect solubilisation prior to oral administration (Choi and McClements, 2020).
32. Formulation with lipid carriers and particles may protect labile molecules from environmental degradation. As many supplements may lose their functional features by oxidation, degradation, and reaction with other materials during processing and storage (Shin et al., 2015), encapsulation within emulsions, micelles, liposomes, or lipid particles has the potential to shield supplement compounds from degradative reactions and increase their stability (Li et al., 2021). As with solubilisation, the ability of a lipidic formulation to enhance the stability of a cargo molecule is a function of the physicochemical properties of the preparation. For instance, larger emulsion droplets led to enhanced stability of curcumin due to a smaller oil-water interfacial area at which the molecule could be degraded (Zou et al., 2015). The size of emulsion droplets also influences the penetration of light, thus modifying the stability of light-sensitive cargo molecules (Choi and McClements, 2020).
33. The characteristics of the interfacial layer between the surfactant and aqueous components of these preparations also effects physical stability and interaction with biological systems. For instance, “the electrical characteristics of the oil droplets [zeta potential] impact the physical stability of nanoemulsions by altering the colloidal interactions acting between the droplets, which impacts their tendency to aggregate with each other.” (Choi and McClements, 2020). The charge of the droplets/particles and their tendency to aggregate may also influence interaction with biological systems, for instance by modulating their adhesion to biological surfaces and the ability of enzymes to adsorb to their surfaces. Although less common in food/feed applications, coating of vesicles and micelles with ligand molecules can be used for targeting and precision delivery.
34. Encapsulation and solubilisation in lipid carriers may protect cargo molecules from degradation in the GI tract and increase their residence time, thus promoting absorption. For instance, certain liposomes may pass through the stomach relatively structurally intact due to the low activity of gastric lipases against phospholipids, thus protecting the liposomal cargo from the low pH of the stomach (Carrière et al., 1992; Liu et al., 2019; Liu et al., 2020). Following transit through the stomach, the liposomal cargo is released under the conditions of the small intestine thus promoting absorption at this site (Tan et al., 2014).
35. Conversely, carrier systems may undergo various physicochemical/structural changes as they transit through the mouth, oesophagus, and stomach with resultant impacts on the bioavailability of their associated cargo. The physical stress of mastication and torsional stomach contraction, coupled with changes in pH and ionic strength may induce particles to aggregate, burst, or swell. Particles may clump/aggregate (also referred to as flocculation), coalesce/partially coalesce back to a two-phase system, undergo Ostwald ripening (large particles grow and small ones shrink due to molecular diffusion of oil molecules through the aqueous phase), be digested, or solubilised (Yao et al., 2014). Similarly, the surface properties of the particles/droplets may undergo various changes through interaction with surface-active components of the GI fluid. These changes include adsorption, co-adsorption, multilayer formation, and digestion/degradation. The nature and extent of such events occurring to a specific formulation has the potential to impact on downstream bioavailability.
Biological mechanisms
36. There are three key pathways by which the lipidic components of colloidal emulsions, micelles, liposomes, and lipid particles may modulate the absorption, bioavailability, and disposition of target molecules following oral administration. These are: “the alteration of the composition and character of the intestinal milieu, the recruitment of intestinal lymphatic drug transport, and the interaction with enterocyte-based transport processes” (Porter et al., 2007). Some of these pathways are general and are shared between different formulations, whereas others depend on the size and/or structural and molecular characteristics of specific particles. The following paragraphs summarise the general mechanisms of increased bioavailability in lipid-based delivery systems and notes some pathways specific to certain formulations (e.g., liposomes and solid lipid nanoparticles). Figure 2 provides a schematic overview of the absorption mechanisms via lipid-based formulations.
Solubilisation in the GI tract
37. Following entry to the small intestine, the large surface area-to-volume ratio and low interfacial tension of microemulsions and other micro- and nano- lipid-based formulations promotes their interaction with components of the intestinal milieu (Fanun, 2012). Here, lipid-based preparations undergo lipolysis by pancreatic lipases; triacylglycerols are converted into free fatty acids (FFAs), monoacylglycerols (MAGs), and diacylglycerols (DAGs), which then leave the droplet/particle and participate in the formation of mixed micelles with endogenous fatty acids and bile salts. Whilst ‘simple mixed micelles’ are formed from endogenous bile salts and phospholipids in the small intestine, in the presence of exogenous lipids, ‘complex mixed micelles’ are formed from the digestion products of these exogenous lipids (MAGs and FFAs), in addition to bile salts, and phospholipids, and contribute to the encapsulation of co-present lipophilic bioactive molecules (Yao et al., 2014).
38. Mixed micelles are a complex mixture of colloidal structures whose formation is driven by the hydrophobic effect. These structures include micelles, vesicles, and liquid crystals that have dimensions in the nanometre range (Müllertz et al., 2012), although their precise composition depends on the concentration of components in the system and the characteristics of ingested lipids and/or surface-active molecules. Many components of complex mixed micelles have bilayer structures, and lipophilic molecules may be entrapped in these layers, thus facilitating their solubilisation and protection in the GI tract.
39. Solubilisation with complex mixed micelles delivers lipophilic molecules across the unstirred water layer of the gut lumen to the apical membrane of enterocytes (Yeap et al., 2013). The unstirred water layer is an aqueous diffusion barrier adjacent to the intestinal membrane that is a potential barrier to the intestinal absorption of compounds. Delivery across this site via complex mixed micelles therefore aids the intestinal absorption of lipophilic molecules. Absorption then occurs via passive and active transport mechanisms (Iqbal and Hussain, 2009). The absorption of lipophilic molecules into enterocytes is also modulated by the co-presence of FFAs, MAGs, phospholipids, and cholesterol (Kotake-Nara and Nagao, 2012). By contributing to the solubilisation of lipophilic compounds in complex mixed micelles, lipid-based formulations fundamentally increase the bioaccessibility of encapsulated compounds – i.e., the fraction of a compound available in a suitable form for absorption – thereby contributing to enhanced bioavailability (McClements, 2018).
40. The formation of complex mixed micelles is one of the key factors driving the increased bioavailability of lipophilic bioactive components formulated in lipid-based delivery systems. The solubilisation capacity of exogenous-endogenous complex mixed micelles that entrap lipophilic bioactives can significantly surpass that of endogenous (‘simple’) mixed micelles alone (Kossena et al. 2004), and their solubilisation capacity is dependent on the type and amount of digestible lipids present in the formulation as well as the characteristics of the bioactive molecule. For instance, the bioaccessibility of carotenoids was enhanced when formulated with long-chain versus medium-chain fatty acids, but no difference was observed with curcumin between these two types of fatty acids (Qian et al., 2012; Ahmed et al., 2012). Carotenoids are too large to fit in complex mixed micelles composed of medium-chain fatty acids (because they are smaller and have reduced curvature), whereas curcumin is small enough to fit into both types, thus explaining this discrepancy (Yao et al., 2014).
41. The size distribution of the particles/droplets in the lipid-based colloids may also impact on the incorporation into mixed micelles and subsequent modulation of bioavailability. For instance, beta-carotene bioaccessibility was increased with decreasing droplet size in an oil-in-water emulsion (Wang et al., 2012). This is likely to be due to increased exposed surface area for digestion by lipases. In this respect, due to their nanoscale size and large surface area to volume ratio, NLCs may be efficiently digested, solubilised, and incorporated into mixed micelles, thus aiding their passage across the unstirred water layer for absorption by enterocytes (Kipp, 2004).
Interactions with enterocytes
42. Lipid particles and/or their digested components may physiologically interact with enterocytes to modulate the absorption of associated lipophilic molecules. For instance, surfactant molecules may directly enhance the permeability of cell membranes and cause the opening of tight junctions, thereby facilitating cellular absorption and paracellular transport of associated compounds, respectively (Benzaria et al., 2013; Zordan-Nudo et al., 1993). This effect is dependent upon the lipid composition of the formulation. For example, olive oil nanoemulsions more effectively increased trans-enterocyte transport of pterostilbene than flaxseed, and oil type also modulated intracellular pterostilbene metabolism in these cells (Sun et al., 2015).
43. Lipidic excipients may also influence the transport pathways into and across enterocytes by affecting the activity and expression levels of transporter proteins. For instance, some studies have suggested that the lipidic components of emulsions may inhibit the apical membrane efflux transporter P-glycoprotein, thereby facilitating the absorption of compounds which are substrates for this transporter (Constantinides and Wasan, 2007; Bogman et al., 2003).
Lymphatic transport and direct uptake mechanisms
44. Intact liposomes that have not been digested and incorporated into complex mixed micelles also interact with intestinal epithelial cells thereby influencing the absorption of their cargo molecules (Liu et al., 2020). For instance, liposomes may adhere to the cell membrane and release their contents for absorption by the cell (Blumenthal et al., 1977; Allen et al., 1981). Liposomes may also directly fuse with the cell membrane, thus delivering their contents intracellularly (Knoll et al., 1988), be intracellularly internalised by endocytosis (Straubinger et al., 1983), or become disrupted through exchanging their lipid components with cell membranes thereby leading to intracellular uptake of their contents (Sandra and Pagano, 1979). The pathways of cellular uptake of liposomes are size dependent: liposomes of approximately 98-160 nm are predominantly internalised by clathrin-dependent uptake, liposomes of approximately 70 nm by clathrin- and dynamin-dependent uptake, and liposomes of approximately 40 nm primarily by dynamin-dependent uptake mechanisms. The size of liposome also affects intracellular trafficking to endosomes and lysosomes (Andar et al., 2013) which therefore may exert important effects on the resultant bioavailability of cargo molecules.
45. Bioactives associated with lipid excipients may enter the lymphatic system after absorption by enterocytes, via packaging into chylomicrons. Within enterocytes the fate of bioactive lipophilic compounds is tied to the metabolism of the associated carrier lipids; whilst lipids with shorter chain lengths (C <12) pass into the portal vein, those with longer chains (C ≥12) are re-esterified into triacylglycerols. These are then packaged into chylomicrons and trafficked into the lymphatic system. Subsequently, the bioactive cargo molecules may be co-shuttled into this pathway (Yáñez et al., 2011; Trevaskis et al., 2008). The association of lipophilic molecules with chylomicrons evades first-pass metabolism and contributes to increased oral bioavailability. Packaging into chylomicrons may also protect associated the cargo molecules from metabolism within enterocytes, also contributing to increased bioavailability (Trevaskis et al., 2008).
46. Undigested (nano)emulsion droplets and lipid nanoparticles may also be absorbed intact, via paracellular and/or transcellular pathways or via M cells (Singh et al., 2017). The size of lipid particles influences their fate within the GI tract. For instance, SLNs may exhibit bio-adhesion to the intestinal wall thus increasing their retention time within the GI tract and potentially leading to increased absorption of encapsulated molecules (Ponchel et al., 1997; Li et al., 2009).
47. Smaller particles are also likely to have increased direct uptake within the GI tract and are absorbed into the lymphatic system via paracellular transport, thereby evading first pass metabolism (Kreuter, 1991). Absorption of SLNs have exhibited a biphasic distribution with early (1 -2 hr) and later (6 – 8 hr) peaks. These peaks may indicate rapid transport into the systemic circulation and release/distribution from organs into the circulation, respectively (Yuan et al., 2007).
Figure 2. Summary of absorption pathways from lipid-based delivery systems. (1) Lipid carriers are digested and incorporated into complex mixed micelles which deliver lipophilic bioactive molecules across the unstirred water layer for absorption by enterocytes. (2) Undigested lipid particles can be directly absorbed via paracellular and transcellular pathways. See the text for more detail. Figure generated by the Secretariat.
Summary
48. Overall, lipid-based formulation of lipophilic bioactive molecules facilitates their solubility within the GI tract and increases the bioaccessible fraction of the associated compound available for absorption. Although the physicochemical and structural features of these preparations, and therefore their fate within the GI tract, is subject to significant heterogeneity, the pathways leading to enhanced absorption depend on partial digestion of the lipid components, disassembly, and reassembly into complex mixed micelles. Mixed micelles contribute to the solubility of associated lipophilic bioactive molecules and deliver them to enterocytes for absorption via passive and active uptake mechanisms. A range of molecular interactions between the lipidic components of these formulations with enterocytes may also modulate uptake of associated bioactive molecules, for instance by enhancing cell permeability through physical and biological mechanisms. Importantly, formulation with lipidic excipients promotes the lymphatic transport of lipophilic molecules via chylomicron packaging in enterocytes and transport through M-cells. Lymphatic transport evades first pass metabolism, thus potentially increasing the fraction of bioactive molecule within the systemic circulation.
Other systems to increase bioavailability
49. Several other systems have been developed to increase the oral bioavailability of poorly soluble/absorbed compounds that do not use lipid-based solubilisation. Such methods include co-formulation with hydrophilic carrier molecules including polysaccharides.
50. Cyclodextrins (CDs) are cyclic oligosaccharides formed by the linkage of D-(+)-glucopytanose units with ɑ-(1,4)-glycosidic bonds. CDs can form complexes with a variety of hydrophobic substances through the incorporation of the entire molecule or part of the molecule into their cavity. The formation of such molecular complexes affects the physicochemical properties of the cargo molecules, including stability, water solubility, and bioavailability (Uekaji and Terao, 2018). Complexing lipophilic supplements with CDs can increase their oral bioavailability, potentially by increasing absorption through the formation of mixed micelles and prolongation of plasma circulation (Terao et al., 2006; Uekaji and Terao, 2018).
51. Other molecules that have been used to complex and solubilise lipophilic molecules include soluble fenugreek fibre and galactomannans (Goh et al., 2020). These systems act to keep lipophilic molecules solubilised within the GI tract and can be used to form particulate carrier vehicles (Cerqueira et al., 2019).
52. Reduction of particle size through micronisation and nanoisation has also been employed to increase solubility of target molecules. Micronisation was developed by the pharmaceutical industry to combat poor bioavailability associated with new chemical entities and has been increasingly adopted within the supplement industry for poorly soluble compounds such as curcumin. Micronisation refers to processes which reduce the average particle size of an active ingredient. Particle size reduction is achieved through comminution and deagglomeration via impact and shear forces, respectively (Kirkwood, 2018). Mechanical milling techniques can produce particles with diameters of around 50-75 µm, whilst ultra-fine grinding methods such as jet milling can result in particles with sizes of <5 µm (Pharmaceutical Technology, 2021).
53. By fragmenting particles into smaller sizes, micronisation increases the surface area to volume ratio area of an active ingredient, thereby increasing its solubility in an appropriate solvent (Savjani et al., 2012). As micronisation increases dissolution speed (Bhalani et al., 2022), this process can increase absorption and hence the bioavailability of compounds with absorption limited by the rate of diffusion (Oh et al., 1995). The poorly soluble nature of many plant-derived compounds has led to investigations of micronisation to increase their oral bioavailability (Xing et al., 2017).
54. The production of nanocrystals/nanosuspesions of active ingredients with particles from 200-400 nm can be achieved by wet milling, nancocrystalisation, or spray drying. Like micronisation, nanoisation takes advantage of increased surface area to volume ratio of particle diminution to increase the solubility of a compound in aqueous media (Tiwari and Takhistov, 2012).
Uncertainties surrounding novel supplement formulations
55. There is a large degree of uncertainty regarding the identity and physicochemical properties of supplements claiming to have enhanced bioavailability that are available on the market. Many of these supplements make generic claims about improved bioavailability but do not indicate the supposed mechanisms underlying this effect and it is not clear what the point of comparison is. Some of these products make precise quantitative claims about their relative absorption, but these statements are generally unreferenced and/or have a lack of associated evidence.
56. Many supplements on the market are advertised as ‘liposomal’, but the physicochemical identities of the supplements in question are not rigorously characterised. For instance, one company (Lipolife) claims that many liposomal supplements available on the market are of a low quality, and that some products advertised as ‘liposomal’ do not contain any liposomes. Their website also argues that the terms ‘liposomal’ and ‘liposome’ do not mean the same thing; whereas ‘liposomes’ refers to spherical phospholipid bilayer structures, ‘liposomal’ may refer to products that contain (phospho)lipids, but which do not necessarily contain liposomes (i.e., they are emulsions) (Lipolife, 2023).
57. Lipolife’s website displays analysis of several commercially available ‘liposomal’ vitamin C products in terms of particle size, polydispersity index (PDI), and nutrient content. Based on the conclusions of their analysis, one product did not contain any liposomes, whilst the particle size and PDI were highly variable between others. Moreover, one product contained approximately 4% of the claimed levels of vitamin C. It should be noted, however, the methods used in this analysis were not reported and the analysed products were from rival companies.
58. Lipolife also offer a service to which consumers can submit liposomal formulations purchased from other companies for analysis of key physicochemical properties. As of 16/02/2023, two supplements have been analysed. One returned generally acceptable results with a low PDI and expected particle size, whereas the other contained particles larger than the expected size for liposomes, a high PDI, and no detectable levels of the advertised supplement (nicotinamide mononucleotide).
59. Although these results need to be interpreted with caution, as the analytical methods were not reported, it indicates consumer uncertainty in the liposomal supplement market with potential significant heterogeneity in the formulation of products advertised as ‘liposomal’, and/or intentional mislabelling.
60. Altrient are another company arguing that many so-called ‘liposomal’ products contain no actual liposomes (Altrient, 2023). Abundance and Health, the distributor and retailer for Altrient, have also performed independent analysis of liposomal products. Their director has stated that analyses conducted in September 2020 demonstrated very low levels of liposomes in two products, and the absence of liposomes in another (Abundanceandhealth, 2021).
61. Although the analysis performed by Abundance and Health has not been published, it prompted an investigation by the Advertising Standards Authority (ASA) in November 2019 into one of the products that was being marketed as ‘liposomal vitamin C’. The company in question explained that the product was an ‘emulsion’ of phospholipids that wrapped around the vitamin C. However, following consultation with the Laboratory of the Government Chemist and scrutiny of the evidence and associated analytical data (particle tracking analysis), the ASA ruled that there was insufficient evidence to convincingly demonstrate the presence of liposomes in the product. A ruling was made against the advert appearing in that form again (Advertising Standards Authority, 2021).
62. As these considerations make clear, there is likely to be significant heterogeneity in the properties of supplements that are advertised as ‘liposomal’ and many of the products available lack testing and characterisation. There are therefore important limitations in our understanding of the identity of the formulations in question.
Market data and projected trends
63. A preliminary analysis was conducted on liposomal supplements available on the internet to gather data about the active ingredients formulated in this way and their relative distribution. The search term “liposomal supplements” returned 649 products from a popular online marketplace The active ingredients of the first 105 of these supplements were listed.
64. This analysis excluded supplements containing >1 active ingredient (e.g., vitamin D3 + vitamin K2). Moreover, some supplements were duplicated in the search results, and may therefore be relatively overrepresented in the analysis. However, vitamin C was overwhelmingly the most common supplement available in a ‘liposomal’ form. Other important supplements included glutathione, vitamin D3, and vitamin B complex. Table 3 provides a summary of the products available and the number of each product.
Table 3. Distribution of liposomal supplement products available from an online retailer from first 105 results.
|
Active ingredient |
Number of products |
|
Vitamin C |
54 |
|
Vitamin B |
11 |
|
Glutathione |
10 |
|
NAD+ |
8 |
|
Vitamin D3 |
6 |
|
NMN |
5 |
|
Multivitamin |
3 |
|
MTHF |
2 |
|
Nicotinamide riboside |
1 |
|
Apigenin |
1 |
|
Magnesium |
1 |
|
Carnosine |
1 |
|
Zinc |
1 |
|
Vitamin K2 |
1 |
|
Total |
105 |
NAD+: Nicotinamide adenine dinucleotide; NMN: Nicotinamide mononucleotide; MTHF: Methyltetrahydrofolate.
65. A search for “nanoencapsulated supplements” returned no products from the online retailer whilst “micellar supplements” primarily returned micellar casein supplements and a few turmeric/curcuminoid preparations. The limitations of performing an approximate market analysis in this way is due to differences in nomenclature and marketing styles.
66. These analyses, therefore, are uncertain. However, more sophisticated market data is available on the market trends for novel supplement formulations. To access this information a report would need to be purchased from a market research company.
67. Three reports have been identified which may be of use in the current assessment. Two contain information about novel formulations across the supplement market, whilst the other contains information specific to the curcumin/turmeric market. Two of the reports (from Research and Markets and Absolute Reports) were identified via communication with the respective company based on specific inquiries by the Secretariat and are not directly advertised online. One of the reports (from Mintel) is advertised online, and the Secretariat have inquired into the contents of this report.
68. The first report is provided by the company Mintel (2022) and is titled “The Future of Vitamins, Minerals, and Supplements 2022.” This report discusses the market trends of novel formulations over the next five years and discusses specific products. The brochure for this report argues that, in five years and beyond, “bioavailable supplements that support optimal absorption will stand out.”
69. The second report is from Research and Markets.[1] It contains base data for 2022 and forecast data for 2023-2028 and segments the nutraceutical/supplement market by formulation type, including liposomal, nanoemulsions, micellar, and nano-encapsulated supplements. It is not clear from the proposal whether this report contains detailed information about the active ingredients that are being formulated in these ways, and their relative proportions within the market.
70. The third report is provided by the company Absolute Reports.[2] This report discusses the turmeric supplement market only and is titled “Europe Turmeric Supplement Market Research Report 2022 (Status and Outlook).” This report contains information about the relative proportion of the market composed by novel formulations, including ‘liposomal’, ‘micellar’, ‘emulsion-based’ and ‘nanoencapsulated’. This report also contains projected trends for these formulations, and some information on the uses of these supplements for specific ailments (e.g., osteoarthritis).
Case studies of supplement formulations with increased bioavailability
71. The following paragraphs outline three case studies of supplement compounds prepared as novel formulations. The case studies are intended to provide empirical pharmacokinetic outcomes of the mechanisms and physiological parameters discussed above and, specifically, to assess how novel formulations of supplement compounds may significantly affect plasma levels of active compounds. Some of these examples, therefore, may have toxicological implications.
72. The case studies are focused on controlled human trials in which novel and standard formulations are compared, rather than on in vivo and/or in vitro data. These studies are not exhaustive and attempt to provide an overview of realistic scenarios of how novel formulations of supplements may impact their bioavailability.
Case study 1: Liposomal vitamin C
73. A significant quantity of the novel formulations on the market appears to be liposomal formulations of vitamin C (see Table 3., above). Liposomal vitamin C supplements therefore provide an informative case study for investigating how novel formulations might impact supplement bioavailability and pharmacokinetics with potential implications for consumers.
74. Due to its potential role in cancer therapy at high doses, a significant amount of attention has been given to the pharmacokinetics of vitamin C. Vitamin C (ascorbate/ascorbic acid) is a hydrophilic compound with complex pharmacokinetics. Its bioavailability is limited by saturable transport mechanisms in the small intestine, its absorption follows a non-linear process, and body levels are dependent on current intakes. Some authors have argued that encapsulation of vitamin C in liposomes may result in a more prolonged release thereby increasing its uptake (Duconge et al., 2008). Liposomal encapsulation may also bypass saturable uptake mechanisms via direct transport into the lymphatic system (Duconge et al., 2008).
75. Liposomal formulation of vitamin C, therefore, is not designed to increase its solubility in the GI tract, as with lipophilic molecules, but to bypassing its transport rate-limited absorption. This observation underscores the importance of investigating novel/alternative formulations of supplements on a case-by-case basis.
76. Despite its significant presence in the market, however, there are only a handful of controlled studies investigating the oral bioavailability of liposomal vitamin C in humans. A couple of these studies were performed with small sample sizes. These studies and their conclusions are summarised below. The majority of these studies (4/6) were conducted in the last two years, indicating an emergent research interest in formulating supplements with increased bioavailability.
77. In an early study from 2008, Hickey et al. investigated the oral pharmacokinetics of standard and liposomal vitamin C. The study contained only two participants, one male and one female. Both subjects received 5 g vitamin C in standard formulation, the female received 5 g and 36 g in liposomal formulation, and the male received 20 g and 36 g in liposomal formulation. These larger doses were administered to test hypotheses about maximum blood levels achievable from oral dosing. Liposomes were composed of phosphatidylcholine (Hickey et al., 2008).
78. In the female subject, the concentration-time curves of plasma vitamin C levels were similar for standard and liposomal formulations (5 g), albeit, with a slightly delayed Tmax (from 100 to approximately 200 minutes). In the male subject, 20 g liposomal vitamin C produced a concentration-time curve with a broader profile than that observed with a 5 g dose of standard vitamin C. In both subjects, administration of 36 g liposomal vitamin C led to plasma levels of approximately 400 µM, higher than that suggested by the NIH (National Institutes of Health) to be possible from oral dosing at the time of the study, and higher than that achieved via oral dosing or 5 g liposomal vitamin C in the present study. Although pharmacokinetic parameters (Cmax and area under the curve; AUC) were not reported for the 36 g liposomal dose, The concentration-time curve suggested that the liposomal vitamin C resulted in slower onsets to peak levels, and broader profiles, than the 5 g standard dose. The authors argued that these findings indicated a more sustained absorption of liposomal vitamin C owing to the physiological handling of liposomes (Hickey et al., 2008).
79. Davis et al. (2016) compared the oral pharmacokinetics of liposomal encapsulated and non-encapsulated vitamin C in 11 older (53±2 years) overweight adults (34.1±1 kg/m2 BMI). The vitamin C dose was 4 g. Liposomes were made with “mixed natural phospholipids” classified as Generally Recognised as Safe (GRAS) ingredients (GRAS is a designation applied to food ingredients by the United States Food and Drugs Administration. It is a designation that a chemical or substance added to food is considered safe by experts under the conditions of its intended use and is therefore exempt from review as an additive).
80. At two-, three-, and four-hours post-administration, plasma vitamin C levels were significantly higher with liposomal vs. non-liposomal vitamin C (p<0.001). The AUC0-4h (the area under the time concentration curve up to 4 hours post administration) was 1.4-fold greater with liposomal vs. non-liposomal vitamin C (10.3±0.9 vs 7.6±0.4 mg/dL h), indicating that oral bioavailability of vitamin C was increased by liposomal formulation. Plasma levels achieved by oral dosing with either standard or liposomal formulation were significantly lower than that achieved by intravenous administration (IV) at all time points (p<0.001). IV vitamin C achieved a Cmax of approximately 27 mg/dL, compared to approximately 3.5 mg/dL for liposomal and approximately 2 mg/dL for standard vitamin C (p values not reported) (Davis et al., 2016).
81. Łukawski et al. (2020) studied the oral pharmacokinetics of liposomal vitamin C compared to unencapsulated vitamin C in 20 healthy participants. Ten participants received a standard formulation of vitamin C, whilst 10 received a liposomal form. The liposomes used in this study were formulated from soybean phosphatidylcholine. Following administration of 10 g vitamin C, the maximum blood concentration reached (Cmax) was higher in those receiving the liposomal vs. non-liposomal formulation (303 µM vs. 180 µM) and the time taken to reach the maximum concentration (Tmax) was longer, by approximately one hour, from 96 to 180 minutes. The half-life was also longer: >6 hours compared to 4 hours. The authors concluded that the results “indicate that the presence of liposomes enhances bioavailability of vitamin C.” The authors further suggested that the increased bioavailability of liposomal vitamin C was related to protection from degradation inside the GI tract which provided a sustained reserve of the compound for absorption.
82. Gopi and Balakrishnan (2021) compared the oral bioavailability of liposomal and non-liposomal vitamin C in 24 healthy adults in a cross-over design trial. Participants received 1 g of vitamin C. Tmax was unaffected by formulation (approximately 3.5 hours), whereas Cmax was increased with the liposomal formulation (5.2 versus 1.2 mg/dL). The AUC0-24h analysis also demonstrated an increase with liposomal vitamin C (55.9 versus 31.5 mg∙h/dL), whilst half-life was increased from 12.4 to 19 hours with the liposomal formulation.
83. Joseph et al. (2021) designed and evaluated the oral pharmacokinetics of a multilamellar surface engineered liposomal vitamin C formulation (in the form of calcium ascorbate). Liposomal surfaces were engineered/modified by impregnation into a fenugreek galactomannan hydrogel in a powder form. All ingredients used were “food grade” and the process was designed to stabilise liposomes from harsh physiological conditions, thereby enabling sustained and increased absorption.
84. Fourteen healthy participants were administered 1 g of vitamin C either in liposomal or non-liposomal forms in a cross-over design. The liposomal formulation resulted in significantly higher plasma vitamin C levels over 12 hours (p<0.05). Liposomal vitamin C in tablet and capsule form resulted in a Cmax of 282 and 273 µM, respectively, versus 52 µM for unformulated control. The half-life was also increased from 3.6 hours with unformulated vitamin C to 8.5 and 7.6 hours for tablet and capsule forms of liposomal vitamin C, respectively. The AUC0-12h was increased by approximately 7-fold with the liposomal versus non-liposomal vitamin C preparation. The authors suggested that the larger increase in the AUC observed in their study versus that seen in other liposomal vitamin C studies was due to the enhanced stability of liposomes embedded in a fibre matrix.
85. Jacob et al. (2021) also evaluated the oral pharmacokinetics of a fibre-reinforced liposomal vitamin C preparation. The fibre was of turmeric origin. Eight participants were administered 150 mg of vitamin C in liposomal or standard formulations, in a cross over design. Liposomal vitamin C increased the Cmax from 1.2 mg/dL to 6.7 mg/dL and increased the AUC0-24h by 5.9-fold. Like Joseph et al. (2021), the authors suggested the enhanced bioavailability of fibre-reinforced liposomal vitamin C was due to the stability of the formulation under physiological conditions.
86. In summary, liposomal preparations of vitamin C appear to increase oral bioavailability as determined by pharmacokinetic studies. The effects of liposomal vitamin C on the AUC0-n and Cmax in the studies discussed above are summarised in Table 4.
Table 4. Summary of effects of liposomal vitamin C on AUC and Cmax versus non-liposomal preparations.
|
Study |
Cmax positive fold difference |
AUC0-n positive fold difference |
|
Davis et al. (2016) |
n.r. |
1.4 |
|
Łukawski et al. (2020) |
1.7 |
1.8 |
|
Gopi and Balakrishnan (2021) |
2.4 |
1.8 |
|
Joseph et al. (2021) |
5.4 |
7 |
|
Jacob et al. (2021) |
5.4 |
5.9 |
n.r.: not reported. Fold differences in Cmax and AUC0-n were calculated by the Secretariat from the original publications.
Case study 2: Curcuminoids
87. Due to their poor oral bioavailability, novel formulations designed to enhance the oral bioavailability of curcuminoids have been extensively studied. However, it should be noted that “while a large number of such formulations are developed in academia and as garage projects, only a few of them are available on the market in one form or another.” (Jamwal, 2018). Nonetheless, from analysis of the scientific literature, grey and white literature, curcumin appears to be a supplement for which novel formulations designed to increase oral bioavailability are in the more advanced stages of formulation research, design, commercialisation, and marketisation (compared to, for instance, CBD). The following paragraphs, therefore, relate primarily to studies investigating the pharmacokinetics of commercially available curcuminoid formulations.
Review by Jamwal, 2018
88. Jamwal (2018) published a review of studies investigating the pharmacokinetics of different curcuminoid formulations and calculated their relative oral bioavailability compared to unformulated curcuminoids. Table 5 provides an overview of Jamwal’s (2018) review and indicates the relative bioavailability of the various formulations. Relative oral bioavailability values were calculated by Jamwal (2018) using the following formula:
(Relative bioavailability = AUC formulation X Dose control)/ (AUC control X Dose formulation).
Table 5. Summary of studies investigating effects of curcuminoid formulation on oral bioavailability. Adapted from Jamwal (2018).
|
Characterisation |
Relative oral bioavailability (positive fold change, from Jamwal, 2018) |
Reference |
|
Phytosomal Emulsion-based (curcumin, soy lecithin, microcrystalline cellulose). |
48 |
Cuomo et al., (2011). |
|
Solid lipid curcumin particles. |
100 |
Gota et al., (2010). |
|
Fenugreek soluble fibre-based delivery system. |
15.8 |
Im et al., (2012). |
|
Dispersed micronized curcuminoids.
|
9.7 |
Madhavi and Kagan, (2014). |
|
Micronised curcumin. |
9 |
Schiborr et al., (2014). |
|
Liquid micelles. |
185 |
Schiborr et al., (2014). |
|
Water-dispersible curcumin complex – Polyvinylpyrrolidone and cellulose based. |
136.3 |
Jäger et al., (2014). |
|
Turmeric essential oil formulation. |
6.9 [see corrigendum to Jamwal, 2018]. |
Antony et al., (2008). |
|
γ-cyclodextrin-based formulation. |
85 |
Purpura et al., (2018). |
|
Colloidal nanoparticles. |
15.9 |
Sasaki et al., (2011). |
89. Overall, the novel formulations summarised by Jamwal (2018) increased the oral bioavailability of curcuminoids compared to administration of unformulated curcuminoids ranging between 6.9 and 185-fold. Of the formulations reviewed, liquid micelles provided the greatest increase in relative bioavailability (185-fold).
90. However, there are important limitations in comparing across these studies. In the first instance, most of the studies reported in Table 5 administered different doses of unformulated vs. formulated curcumin, and thus required dose-normalisation to extrapolate relative oral bioavailabilities. Some studies indicated that curcuminoid pharmacokinetics are non-linear (Kocher et al., 2015), suggesting that this method may misrepresent fold-changes in bioavailability between preparations (Flory et al., 2021).
91. There was significant variation in the preparative and analytical methods used for detection of plasma curcuminoids and their metabolites. Some of the studies measured levels of free curcuminoids, whereas others quantified conjugated curcumin. Conjugated curcumin is the primary metabolite present in plasma; however, it is less pharmacologically active than the free compound. There were also differences in which metabolites were analysed (curcumin, demethoxycurcumin - DMC, bisdemethoxycurcumin - BDMC, tetrahydrocurcumin - THC), and there is ongoing debate about the relative impact of these metabolites on toxicity. Differences were also apparent in the detection and quantification methods; whilst some studies used high-performance liquid chromatography (stand-alone), others used liquid chromatography-mass spectrometer-based determination.
92. Other important differences related to the clinical trial design including fasting status and food intake after administration of the curcuminoids, which may have important effects on curcumin absorption. There were also differences in the race/ethnicity composition and gender balance of the various cohorts. Some studies have reported sex-differences in the absorption of curcuminoids which is important to consider.
Other studies
93. Several studies not reported by Jamwal (2018) have also investigated the pharmacokinetics of curcuminoid formulations designed to increase oral bioavailability in human subjects. The following paragraphs summarise some of the key findings from these studies. The studies included here were those comparing the pharmacokinetics of oral curcuminoids in standard preparations versus novel formulations in healthy human subjects.
Lipid-based formulations
94. Kocher et al., (2015) studied the effects of micellarisation on curcumin pharmacokinetics in healthy volunteers The effects of the adjuvant phytochemicals sesamin, ferulic acid, naringenin, and xanthohumol were also investigated. The study included 23 healthy volunteers administered 98 mg total curcuminoids and was designed as a cross-over trial with one-week washout periods between subsequent treatments.
95. Curcumin, DMC, and BDMC levels were quantified from plasma. The oral bioavailability of total free curcumin was increased by formulation with phytochemicals, as micelles, and as micelles with phytochemicals by 8-fold, 88-fold, and 73-fold, respectively (comparing the AUC to the control group administered unformulated curcumin). Micellar formulation also increased the AUC of curcumin metabolites DMC and BDMC by 848 and 159-fold, respectively, relative to unformulated curcumin. Overall, micelles were effective at increasing curcumin absorption, and this effect was not further increased by adjuvant phytochemical micelles.
96. Asher et al. (2016) used a crossover study design to compare the pharmacokinetics of unformulated curcumin with that of a curcumin-phosphatidylcholine formulation in 12 healthy subjects. Although the physicochemical properties of the phosphatidylcholine complex used were not reported, the Secretariat has assumed this is likely to be a colloidal dispersion of curcumin-phosphatidylcholine. The authors examined plasma and colorectal tissue levels of curcuminoids after administration of 1000 mg unformulated curcuminoids or 385 mg of curcumin-phosphatidylcholine complex once daily for 7 days. Plasma samples were taken immediately prior to the last dose, and then 11 times over 24 hours following the last dose.
97. Tmax was shorter for phosphatidylcholine-curcumin complex versus unformulated curcumin (64 minutes versus 216 minutes for curcumin, respectively). Dose-adjusted AUC0-24h analysis demonstrated that curcumin, DMC, and BDMC (conjugated forms) plasma levels were increased 8.8, 2.9, and 3.0-fold, respectively, with phosphatidylcholine-curcumin versus unformulated curcumin. Curcumin (conjugated and free), DMC (conjugated only), and BDMC (conjugated only) were also detected in rectal mucosa tissue, but their levels were not different between the formulations.
98. Panda et al. (2019) investigated the oral pharmacokinetics of curcumin formulated as ‘Curene®’ ®’ versus two reference curcumin formulations – standardised 95% curcuminoids and CP-01, a curcumin formulation containing turmeric volatile oil. Curene® is a proprietary curcumin formulation that, according to the authors, forms an “emulsion similar to liposomes upon contact with the aqueous environment [of] intestinal fluids” (Panda et al., 2019), suggesting a S(M)EDDS-like mechanism.
99. Three grams of each curcumin formulation were administered to 12 healthy male subjects split into 3 groups (4 subjects per formulation) and 10 blood samples were collected from point of administration up to 24 hours post-administration. Cmax of free curcumin from the Curene®-curcumin formulation was significantly higher than for control curcumin (1546 vs. 86 and 190 pg/ml for standardised curcuminoids and CP-01, respectively; p<0.05), with no change in Tmax. Compared to standardised curcuminoids and CP-01, AUC0-24h was increased by 31 and 14-fold, respectively, (from 207 and 445 pg∙h/ml, respectively, to 6303 pg∙h/ml; p<0.05).
100. Briskey et al. (2019) compared the oral pharmacokinetics of a novel surfactant, polar-lipids, and solvent-based dispersion curcumin formulation to that of a standard curcumin preparation in 7 healthy human subjects. The so-called LipiSperse® technology is added to an aqueous suspension of curcumin crystals. The surfactant and lipid-based product then forms a coat around the curcumin crystals, coating them, preventing agglomeration, and increasing aqueous solubility.
101. Curcumin formulated with LipiSperse® led to increases in the Cmax and AUC0-6h for curcumin, DMC, and BDMC compared to standard curcumin. In a crossover trial with 5 healthy subjects, curcumin Cmax was increased 3-fold, from 215 to 691 ng/mL (p<0.05) and total AUC0-6h was increased 2.0-foldp<0.05). Tmax was unchanged between preparations (1 hour). In a parallel study design with 8 healthy subjects, curcumin total AUC0-6 was 2.3-fold higher in those receiving Lipisperse® curcumin and Cmax was increased by 4.4-fold (151 vs 658 ng/mL; AUC and Cmax p<0.05).
102. Fança-Berthon et al. (2021) compared the oral pharmacokinetics of unformulated curcumin, liquid micellar, phytosomal, and dried-colloidal curcumin formulations in 30 healthy subjects. Different doses of each formulation were used and in accordance with the supplier’s daily recommended doses (1500 mg unformulated curcumin, 1000 mg phytosomal curcumin, 1000 mg liquid micellar curcumin, 300 mg dried-colloidal curcumin). The authors argued that this approach provided meaningful data that could be applied to exposures expected through the real-world use of these products.
103. For non-dose adjusted analysis, the AUC0-24h of total curcuminoids from the liquid micellar formulation were significantly higher than the group receiving unformulated curcumin (control group; p<0.0001). When AUC0-24h was adjusted for dose, plasma curcuminoids were also significantly increased with liquid micellar, dried-colloidal, and phytosomal curcumin formulations (136, 73, and 13 ng∙h/ml/mg, respectively versus 3.7 ng∙h/ml/mg for the control group; p<0.0001 for each).
104. A 2022 study by Kanae et al. investigated the pharmacokinetics of orally administered curcumin in four different formulations: unformulated curcumin extract, curcumin mixed with squalene, curcumin mixed with docosahexaenoic acid and solid lipid curcumin particles (SLCP). Pharmacokinetics of all four preparations were compared separately in 10 Japanese individuals (5 male and 5 female) >20 years and <65 years of age. A 7-day washout period was observed between trials (Kanae et al., 2022).
105. Higher doses of unformulated curcuminoids (260 mg, control group) were administered than for formulated curcuminoids (SLCP: 88mg, squalene: 82 mg, docosahexaenoic acid: 79 mg) and pharmacokinetic parameters were normalised to curcuminoid doses for the various formulations. Conjugated curcuminoids were detected after glucuronidase/β-sulfatase pre-treatment of plasma samples. The Tmax of curcumin was not significantly changed between the formulations (p>0.05), but those of DMC and BDMC were significantly shorter with SLCP, docosahexaenoic acid, and squalene formulations compared to the control group (p<0.05).
106. Plasma levels of curcumin and total curcuminoids were higher with the novel formulations at all time points (1 – 8 hours), whilst plasma levels of DMC and BDMC were higher at earlier time points (1 -2 hours), compared to control. The dose-normalised AUC0-8h of curcumin was significantly increased in all the novel formulations compared to the control: 0.43, 0.45, and 0.55 ng/ml.h/mg for solid lipid particles, squalene, and docosahexaenoic acid, respectively, versus 0.19 ng/ml.h/mg for control (p<0.01, p<0.05, and p<0.01, respectively).
107. The dose normalised Cmax of curcumin was also significantly higher for all the novel preparations versus unformulated curcuminoids: 0.09, 0.09 and 0.12 ng/ml/mg for solid lipid particles, squalene, and docosahexaenoic acid, respectively, versus 0.05 for control (p<0.05, p<0.05, and p<0.01, respectively). This amounted to a relative increase of curcumin absorption of 2.2, 2.3 and 2.8-fold for solid lipid particles, squalene, and docosahexaenoic acid preparations, respectively. The AUC0-8h of DMC and BDMC were not different for the novel preparations versus control, whereas their Tmax was significantly shortened for all the preparations (p<0.05). The only sex difference observed was a significantly higher dose normalised Cmax for DMC in men administered the standard curcuminoid preparation (p=0.04).
Dispersion technologies
108. Sunagawa et al. (2015) investigated the oral bioavailability of Theracurmin® (182 mg), a colloidal submicron-particle formulation of curcumin, in healthy human subjects compared to liposomal (Meriva®; 152 mg) and micronised curcumin mixed with turmeric essential oils (BCM-95; 279 mg). Theracurmin® is a proprietary technology, and an earlier study investigating this formulation (Sasaki et al., 2011) was included in Jamwal’s (2018) review, who calculated an increase in relative oral bioavailability over unformulated curcumin of 15.9-fold. Theracurmin® is composed of “curcumin dispersed with colloidal submicron-particles” (Sunagawa et al., 2015). This colloidal dispersion is based on the water-soluble polysaccharide gum ghatti that has emulsifying characteristics and can increase the water solubility of lipophilic compounds. To produce Theracurmin®, curcumin powder was added to a gum ghatti water solution, ground by a wet grinding mill, and dispersed by a high-pressure homogeniser (Sunagawa et al., 2015).
109. The Sunagawa et al., (2015) study was designed as a 3-way crossover with nine subjects with a 7-day washout period between administration of the different formulations. Theracurmin® resulted in a higher curcumin Cmax (287.2 ng/mL) than BCM-95 and liposomal curcumin of10.7 and 5.6-fold, respectively(p<0.05). AUC0-6h for Theracurmin® was significantly higher than that of BCM-95 and liposomal curcumin by 16.1 and 5.6-fold (p<0.05), respectively, whilst the AUC0-24h was 11 and 4.6-fold higher, respectively (p<0.05).
110. Panda et al. (2021) studied the oral bioavailability of a “novel dispersible” curcuminoid extract compared to a standard curcumin extract. The extract under study was the proprietary CURCUGEN an oleoresin-based turmeric formulation that derives its dispersible properties from turmeric-native polar resins, turmeric essential oils, and turmeric polysaccharides. This formulation preserves the “food-state” ratio of curcuminoids (i.e., the natural ratio of DMC and BDMC), as opposed to standardised curcumin extracts.
111. The oral bioavailability of CURCUGEN was studied in a 2-way crossover trial in 17 healthy male subjects. Plasma levels of free and total curcumin, total DMC, BDMC, curcuminoids, and THC were quantified up to 24 hours post administration. CURCUGEN significantly increased levels of free and total curcumin, and all the curcumin metabolites studied (p<0.05). Based on AUC0-24h, plasma levels of all curcuminoids analysed were significantly increased (p<0.05): free curcumin (39-fold), total curcumin (50-fold), DMC (44-fold), BDMC (47-fold), total curcuminoids (53-fold), and THC (31-fold).
Comparative studies
112. Flory et al. (2021) argued that, owing to non-linear pharmacokinetics, comparing oral bioavailability of curcuminoid formulations administered at different doses by using the relative AUC method is flawed. A number of studies discussed in the previous sections utilised the relative AUC method, and this may therefore be a consideration when interpreting those studies.
113. Flory et al.’s (2021) comparative study compared the effects of different curcuminoid formulations on oral bioavailability using the same administered dose of total curcuminoids between formulations. They compared the pharmacokinetics of seven curcumin formulations designed to increase oral bioavailability with that of native curcumin: micellar, γ-cyclodextrin formulation, phytosomal, submicron-particle, with adjuvants (piperine), with turmeric oil, and liposomal. Preparations were administered at identical doses of curcumin (207 mg) in 12 individuals (6 male, 6 female) per group in a cross-over design.
114. Plasma levels of curcumin were measured over 24 hours. Only the administration of micellar curcumin and γ-cyclodextrin-formulated curcumin led to increases in the AUC0-24h (57-fold and 30-fold, respectively). Micellar curcumin also significantly increased the AUC0-24h relative to γ-cyclodextrin-formulated curcumin (p<0.05). Females had significantly higher AUC0-24h than males after uptake of micellar curcumin (p<0.05). Phytosomal and submicron-particle curcumin led to non-significant increases in the AUC0-24h of 7.5- and 6.5-fold, respectively.
115. In vitro digestive assays demonstrated that sub-micron particles, micellar, and γ-cyclodextrin-formulated curcumin had the highest digestive stabilities (109%, 102% and 73%, respectively). In those same assays, solubility and micellisation efficiency were highest for micellar and γ-cyclodextrin formulations; micellar and γ-cyclodextrin curcumin had solubilities of 80% and 33%, respectively, whilst micellisation efficiency was 55% and 23%, respectively (calculated as “mass curcumin in mixed micellar fraction/mass curcumin in raw material”). Bioaccessibility studies in Caco-2 cells (a human colorectal model) suggested that apparent permeability did not differ between the formulations.
116. Overall, Flory et al., (2021) argued that the increased oral bioavailability of micellar and γ-cyclodextrin-formulated curcumin preparations resulted from increased pre-digestive stability and post-digestive solubilisation in gastrointestinal conditions. Increased transport across the epithelium or inhibition of biotransformation and/or epithelial efflux pumps had no effects on oral curcumin bioavailability.
117. The study by Flory et al. (2021) suggests that comparing relative oral bioavailability of curcumin formulations administered at different doses may be misrepresentative. There are also limitations, therefore, in directly comparing between different studies that used different doses. The magnitude of this effect is likely to be exacerbated when there are large differences in doses, and when analysis of plasma curcuminoids is close to or at the limit of detection.
118. Despite these methodological limitations, the literature suggests that novel formulations of curcumin in lipid-based and dispersion systems have the potential to increase oral bioavailability of curcumin and its metabolites. Table 6 provides a summary of the curcumin formulations that led to increased bioavailability in the above studies. The table lists the increase in bioavailability as defined by increased fold changes in Cmax and AUC0-n for curcumin only, as calculated in the respective publications.
Table 6. Summary of curcumin formulations increasing curcumin AUC and Cmax in healthy human studies. Preparations that did not affect AUCs as part of the same study are not included in the table.
|
Formulation |
Cmax positive fold difference (curcumin) |
AUC0-n positive fold difference (curcumin) |
Study |
|
SLCP |
2 |
2 |
Kanae et al. (2022). |
|
Micelle Micelle |
216 84 |
88 37 |
Kocher et al. (2015). Franca-Berthon et al. (2021). |
|
Phytosomal Phytosomal |
1.2a 203 |
9 57 |
Asher et al. (2016). Flory et al. (2021). |
|
Aquesome® |
18 |
31 |
Panda et al. (2019). |
|
LipiSperse® |
3 |
2 |
Briskey et al. (2019). |
|
Dried colloidal |
23 |
20 |
Franca-Berthon et al. (2021). |
|
Squalene-curcumin preparation. |
2 |
2 |
Kanae et al. (2022). |
|
Docosahexaenoic acid-curcumin preparation. |
2 |
3 |
Kanae et al. (2022). |
|
Colloidal submicron |
11 |
11 |
Sunagawa et al. (2021). |
|
Dispersible form (CURCUGEN) |
25 |
50 |
Panda et al. (2021). |
|
γ-cyclodextrin curcumin |
56 |
30 |
Flory et al. (2021). |
AUC fold differences were calculated by the secretariat based on presented data. Where available, fold differences were calculated from total curcumin plasma levels, and from the AUC for the longest defined time period. SLCP: solid lipid curcumin particles; n.r.: not reported; a values are not dose-normalised and are from administration of 4000 mg standard and 400 mg phytosomal curcumin.
Case study 3: Cannabidiol
Background
119. Cannabidiol (CBD) is a highly hydrophobic molecule known to be of relatively low oral bioavailability, with reports suggesting an average of approximately 6% (Millar et al., 2020). Moreover, a large degree of inter-individual variation exists in the absorption of CBD (Millar et al., 2018) and absorption is modified by feeding state (Silmore et al., 2021; Mozaffari et al., 2021).
120. Preparation of CBD also affects its bioavailability. For instance, Williams et al. (2021) demonstrated that oral bioavailability differed between five different CBD formulations. A preparation comprising 5% CBD concentrated liquid (containing medium-chain triglyceride (MCT) oil, gum arabic, and citric acid in reverse osmosis water) evoked the shortest Tmax, highest Cmax, and largest AUC0-4h, whilst CBD powder suspended in reverse osmosis water had the lowest oral bioavailability.
121. The most bioavailable CBD preparation in Williams et al.’s (2021) study was formulated with medium-chain triglyceride (MCT) oil, citric acid, and gum arabic in reverse osmosis water. The authors suggested that the presence of gum Arabic and medium-chain triglyceride (MCT) oil may have aided CBD solubilisation in the GI tract and therefore enhanced absorption.
122. However, whilst formulation type can affect the bioavailability of CBD, questions remain as to how this translates into the supplement market and the precise products to which consumers might be exposed. CBD is widely consumed as a supplement in the UK and is available in a variety of formulations, for instance as oils, tinctures, capsules, in beverages, and in food. It is potentially misrepresentative, therefore, to speak of a ‘standard’ formulation of CBD.
123. However, a preliminary analysis conducted by the Secretariat of 51 CBD supplements available from the online market suggests a large portion of CBD supplements (29/51) are formulated with medium-chain triglyceride (MCT) oil as a carrier. The most common delivery method is oral oil drops: 25 out of the 51 products are formulated as oral oil drops, 19 of which use medium-chain triglyceride (MCT) oil as a carrier, with the remainder using either hemp seed oil or rice brain oil. Gummies are the second most common formulation (12/50) whilst oral sprays and capsules comprise the remainder.
124. One entry for micellar CBD was found. The proprietary formulation of this product was NovaSOL®, and the pharmacokinetics of curcumin formulated in this way have been studied in control settings (see above section). There were no other indications for CBD formulated in ways to increase oral bioavailability on a preliminary search of the online market, but a further general internet search identifies several possible products on the market.
Studies investigating the oral pharmacokinetics of CBD formulations
125. Alternative formulations have been designed to increase oral CBD bioavailability and alter its pharmacokinetic profile. Owing to potential application of CBD to treat symptoms of disease, a large amount of this research has been based on development for pharmaceutical indications. Unlike curcumin, however, there are currently only a few clear examples of novel formulation products available on or destined for the supplement/nutraceutical market (or to wholesalers/white label who supply this market).
126. However, some of the CBD formulations in development as academic and/or pharmaceutical projects have used food-grade ingredients to design preparations that could conceivably be adopted by supplement manufacturers. Additionally, owing to the regulatory status of CBD, some of these applications and/or products occupy a grey area between the pharmaceutical and supplement markets, and their penetrations into either space is possible.
127. Based on this reasoning, the following paragraphs summarise key studies investigating formulations of CBD with increased bioavailability in human subjects. The studies are selected to indicate possible formulations that might increase bioavailability and offer a ‘horizon scanning’ perspective on formulations that, owing to their formulation characteristics, might conceivably penetrate the CBD supplement market in the future.
128. Hobbs et al. (2020) investigated the relative oral bioavailability of two commercially available CBD formulations: ‘water-soluble’ and ‘lipid-soluble’ powders in 10 healthy subjects in a randomised parallel arm study. Volunteers were administered 30 mg CBD, which was suggested to be a ‘standard’ dose based on available products. The water-soluble powder had Cmax of 2.82 ng/mL and a Tmax of 90 min. The abstract to this study states that the water-soluble powder was approximately 4.5-fold more bioavailable than the lipid-soluble form.
129. De Prá et al. (2021) prepared a self-emulsifying drug delivery system (SEDDS) designed to increase the oral delivery of CBD. As described in the above section, SEDDS are lipid-based preparations of active ingredients formulated with lipids, surfactants, and/or co-surfactants that self-emulsify upon contact with the aqueous conditions of the GI tract to form mixed micelles and potentially increase absorption of lipophilic compounds (Pouton and Porter, 2008). The CBD SEDDS was prepared with polyoxyl 40 castor oil as the emulsifier and polyethylene glycol 400 as the co-emulsifier, both of which are food-grade ingredients.
130. The De Prá et al., (2021) study also investigated the effects of partially hydrolysed long-chain triglycerides (GML) as an excipient on the oral bioavailability of CBD. GML is composed of a mixture of mono-, di-, and triglycerides, which may improve the solubility of CBD via promoting mixed micelle formation. CBD formulated with medium-chain triglyceride (MCT) oil was used as the reference preparation. In vitro digestion studies demonstrated that the majority of CBD from the SEDDS remained partitioned in the aqueous phase post-digestion, suggesting a complete solubilisation under these conditions. Only a low percentage of CBD from the other two preparations, however, was recovered in the aqueous phase.
131. The preparations were investigated in a controlled trial comprising 11 (analysed) subjects. The trial was designed as a three-arm crossover study with 7-day washout periods between administration of subsequent formulations. These human pharmacokinetic studies demonstrated that SEDDS CBD formulation led to an increased Cmax and AUC0-12h versus the medium-chain triglyceride (MCT) formulations (2 and 1.5-fold, respectively). GML preparation also increased the Cmax and AUC0-12h by 1.9-and 1.3-fold, respectively. Both the SEDDS and GML formulations also decreased the Tmax of plasma CBD levels (1.7 and 1.6 hours, respectively, versus 4.3 hours). The authors concluded that the “bioavailability of [CBD] is significantly influenced by the physicochemical characteristics of [excipient] lipids, the length of the fatty acid chain, and its susceptibility to digestion.”
132. Knaub et al. (2019) also investigated the effect of a SEDDS on the oral pharmacokinetics of CBD. Their SEDDS was based on the VESIsorb® technology, a proprietary SEDDS system for which commercial ubiquinol formulations are already available on the market. The VESIsorb® SEDDS is comprised of “food emulsifiers, edible vegetable oils and fatty acids.”
133. Bioavailability was studied in sixteen healthy volunteers who were administered 25 mg CBD either formulated with medium-chain triglyceride (MCT) oil or with the SEDDS in a cross-over study design. SEDDS-CBD significantly increased oral bioavailability as indicated by increases in the Cmax and AUC0-24h of 4.4- and 1.7-fold, respectively (p<0.0001 and p=0.0021). Tmax was also reduced from 3h to 1h with the SEDDS versus medium-chain triglyceride (MCT) oil CBD.
134. In interpreting their findings, Knaub et al., (2019) suggested that the increased oral bioavailability of CBD formulated with a SEDDS is due to the formation of droplets that solubilise CBD in the GI tract that deliver the molecule to enterocytes for absorption. Moreover, lymphatic transport, which bypasses the first-pass effect known to limit oral bioavailability of CBD, may also play a role.
135. Izgelov et al. (2020) compared the oral bioavailability of 90 mg CBD powder (no dissolution vehicle), CBD dissolved in sesame oil, and CBD formulated in a self-nano-emulsifying drug delivery system (SNEDDS) in a three-way crossover trial in 12 healthy subjects. The SNEDDS was composed of ethanol, soy lecithin, and surfactants (Tween 20, Span 80, and Kolliphor RH40).
136. CBD formulated in lipid-based systems was more bioavailable than CBD powder: Cmax was increased 22.5-fold and 17.5-fold with the SNEDDS and sesame oil CBD preparations, respectively, whilst AUC0-24h was increased approximately 8-fold for each formulation compared to the CBD powder. The SNEDDS also reduced Tmax and its associated variability (2 hours, versus 4 hours and 8.4 hours for sesame oil CBD and powder CBD, respectively). Sub-analysis of the sesame oil CBD time-concentration curves suggested the existence of two absorption behaviours in different groups of subjects; an ‘early’ and ‘delayed’ absorption population. Izgelov et al., (2020) suggested that the SNEDDS CBD formulation provided a less variable absorption profile owing to the consistent physicochemical parameters of the resultant emulsion compared to the sesame oil CBD preparation.
137. Patrician et al. (2019) investigated the oral bioavailability of a novel CBD formulation called ‘TurboCBD’ in a double-blinded, placebo controlled cross-over design with 12 participants. 45 mg or 90 mg CBD was administered. circulating CBD levels were higher with the TurboCBD 90 mg group at both 90 and 120 minutes compared with the 90 mg control (p<0.05). Total area under the curve tended to be higher with TurboCBDTM 90 mg compared with 90 mg standard dose but did not reach statistical significance (10,865 ng/mL vs. 7,114 ng/mL; p=0.088). The authors concluded that TurboCBD had a higher bioavailability than a standard CBD preparation.
138. A pilot study from Blair (2020) reported on the pharmacokinetics of liposomal CBD in a cross-over trial with 15 healthy subjects compared to a control non-liposomal formulation. Ten mg of CBD were administered, and CBD blood levels were measured at 1-hour post-ingestion. Mean plasma CBD levels were higher with administration of liposomal versus non-liposomal CBD (1.77 ng/ml versus 0.24 ng/ml). Moreover, whilst CBD in plasma was detected in 6/15 participants administered non-liposomal CBD, it was detected in all of those (15/15) receiving liposomal CBD.
139. In summary, several bioavailable formulations of CBD appear to be emerging in academic research, and a number of these are tied to commercial interest for supplement formulation. A summary of the effects of the CBD formulations on the Cmax and AUC in the studies discussed above is presented in Table 7.
Table 7. Effects of CBD formulations on AUC and Cmax in healthy human subjects.
|
Bioavailable formulation |
Reference formulation |
Cmax positive fold difference |
AUC0-n positive fold difference |
Study |
|
Water-soluble CBD. |
Lipid-soluble CBD. |
n.r.a |
4.5 |
Hobbs et al. (2020). |
|
GML CBD |
MCT CBD |
1.8 |
1.3 |
De Prá et al. (2021). |
|
SEDDS CBD |
MCT CBD |
2.0 |
1.5 |
De Prá et al. (2021). |
|
SEDDS CBD (VESIsorb®) |
MCT CBD |
4.4 |
1.7 |
Knaub et al. (2019). |
|
Sesame oil CBD |
Powder CBD |
17.5 |
8.3 |
Izgelov et al. (2020). |
|
SNEDDS CBD |
Powder CBD |
22.5 |
7.6 |
Izgelov et al. (2020). |
|
Liposomal CBD |
‘non-liposomal’ CBD |
7.4b |
7.4b |
Blair (2020). |
AUC and Cmax fold differences were calculated by the secretariat based on presented data. AUC fold differences were calculated from the longest defined time period. a Abstract only retrieved. b from baseline-1 hour only (i.e., ‘Cmax’ by definition, but only one time point tested).
Toxicology studies with novel supplement formulations
140. The increased bioavailability of supplements formulated in novel ways as discussed above may have important toxicological implications. As EFSA (2018) state in their ‘guidance for risk assessment on nanotechnologies’: “If nanoencapsulates function as intended…there will be increased bioavailability (systemic exposure) of the encapsulated material. This represents a potential concern since health-based guidance values are currently set based on the external rather than the internal dose and may no longer provide an appropriate level of protection to the consumer.” HBGVs, therefore, may need reassessing in light of specific formulations and relative bioavailabilities. This also suggests that exposure assessments based on standard formulations may potentially underestimate exposure from novel formulations.
141. In addition to increases in bioavailability, novel supplement formulations may alter toxicological profiles through other toxicokinetic parameters such as alterations in tissue distribution, or via changes in physiology not reported in the studies reviewed above. Furthermore, there may be formulation specific toxicological effects that cannot be extrapolated from toxicology studies using standard formulations of a given compound / supplement. For instance, it is reported that administration of vitamin C may enhance iron absorption which may be of concern for individuals with hemochromatosis or heterozygous for this disorder (EVM, 2008). However, the mechanism of vitamin C enhanced iron absorption occurs through the chelation of ferric iron which increases the solubility of the latter (Lynch and Cook, 1980), an effect which may be less relevant for encapsulated vitamin C formulations which are less able to physically interact with iron (example formulated by the Secretariat). These considerations suggest the need for case-by-case evaluation of specific formulations with respect to their potential toxicological implications beyond effects on bioavailability.
142. An initial review of toxicological effects which may be related to increased bioavailability, or specific to novel formulations of the compounds reviewed above was conducted using a literature search. The full results of this literature search, including the search strings used, number of retrieved results, and brief summaries of in vitro and in vivo studies are presented in appendix 1. This was not designed as a comprehensive review of toxicological effects related to novel formulations but intended as a preliminary scoping exercise to guide future assessments and/or to identify data gaps.
143. No studies investigating toxicological effects of novel formulations of vitamin C or CBD were identified. This reflects the lack of knowledge regarding the safety of novel formulations in general. Thus, although toxicological data exists on these active compounds, there are significant data gaps as to how these toxicological profiles may be altered when they are formulated in the ways discussed above.
144. Several studies investigating the toxicology of novel curcumin formulations in experimental systems and/or human subjects were retrieved. The majority of these studies investigated the toxicity of novel curcumin formulation in in vitro and/or in vivo systems, and three studies investigated effects in human subjects. Owing to the scope and aims of the current discussion paper, the in vitro and in vivo preclinical studies are summarised briefly in appendix 1 and include cytotoxicity assessments in a number of primary cells and cell lines and toxicological studies in rats and mice. A number of these studies might be of interest for inclusion in future discussion papers. The studies in human subjects are summarised below.
Curcumin
Human studies
145. Storka et al. (2015) investigated the safety and tolerability of a liposomal curcumin in healthy human subjects in a randomised dose escalation study. Subjects were administered liposomal curcumin intravenously at either 10, 20, 40, 80, 120, 180, 240, 320 or 400 mg/m2. Because of adverse reactions to mean red blood cellular volume and the formation of echinocytes only two subjects received 400 mg/m2. Red blood cell echinocyte formation was dose-dependent, detectable at a threshold dose of 120 mg/m2, without clinical symptoms, transient, and fully recoverable at 6 hours post infusion. Increases in mean red blood cellular volume were observed in two subjects administered 400 mg/m2 and did not associate with markers of haemolysis but did associate with increased venous serum lactate concentrations (maximum of 3.7 mmol/L versus normal range of ≤ 2.2 mmol/L). Twenty five subjects also experienced at least 1 adverse event; there were 49 adverse events in total, 11 of which were moderate and 38 of which were mild. Based on the dose-dependent, transient, and reversible effects on echinocyte formation and increases in mean red blood cell volume, the authors concluded that “a single intravenous dose of liposomal curcumin is considered safe up to a dose of 120 mg/m2 when infused over a period of 2 hours.”
146. In another dose escalation study, Greil et al. (2018) assessed the safety, tolerability, and efficacy of liposomal curcumin in 32 patients with locally advanced or metastatic cancer. Liposomal curcumin was administered intravenously weekly for 8 weeks, and the dose was increased from 100 mg/m2 over 8 hours to 300 mg/m2 over 6 hours. Twenty-six patients successfully completed dose-escalation without dose-limiting toxicity. However, the number of adverse events related to the treatment increased with doses at 300 mg/m2. One patient receiving 300 mg/m2 developed haemolysis, three patients treated with this dose displayed haemoglobin decreases without signs of haemolysis, whilst one patient exhibited definite haemolysis. Out of a total of 143 adverse events, 34 were considered related to the treatment and the remainder to underlying disease. Two of these events, facial oedema, and anaemia, were considered serious. Echinocytes were also observed in one patient. Although adverse events were observed, this was a study population with advanced cancer disease, many of whom had exhausted other lines of treatment and all of whom were taking other medication at the time of the study, and therefore these events may not be generalisable to other populations.
147. It should be noted that the two above studies (Storka et al., 2015 and Greil et al., 2018) administered liposomal curcumin via intravenous infusion. This may have resulted in plasma levels that would not be achieved by oral dosing alone.
148. Kocher et al. (2016) investigated the safety of micellar curcuminoids in moderately hyperlipidaemic individuals. Subjects consumed 294 mg of micellar curcuminoids or placebo per day for 5 weeks. Neither blood lipids, nor markers of inflammation, glucose and iron homeostasis, or liver enzymes differed between curcuminoid and placebo interventions. The authors concluded the intervention was safe.
Summary and discussion
149. The low oral bioavailability of certain supplement compounds has led to efforts to design novel formulations to increase their absorption and reduce their first-pass metabolism. Formulations composed of food-grade surfactants and lipid excipients engineered as micellar, liposomal, emulsion-based, and lipid nanoparticle systems are emerging in the supplement market. Such systems may increase oral bioavailability by solubilising lipophilic molecules and increasing their bioaccessibility, promoting lymphatic transport, and contributing to direct uptake via paracellular mechanisms.
150. The three case studies discussed above suggest that alternative novel formulations of vitamin C, curcuminoids, and CBD greatly alter their pharmacokinetics. An increase in oral bioavailability for novel formulations relative to standard/unformulated supplement was generally recorded, as determined by standard pharmacokinetic parameters (AUC0-n, Cmax). However, it is difficult to compare between these studies as they used different doses and reference formulations. Several of the curcumin studies administered different doses of reference and experimental formulation and used dose-normalisation to compare the relative bioavailability. The pharmacokinetics of curcumin are potentially non-linear, suggesting that this approach may misrepresent fold changes in bioavailability (Flory et al., 2021).
151. EFSA (2018) also note in their ‘guidance for risk assessment on nanotechnologies’ that “the amounts of the shell [i.e., lipid coatings etc.; see Table 1] components derived from food materials for use in delivery systems are generally far lower than their normal intake from dietary sources or other approved uses. As such there would be little concern over the shell components, unless these were neither normal constituents of the body or approved food additives.” However, given the interaction between these carrier systems and biological molecules and the formation of “new biological identities” (Giulimondi et al., 2019) in these systems, the inherent toxicity of these delivery systems is an area of uncertainty, for instance, with respect to immunological interactions (Inglut et al., 2020).
152. Finally, it is noted that a large proportion of the studies discussed above were potentially related to commercial interests: some studies were undertaken solely by the companies developing novel formulations, whilst others were conducted as collaborations between interested companies and academic institutions. Moreover, journals have case-by-case guidelines for declaring interests. Thus, whilst some of the presented studies did not report commercial/competing interests per se, declaration of funding presented in those same studies links that research to commercial interests. Based on these considerations, a likely reporting bias may be present in this literature, skewing the presented findings towards positive effects.
Questions for the Committee
153. Members are asked to please consider the following questions:
1) What further and/or specific information would Members like to see regarding novel formulations of supplement compounds?
2) Would Members like to see a more detailed study (i.e., exposure assessment and risk characterisation) of one or more of the included cases? Which pharmacokinetic parameters and calculations would be most useful?
3) Would Members find further case studies/examples useful? If so, are there any specific formulations and/or supplement compounds of interest to the COT?
4) Do Members believe purchasing a report reviewing the (projected) market trends for novel supplement formulations would aid in future evaluation of the risks associated with these formulations? If so, what kind of report do Members consider would be most useful? The reports identified in the current paper are each in the price range of £2,000 - £4,000. Beyond the reports indicated in this paper, are there any other data that would be deemed particularly valuable?
5) Regarding the toxicological evidence presented related specifically to novel formulations of supplements, what kinds of information would Members consider necessary for allowing meaningful evaluation of their safety?
6) Do Members consider that, where they exist, current HBGVs are protective for supplements formulated in the novel ways discussed in this paper? If not, what information would the Committee like to see to derive HGBVs in the future?
7) Would Members like to see more detailed information regarding conflicts of interest in the presented studies or a subset thereof?
8) Do Members have any other comments and/or questions about the paper in general?
Secretariat
April 2023
Abbreviations
|
ASA |
Advertising Standards Authority |
|
AUC0-n |
Integrated area under the time-concentration curve following administration of a compound from 0 to n hours. |
|
BCM-95 |
Curcumin formulation composed of 95% standardised curcuminoids and turmeric essential oils. |
|
BDMC |
Bisdemethoxycurcumin |
|
BMI |
Body mass index. |
|
CBD |
Cannabidiol |
|
Cmax |
Highest plasma concentration achieved following administration of a compound. |
|
COT |
The Committee on Toxicity of Chemicals in Food, Consumer Products, and the Environment |
|
DAGs |
Diacylglycerols |
|
DMC |
Demethoxycurcumin |
|
FFA |
Free fatty acids. |
|
GIT |
Gastrointestinal tract. |
|
GRAS |
Generally recognised as safe. |
|
HBGV |
Health-based guidance value. |
|
LCFA |
Long chain fatty acids. |
|
MAGs |
Monoacylglycerols |
|
MCT |
Medium chain triglycerides. |
|
MTHF |
Methyltetrahydrofolate |
|
NAD+ |
Nicotinamide adenine dinucleotide. |
|
NIH |
National Institutes of Health |
|
NLC |
Nanostructured lipid carriers. |
|
NMN |
Nicotinamide mononucleotide. |
|
PDI |
Polydispersity index. |
|
SE(M/N)DDS |
Self-emulsifying (micro/nano) drug delivery system. |
|
SLCP |
Solid lipid curcumin particles. |
|
SLN |
Solid lipid nanoparticles. |
|
THC |
Tetrahydrocurcumin (c.f. the cannabinoid tetrahydrocannabinol ‘THC’, is not discussed in this paper) |
|
Tmax |
Timepoint following administration of a compound at which highest plasma concentration is achieved. |
Glossary
Amphipathic – Refers to the property of a molecule containing both polar and nonpolar portions in its structure. The property of amphipathicity allows molecules, such as surfactants, to self-organise to create surfaces and structures that interface (or form a ‘surface’) between aqueous and polar solvents.
Bioaccessibility – the fraction of the total amount of a substance that is potentially available for absorption.
Biotransformation – The transformation of compounds by the body, often catalysed by enzymes. Biotransformation processes tend to produce compounds of higher water solubility, thereby increasing excretion. Biotransformation processes may produce compounds of lower or higher biological activity (detoxification vs. bioactivation, respectively).
Cargo molecules – These are the bioactive molecules formulated in novel ways and that are often encapsulated within nanostructures (e.g., micellar, liposomal, etc). The cargo are the molecules with the desired and/or advertised effect, the active ingredient.
Colloid – A substance containing one phase dispersed as droplets or other structures (e.g., spheres, planes, or cylinders) within another. Colloids are stable and do not settle, and examples include milk, gels, and sols. Emulsions are a specific type of colloid of two immiscible fluids with one fluid dispersed in the other, for instance, oil in water.
Comminution - The reduction of solid materials from one average particle size to a smaller average particle size by physical forces including crushing and grinding. In combination with deagglomeration, which separates clumped/agglomerated particles, comminution contributes to particles size reduction in micronisation techniques.
Intestinal milieu – the physiological conditions prevailing in the GIT including chemical composition, temperature, pH, ionic strength etc. Important modifiers to the milieu include fed state and presence/absence of exogenous lipids which stimulates the release of bile salts and lipases.
Liposomes – Vesicular structures composed of phospholipid bilayers with a similar structure to the cell membrane. Liposomes may contain one (unilamellar) or more (multilamellar) bilayers and may contain intravesicular vesicles.
LogP – The partition coefficient of distribution coefficient of a solute between water and octanol. LogP is calculated as the logarithm of the ratio of the concentrations of a given compound in the two immiscible phases. Molecules with a LogP of zero are equally partitioned between the aqueous and polar phase, those with a positive LogP are preferentially dissolved in the polar phase, and those with a negative LogP are preferentially dissolved in the aqueous phase. Because the value is logarithmic, a LogP of 1 means there is a 10:1 partitioning in the polar compared the aqueous phase.
Micelles – structures formed when an amphipathic substance (e.g., phospholipids) are added to an aqueous solution above a critical concentration known as the ‘critical micelle concentration’. Micelles spontaneously form as spherical structures in which the hydrophilic heads face inward to form the core, and the hydrophobic tails face outward to form the corona of the micelle. In lipid solution reverse micelles can form, in which the tail and head directions are reversed. When lipophilic molecules are added to the solution (e.g., supplement compounds), they associate with the hydrophobic portion of the micelle, to form what are sometimes referred to as ‘swollen micelles.’
Micro/nanoemulsion – a thermodynamically stable (microemulsion) or unstable (nanoemulsion) colloidal mixture of one phase (e.g., oil) dispersed in another (e.g., water), stabilised by an interfacial film of surface-active molecules.
Nanostructured lipid carriers – These are nanosized lipid droplets (20-200 nm) with a lipid core composed of two lipids, one liquid and one solid/crystalline. They may have complex internal structures, including the presence of nano-compartments of liquid lipid. NLCs exhibit reduced phase-transition in the lipid/oil compartment than SLNs and have larger intermolecular spaces. They are designed to increase loading capacity and stability of cargo molecules.
‘Novel’ formulations – formulations of supplements that have been designed specifically to increase the oral bioavailability of the active compound. Such formulations include emulsions, micelles, lipid nanoparticles, and other solubility enhancing systems.
Self-emulsifying (micro/nano) drug delivery system (S(M/N)EDDS) - Mixtures of oils, surfactants, solvents, and drug substance that spontaneously form oil-in-water (micro/nano) emulsions when introduced into aqueous phases under gentle agitation.
Solid lipid nanoparticles – These are nanosized (20-200 nm) lipid droplets dispersed in aqueous media and stabilised by an interfacial film of surface-active molecules. The core of these droplets is composed of partially or fully crystalline lipid, rather than liquid lipids as in emulsion systems.
Unilamellar- Refers to the membrane properties of liposomes. Unilamellar vesicles are bounded by a single bilayer of amphiphilic molecules (e.g., phospholipids, block copolymers). Multilamellar vesicles have several bilayers arranged on concentric rings within the largest vesicle.
References
Abundance and Health., 2021. Altrient C Welcomes Latest ASA Ruling on Clarity of Liposomal Vitamin C Marketing. Available at. Accessed 16th February 2023.
Advertising Standards Authority., 2021. ASA Ruling on YourZooki Ltd. Available at YourZooki Ltd - ASA | CAP Accessed 31st January 2023.
Ahmed, K., Li, Y., McClements, D.J., Xiao, H., 2012. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chemistry 132, 799–807. https://doi.org/10.1016/j.foodchem.2011.11.039
Ajeeshkumar, K.K., Aneesh, P.A., Raju, N., Suseela, M., Ravishankar, C.N., Benjakul, S., 2021. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Comprehensive Reviews in Food Science and Food Safety 20, 1280–1306. https://doi.org/10.1111/1541-4337.12725 [Abstract only]
Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S.W., Zarghami, N., Hanifehpour, Y., Samiei, M., Kouhi, M., Nejati-Koshki, K., 2013. Liposome: classification, preparation, and applications. Nanoscale Research Letters 8, 102. https://doi.org/10.1186/1556-276X-8-102
Allen, T.M., McAllister, L., Mausolf, S., Gyorffy, E., 1981. Liposome-cell interactions A study of the interactions of liposomes containing entrapped anti-cancer drugs with the EMT6, S49 and AE1 (transport-deficient) cell lines. Biochimica et Biophysica Acta (BBA) - Biomembranes 643, 346–362. https://doi.org/10.1016/0005-2736(81)90080-8
Ali, A., Ahmad, U., Akhtar, J., Badruddeen, Khan, M.M., 2019. Engineered nano scale formulation strategies to augment efficiency of nutraceuticals. Journal of Functional Foods 62, 103554. https://doi.org/10.1016/j.jff.2019.103554.
Altrient. (n.d.) Real Results. Available at Real Results | Altrient Accessed 16th February 2023.
Andar, A.U., Hood, R.R., Vreeland, W.N., Devoe, D.L., Swaan, P.W., 2014. Microfluidic preparation of liposomes to determine particle size influence on cellular uptake mechanisms. Pharm Res 31, 401–413. https://doi.org/10.1007/s11095-013-1171-8
Antony, B., Merina2, B., Iyer2, V.S., Judy2, N., Lennertz2, K., Joyal2, S., 2008. A Pilot Cross-Over Study to Evaluate Human Oral Bioavailability of BCM-95? CG (BiocurcumaxTM), A Novel Bioenhanced Preparation of Curcumin. Indian Journal of Pharmaceutical Sciences 70, 445. https://doi.org/10.4103/0250-474X.44591
Asher, G.N., Xie, Y., Moaddel, R., Sanghvi, M., Dossou, K.S.S., Kashuba, A.D.M., Sandler, R.S., Hawke, R.L., 2017. Randomized Pharmacokinetic Crossover Study Comparing 2 Curcumin Preparations in Plasma and Rectal Tissue of Healthy Human Volunteers. The Journal of Clinical Pharmacology 57, 185–193. https://doi.org/10.1002/jcph.806
Aswathanarayan, J.B., Vittal, R.R., 2019. Nanoemulsions and Their Potential Applications in Food Industry. Frontiers in Sustainable Food Systems 3.
Benzaria, A., Chevalier-Lucia, D., Picart-Palmade, L., Hue, P., López-Pedemonte, T., Dumay, E., 2013. Intracellular fate of retinyl acetate-loaded submicron delivery systems by in vitro intestinal epithelial cells: a comparison between whey protein-stabilised submicron droplets and micelles stabilised with polysorbate 80. Food Research International 51, 679–692.
Bhalani, D.V., Nutan, B., Kumar, A., Singh Chandel, A.K., 2022. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 10, 2055. https://doi.org/10.3390/biomedicines10092055
Blair, E., Labs, V., Collins, F., 2021. Liposomal Cannabidiol Delivery: A Pilot Study. American Journal of Endocannabinoid Medicine 2, 1, 19-21.
Blumenthal, R., Weinstein, J.N., Sharrow, S.O., Henkart, P., 1977. Liposome--lymphocyte interaction: saturable sites for transfer and intracellular release of liposome contents. Proceedings of the National Academy of Sciences 74, 5603–5607. https://doi.org/10.1073/pnas.74.12.5603
Bogman, K., Zysset, Y., Degen, L., Hopfgartner, G., Gutmann, H., Alsenz, J., Drewe, J., 2005. P-glycoprotein and surfactants: effect on intestinal talinolol absorption. Clin Pharmacol Ther 77, 24–32. https://doi.org/10.1016/j.clpt.2004.09.001
Briskey, D., Sax, A., Mallard, A.R., Rao, A., 2019. Increased bioavailability of curcumin using a novel dispersion technology system (LipiSperse®). Eur J Nutr 58, 2087–2097. https://doi.org/10.1007/s00394-018-1766-2
Carrière, F., Moreau, H., Raphel, V., Laugier, R., Benicourt, C., Junien, J.L., Verger, R., 1991. Purification and biochemical characterization of dog gastric lipase. Eur J Biochem 202, 75–83. https://doi.org/10.1111/j.1432-1033.1991.tb16346.x
Cerqueira, M.A., Pinheiro, A.C., Pastrana, L.M., Vicente, A.A., 2019. Amphiphilic Modified Galactomannan as a Novel Potential Carrier for Hydrophobic Compounds. Front. Sustain. Food Syst. 3, 17. https://doi.org/10.3389/fsufs.2019.00017
Choi, S.J., McClements, D.J., 2020. Nanoemulsions as delivery systems for lipophilic nutraceuticals: strategies for improving their formulation, stability, functionality and bioavailability. Food Sci Biotechnol 29, 149–168. https://doi.org/10.1007/s10068-019-00731-4
Committee on Toxicity of Chemicals in Food, Consumer Products, and the Environment. 2022a. Second draft statement on the potential risk to human health of turmeric and curcumin supplements. Available at Second draft statement on the potential risk to human health of turmeric and curcumin supplements | Committee on Toxicity (food.gov.uk)
Committee on Toxicity of Chemicals in Food, Consumer Products, and the Environment. 2022b. Discussion paper on the potential risk to human health of turmeric and curcumin supplements. Available at Discussion paper on the potential risk to human health of turmeric and curcumin supplements | Committee on Toxicity (food.gov.uk)
Committee on Toxicity of Chemicals in Food, Consumer Products, and the Environment. 2023. 2022 Annual Report of the COT. Annex 5: Glossary. Available at Annex 5 Glossary (food.gov.uk) Accessed 4th May 2023.
Constantinides, P.P., Wasan, K.M., 2007. Lipid formulation strategies for enhancing intestinal transport and absorption of P-glycoprotein (P-gp) substrate drugs: in vitro/in vivo case studies. J Pharm Sci 96, 235–248. https://doi.org/10.1002/jps.20780 [Abstract only]
Cuomo, J., Appendino, G., Dern, A.S., Schneider, E., McKinnon, T.P., Brown, M.J., Togni, S., Dixon, B.M., 2011. Comparative Absorption of a Standardized Curcuminoid Mixture and Its Lecithin Formulation. J. Nat. Prod. 74, 664–669. https://doi.org/10.1021/np1007262
Davis, J.L., Paris, H.L., Beals, J.W., Binns, S.E., Giordano, G.R., Scalzo, R.L., Schweder, M.M., Blair, E., Bell, C., 2016. Liposomal-encapsulated Ascorbic Acid: Influence on Vitamin C Bioavailability and Capacity to Protect Against Ischemia–Reperfusion Injury. Nutr Metab Insights 9, 25–30. https://doi.org/10.4137/NMI.S39764
De Prá, M.A.A., Vardanega, R., Loss, C.G., 2021. Lipid-based formulations to increase cannabidiol bioavailability: In vitro digestion tests, pre-clinical assessment and clinical trial. Int J Pharm 609, 121159. https://doi.org/10.1016/j.ijpharm.2021.121159
Duconge, J., Miranda-Massari, J.R., Gonzalez, M.J., Jackson, J.A., Warnock, W., Riordan, N.H., 2008. Pharmacokinetics of vitamin C: insights into the oral and intravenous administration of ascorbate. P R Health Sci J 27, 7–19.
EFSA., 2018. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA Journal 16, e05327. https://doi.org/10.2903/j.efsa.2018.5327
Expert Group on Vitamins and Minerals, 2003. Safe Upper Levels for Vitamins and Minerals. Available at vitmin2003.pdf (food.gov.uk) Accessed 13th April 2023.
Fança-Berthon, P., Tenon, M., Bouter-Banon, S.L., Manfré, A., Maudet, C., Dion, A., Chevallier, H., Laval, J., van Breemen, R.B., 2021. Pharmacokinetics of a Single Dose of Turmeric Curcuminoids Depends on Formulation: Results of a Human Crossover Study. The Journal of Nutrition 151, 1802–1816. https://doi.org/10.1093/jn/nxab087
Fanun, M., 2012. Microemulsions as delivery systems. Current Opinion in Colloid & Interface Science 17, 306–313. https://doi.org/10.1016/j.cocis.2012.06.001
Flory, S., Sus, N., Haas, K., Jehle, S., Kienhöfer, E., Waehler, R., Adler, G., Venturelli, S., Frank, J., 2021. Increasing Post-Digestive Solubility of Curcumin Is the Most Successful Strategy to Improve its Oral Bioavailability: A Randomized Cross-Over Trial in Healthy Adults and In Vitro Bioaccessibility Experiments. Molecular Nutrition & Food Research 65, 2100613. https://doi.org/10.1002/mnfr.202100613
Ganesan, P., Ramalingam, P., Karthivashan, G., Ko, Y.T., Choi, D.-K., 2018. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. IJN 13, 1569–1583. https://doi.org/10.2147/IJN.S155593
Garti, N., Aserin, A., 2012. 9 - Micelles and microemulsions as food ingredient and nutraceutical delivery systems, in: Garti, Nissim, McClements, D.J. (Eds.), Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals, Woodhead Publishing Series in Food Science, Technology and Nutrition. Woodhead Publishing, pp. 211–251. https://doi.org/10.1533/9780857095909.3.211 [Abstract only]
Goh, J., Menke, W., Herrick, L.P., Campbell, M.S., Abel, M.G., Fleenor, B.S., Bergstrom, H.C., 2020. Examination of Curcumin and Fenugreek Soluble Fiber Supplementation on Submaximal and Maximal Aerobic Performance Indices. J Funct Morphol Kinesiol 5, 34. https://doi.org/10.3390/jfmk5020034
Gopi, S., Balakrishnan, P., 2021. Evaluation and clinical comparison studies on liposomal and non-liposomal ascorbic acid (vitamin C) and their enhanced bioavailability. J Liposome Res 31, 356–364. https://doi.org/10.1080/08982104.2020.1820521
Gota, V.S., Maru, G.B., Soni, T.G., Gandhi, T.R., Kochar, N., Agarwal, M.G., 2010. Safety and Pharmacokinetics of a Solid Lipid Curcumin Particle Formulation in Osteosarcoma Patients and Healthy Volunteers. J. Agric. Food Chem. 58, 2095–2099. https://doi.org/10.1021/jf9024807 [Abstract only]
Greil, R., Greil-Ressler, S., Weiss, L., Schönlieb, C., Magnes, T., Radl, B., Bolger, G.T., Vcelar, B., Sordillo, P.P., 2018. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemother Pharmacol 82, 695–706. https://doi.org/10.1007/s00280-018-3654-0
Giulimondi, F., Digiacomo, L., Pozzi, D., Palchetti, S., Vulpis, E., Capriotti, A.L., Chiozzi, R.Z., Laganà, A., Amenitsch, H., Masuelli, L., Peruzzi, G., Mahmoudi, M., Screpanti, I., Zingoni, A., Caracciolo, G., 2019. Interplay of protein corona and immune cells controls blood residency of liposomes. Nat Commun 10, 3686. https://doi.org/10.1038/s41467-019-11642-7
Gursoy, N.R., Benita, S., 2004. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomedicine & Pharmacotherapy 58, 173–182. https://doi.org/10.1016/j.biopha.2004.02.001
Haley, B., Frenkel, E., 2008. Nanoparticles for drug delivery in cancer treatment. Urologic Oncology: Seminars and Original Investigations, The Potential of Nanotechnology in Urologic Oncology 26, 57–64. https://doi.org/10.1016/j.urolonc.2007.03.015
Hickey, S., Roberts, H.J., Miller, N.J., 2008. Pharmacokinetics of oral vitamin C. Journal of Nutritional & Environmental Medicine 17, 169–177. https://doi.org/10.1080/13590840802305423
Hobbs, J.M., Vazquez, A.R., Remijan, N.D., Trotter, R.E., McMillan, T.V., Freedman, K.E., Wei, Y., Woelfel, K.A., Arnold, O.R., Wolfe, L.M., Johnson, S.A., Weir, T.L., 2020. Evaluation of pharmacokinetics and acute anti-inflammatory potential of two oral cannabidiol preparations in healthy adults. Phytotherapy Research 34, 1696–1703. https://doi.org/10.1002/ptr.6651
Huang, Q., Yu, H., Ru, Q., 2010. Bioavailability and Delivery of Nutraceuticals Using Nanotechnology. Journal of Food Science 75, R50–R57. https://doi.org/10.1111/j.1750-3841.2009.01457.x
Im, K., Ravi, A., Kumar, D., Kuttan, R., Maliakel, B., 2012. An enhanced bioavailable formulation of curcumin using fenugreek-derived soluble dietary fibre. Journal of Functional Foods 4, 348–357. https://doi.org/10.1016/j.jff.2012.01.004
Imran, M., Revol-Junelles, A.-M., Paris, C., Guedon, E., Linder, M., Desobry, S., 2015. Liposomal nanodelivery systems using soy and marine lecithin to encapsulate food biopreservative nisin. LWT - Food Science and Technology 62, 341–349. https://doi.org/10.1016/j.lwt.2014.12.046
Inglut, C.T., Sorrin, A.J., Kuruppu, T., Vig, S., Cicalo, J., Ahmad, H., Huang, H.-C., 2020. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials (Basel) 10, 190. https://doi.org/10.3390/nano10020190
Iqbal, J., Hussain, M.M., 2009. Intestinal lipid absorption. American Journal of Physiology-Endocrinology and Metabolism 296, E1183–E1194. https://doi.org/10.1152/ajpendo.90899.2008
Izgelov, D., Davidson, E., Barasch, D., Regev, A., Domb, A.J., Hoffman, A., 2020. Pharmacokinetic investigation of synthetic cannabidiol oral formulations in healthy volunteers. European Journal of Pharmaceutics and Biopharmaceutics 154, 108–115. https://doi.org/10.1016/j.ejpb.2020.06.021
Jacob, J., Sukumaran, N.P., Jude, S., 2021. Fiber-Reinforced-Phospholipid Vehicle-Based Delivery of l -Ascorbic Acid: Development, Characterization, ADMET Profiling, and Efficacy by a Randomized, Single-Dose, Crossover Oral Bioavailability Study. ACS Omega 6, 5560–5568. https://doi.org/10.1021/acsomega.0c05963
Jäger, R., Lowery, R.P., Calvanese, A.V., Joy, J.M., Purpura, M., Wilson, J.M., 2014. Comparative absorption of curcumin formulations. Nutrition Journal 13, 11. https://doi.org/10.1186/1475-2891-13-11
Jamwal, R., 2018. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. Journal of Integrative Medicine 16, 367–374. https://doi.org/10.1016/j.joim.2018.07.001
Corrigendum to “Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers” [J. Integr. Med. 16(6) (2018) 367-374] Corrigendum to "Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers" [J. Integr. Med. 16(6) (2018) 367-374] - PubMed (nih.gov) (accessed 2.1.23).
Jones, D., Caballero, S., Davidov-Pardo, G., 2019. Chapter Six - Bioavailability of nanotechnology-based bioactives and nutraceuticals, in: Lim, L.-T., Rogers, M. (Eds.), Advances in Food and Nutrition Research, Food Applications of Nanotechnology. Academic Press, pp. 235–273. https://doi.org/10.1016/bs.afnr.2019.02.014 [Abstract only]
Joseph, A., Kumar, D., Balakrishnan, A., Shanmughan, P., Maliakel, B., Im, K., 2021. Surface-engineered liposomal particles of calcium ascorbate with fenugreek galactomannan enhanced the oral bioavailability of ascorbic acid: a randomized, double-blinded, 3-sequence, crossover study. RSC Adv. 11, 38161–38171. https://doi.org/10.1039/D1RA06483E
Kanae, H., Teshima, K., Shiroma, T., Noguchi, K., 2022. Pharmacokinetics of a single dose of novel curcumin formulations mixed with fish oils in healthy humans. Bioscience, Biotechnology, and Biochemistry 86, 1688–1694. https://doi.org/10.1093/bbb/zbac161
Kharat, M., McClements, D.J., 2019. Recent advances in colloidal delivery systems for nutraceuticals: A case study – Delivery by Design of curcumin. Journal of Colloid and Interface Science 557, 506–518. https://doi.org/10.1016/j.jcis.2019.09.045
Kipp, J.E., 2004. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int J Pharm 284, 109–122. https://doi.org/10.1016/j.ijpharm.2004.07.019
Kirkwood, K., 2018. Using Micronization to Increase Bioavailability of Poorly Soluble Active Pharmaceutical Ingredients. Available at Using Micronization to Increase Bioavailability of Poorly Soluble Active Pharmaceutical Ingredients (linkedin.com) [Accessed 31st January 2023).
Knaub, K., Sartorius, T., Dharsono, T., Wacker, R., Wilhelm, M., Schön, C., 2019. A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb® Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects. Molecules 24, 2967. https://doi.org/10.3390/molecules24162967
Knoll, G., Burger, K.N., Bron, R., van Meer, G., Verkleij, A.J., 1988. Fusion of liposomes with the plasma membrane of epithelial cells: fate of incorporated lipids as followed by freeze fracture and autoradiography of plastic sections. Journal of Cell Biology 107, 2511–2521. https://doi.org/10.1083/jcb.107.6.2511
Kocher, A., Schiborr, C., Behnam, D., Frank, J., 2015. The oral bioavailability of curcuminoids in healthy humans is markedly enhanced by micellar solubilisation but not further improved by simultaneous ingestion of sesamin, ferulic acid, naringenin and xanthohumol. Journal of Functional Foods 14, 183–191. https://doi.org/10.1016/j.jff.2015.01.045
Kocher, A., Bohnert, L., Schiborr, C., Frank, J., 2016. Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals. Mol Nutr Food Res 60, 1555–1563. https://doi.org/10.1002/mnfr.201501034
Kossena, G.A., Charman, W.N., Boyd, B.J., Dunstan, D.E., Porter, C.J.H., 2004. Probing drug solubilization patterns in the gastrointestinal tract after administration of lipid‐based delivery systems: A phase diagram approach. JPharmSci 93, 332–348. https://doi.org/10.1002/jps.10554 [Abstract only]
Kotake-Nara, E., Nagao, A., 2012. Effects of Mixed Micellar Lipids on Carotenoid Uptake by Human Intestinal Caco-2 Cells. Bioscience, Biotechnology, and Biochemistry 76, 875–882. https://doi.org/10.1271/bbb.110777
Kreuter, J., 1991. Peroral administration of nanoparticles. Advanced Drug Delivery Reviews, Gastrointestinal Tract as a Site for Drug Delivery 7, 71–86. https://doi.org/10.1016/0169-409X(91)90048-H
Kulthe, S.S., Choudhari, Y.M., Inamdar, N.N., Mourya, V., 2012. Polymeric micelles: authoritative aspects for drug delivery. Designed Monomers and Polymers 15, 465–521. https://doi.org/10.1080/1385772X.2012.688328
Li, G., Zhang, Z., Liu, H., Hu, L., 2021. Nanoemulsion-based delivery approaches for nutraceuticals: fabrication, application, characterization, biological fate, potential toxicity and future trends. Food Funct. 12, 1933–1953. https://doi.org/10.1039/D0FO02686G [Abstract only]
Li, H., Zhao, X., Ma, Y., Zhai, G., Li, L., Lou, H., 2009. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. Journal of Controlled Release 133, 238–244. https://doi.org/10.1016/j.jconrel.2008.10.002
Lipolife, Product Analysis (2023). Available at Market Snapshot | lipolife Accessed 16th February 2023.
Liu, P., Chen, G., Zhang, J., 2022. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 27, 1372. https://doi.org/10.3390/molecules27041372
Liu, W., Hou, Y., Jin, Y., Wang, Y., Xu, X., Han, J., 2020. Research progress on liposomes: Application in food, digestion behavior and absorption mechanism. Trends in Food Science & Technology 104, 177–189. https://doi.org/10.1016/j.tifs.2020.08.012
Liu, W., Ye, A., Han, F., Han, J., 2019. Advances and challenges in liposome digestion: Surface interaction, biological fate, and GIT modeling. Advances in Colloid and Interface Science 263, 52–67. https://doi.org/10.1016/j.cis.2018.11.007
Łukawski, M., Dałek, P., Borowik, T., Foryś, A., Langner, M., Witkiewicz, W., Przybyło, M., 2020. New oral liposomal vitamin C formulation: properties and bioavailability. J Liposome Res 30, 227–234. https://doi.org/10.1080/08982104.2019.1630642
Lynch, S.R., Cook, J.D., 1980. Interaction of vitamin C and iron. Ann N Y Acad Sci 355, 32–44. https://doi.org/10.1111/j.1749-6632.1980.tb21325.x
Madhavi, D., Kagan, D., 2014. Bioavailability of a Sustained Release Formulation of Curcumin. Integr Med (Encinitas) 13, 24–30.
Mason, T.G., Wilking, J.N., Meleson, K., Chang, C.B., Graves, S.M., 2006. Nanoemulsions: formation, structure, and physical properties. J. Phys.: Condens. Matter 18, R635. https://doi.org/10.1088/0953-8984/18/41/R01
McClements, D.J., 2018. Enhanced delivery of lipophilic bioactives using emulsions: a review of major factors affecting vitamin, nutraceutical, and lipid bioaccessibility. Food Funct. 9, 22–41. https://doi.org/10.1039/C7FO01515A
McClements, D.J., 2012. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter 8, 1719–1729. https://doi.org/10.1039/C2SM06903B
McClements, D.J., Li, F., Xiao, H., 2015. The Nutraceutical Bioavailability Classification Scheme: Classifying Nutraceuticals According to Factors Limiting their Oral Bioavailability. Annual Review of Food Science and Technology 6, 299–327. https://doi.org/10.1146/annurev-food-032814-014043
Millar, S.A., Stone, N.L., Yates, A.S., O’Sullivan, S.E., 2018. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Frontiers in Pharmacology 9.
Mintel. 2022. The Future of Vitamins, Minerals, and Supplements 2022. Available at. The Future of Vitamins, Minerals and Supplements 2022 (mintel.com) Accessed 9th May 2023.
Mozaffari, K., Willette, S., Lucker, B.F., Kovar, S.E., Holguin, F.O., Guzman, I., 2021. The Effects of Food on Cannabidiol Bioaccessibility. Molecules 26, 3573. https://doi.org/10.3390/molecules26123573
Müller, R.H., Mäder, K., Gohla, S., 2000. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics 50, 161–177. https://doi.org/10.1016/S0939-6411(00)00087-4
Müller, R.H., Radtke, M., Wissing, S.A., 2002. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm 242, 121–128. https://doi.org/10.1016/s0378-5173(02)00180-1
Müllertz, A., Fatouros, D.G., Smith, J.R., Vertzoni, M., Reppas, C., 2012. Insights into intermediate phases of human intestinal fluids visualized by atomic force microscopy and cryo-transmission electron microscopy ex vivo. Mol Pharm 9, 237–247. https://doi.org/10.1021/mp200286x
Oh, D.-M., Curl, R.L., Yong, C.-S., Amidon, G.L., 1995. Effect of micronization on the extent of drug absorption from suspensions in humans. Arch. Pharm. Res. 18, 427–433. https://doi.org/10.1007/BF02976347
Pagano, R.E., Weinstein, J.N., 1978. Interactions of Liposomes with Mammalian Cells. Annual Review of Biophysics and Bioengineering 7, 435–468. https://doi.org/10.1146/annurev.bb.07.060178.002251
Panda, S.K., Somashekara -, Parachur, V.A., Mohanty, N., Swain, T., Sahu, S., 2019. A Comparative Pharmacokinetic Evaluation of a Bioavailable Curcumin Formulation Curene® with Curcumin Formulation Containing Turmeric Volatile Oil and Standard Curcuminoids 95% in Healthy Human Subjects. Functional Foods in Health and Disease 9, 134–144. https://doi.org/10.31989/ffhd.v9i2.548
Panda, S.K., Nirvanashetty, S., Missamma, M., Jackson-Michel, S., 2021. The enhanced bioavailability of free curcumin and bioactive-metabolite tetrahydrocurcumin from a dispersible, oleoresin-based turmeric formulation. Medicine (Baltimore) 100, e26601. https://doi.org/10.1097/MD.0000000000026601
Patrician, A., Versic-Bratincevic, M., Mijacika, T., Banic, I., Marendic, M., Sutlović, D., Dujić, Ž., Ainslie, P.N., 2019. Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study. Adv Ther 36, 3196–3210. https://doi.org/10.1007/s12325-019-01074-6
Ponchel, G., Montisci, M.-J., Dembri, A., Durrer, C., Duchêne, D., 1997. Mucoadhesion of colloidal particulate systems in the gastro-intestinal tract. European Journal of Pharmaceutics and Biopharmaceutics 44, 25–31. https://doi.org/10.1016/S0939-6411(97)00098-2
Porter, C.J.H., Trevaskis, N.L., Charman, W.N., 2007. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov 6, 231–248. https://doi.org/10.1038/nrd2197
Pouton, C.W., Porter, C.J.H., 2008. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev 60, 625–637. https://doi.org/10.1016/j.addr.2007.10.010
Purpura, M., Lowery, R.P., Wilson, J.M., Mannan, H., Münch, G., Razmovski-Naumovski, V., 2018. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur J Nutr 57, 929–938. https://doi.org/10.1007/s00394-016-1376-9
Qian, C., Decker, E.A., Xiao, H., McClements, D.J., 2012. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chemistry 135, 1440–1447. https://doi.org/10.1016/j.foodchem.2012.06.047
Sandra, A., Pagano, R., 1979. Liposome-cell interactions. Studies of lipid transfer using isotopically asymmetric vesicles. The Journal of biological chemistry.
Sasaki, H., Sunagawa, Y., Takahashi, K., Imaizumi, A., Fukuda, H., Hashimoto, T., Wada, H., Katanasaka, Y., Kakeya, H., Fujita, M., Hasegawa, K., Morimoto, T., 2011. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biological and Pharmaceutical Bulletin 34, 660–665. https://doi.org/10.1248/bpb.34.660
Savjani, K.T., Gajjar, A.K., Savjani, J.K., 2012. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm 2012, 195727. https://doi.org/10.5402/2012/195727
Schiborr, C., Kocher, A., Behnam, D., Jandasek, J., Toelstede, S., Frank, J., 2014. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Molecular Nutrition & Food Research 58, 516–527. https://doi.org/10.1002/mnfr.201300724
Sharma, A., 1997. Liposomes in drug delivery: Progress and limitations. International Journal of Pharmaceutics 154, 123–140. https://doi.org/10.1016/S0378-5173(97)00135-X
Shen, Z., Fisher, A., Liu, W.K., Li, Y., 2018. 1 - PEGylated “stealth” nanoparticles and liposomes, in: Parambath, A. (Ed.), Engineering of Biomaterials for Drug Delivery Systems, Woodhead Publishing Series in Biomaterials. Woodhead Publishing, pp. 1–26. https://doi.org/10.1016/B978-0-08-101750-0.00001-5
Shin, G.H., Kim, J.T., Park, H.J., 2015. Recent developments in nanoformulations of lipophilic functional foods. Trends in Food Science & Technology 46, 144–157. https://doi.org/10.1016/j.tifs.2015.07.005
Siano, D.B., 1983. The swollen micelle—microemulsion transition. Journal of Colloid and Interface Science 93, 1–7. https://doi.org/10.1016/0021-9797(83)90377-6
Silmore, L.H., Willmer, A.R., Capparelli, E.V., Rosania, G.R., 2021. Food effects on the formulation, dosing, and administration of cannabidiol (CBD) in humans: A systematic review of clinical studies. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 41, 405–420. https://doi.org/10.1002/phar.2512
Singh, Y., Meher, J.G., Raval, K., Khan, F.A., Chaurasia, M., Jain, N.K., Chourasia, M.K., 2017. Nanoemulsion: Concepts, development and applications in drug delivery. Journal of Controlled Release 252, 28–49. https://doi.org/10.1016/j.jconrel.2017.03.008
Storka, A., Vcelar, B., Klickovic, U., Gouya, G., Weisshaar, S., Aschauer, S., Bolger, G., Helson, L., Wolzt, M., 2015. Safety, tolerability and pharmacokinetics of liposomal curcumin in healthy humans. Int J Clin Pharmacol Ther 53, 54–65. https://doi.org/10.5414/CP202076
Straubinger, R.M., Hong, K., Friend, D.S., Papahadjopoulos, D., 1983. Endocytosis of liposomes and intracellular fate of encapsulated molecules: Encounter with a low pH compartment after internalization in coated vesicles. Cell 32, 1069–1079. https://doi.org/10.1016/0092-8674(83)90291-X [Abstract only]
Sun, Y., Xia, Z., Zheng, J., Qiu, P., Zhang, L., McClements, D.J., Xiao, H., 2015. Nanoemulsion-based delivery systems for nutraceuticals: Influence of carrier oil type on bioavailability of pterostilbene. Journal of Functional Foods 13, 61–70. https://doi.org/10.1016/j.jff.2014.12.030
Sunagawa, Y., Hirano, S., Katanasaka, Y., Miyazaki, Y., Funamoto, M., Okamura, N., Hojo, Y., Suzuki, H., Doi, O., Yokoji, T., Morimoto, E., Takahashi, T., Ozawa, H., Imaizumi, A., Ueno, M., Kakeya, H., Shimatsu, A., Wada, H., Hasegawa, K., Morimoto, T., 2015. Colloidal Submicron-Particle Curcumin Exhibits High Absorption Efficiency—A Double-Blind, 3-Way Crossover Study—. Journal of Nutritional Science and Vitaminology 61, 37–44. https://doi.org/10.3177/jnsv.61.37
Tamjidi, F., Shahedi, M., Varshosaz, J., Nasirpour, A., 2013. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innovative Food Science & Emerging Technologies 19, 29–43. https://doi.org/10.1016/j.ifset.2013.03.002
Tan, C., Zhang, Y., Abbas, S., Feng, B., Zhang, X., Xia, S., 2014. Modulation of the carotenoid bioaccessibility through liposomal encapsulation. Colloids and Surfaces B: Biointerfaces 123, 692–700. https://doi.org/10.1016/j.colsurfb.2014.10.011
Tanya, M., Hari K.S.L., 2017. SMEDDS/SNEDDS: AnEmerging Technique to Solubility Enhancement for the Pharmaceutical Industry. WJPPS 317–336. https://doi.org/10.20959/wjpps20177-9493
Terao, K., Nakata, D., Fukumi, H., Schmid, G., Arima, H., Hirayama, F., Uekama, K., 2006. Enhancement of oral bioavailability of coenzyme Q10 by complexation with γ-cyclodextrin in healthy adults. Nutrition Research 26, 503–508. https://doi.org/10.1016/j.nutres.2006.08.004
Thompson, A., Mozafari, M., Singh, H., 2007. The properties of liposomes produced from milk fat globule membrane material using different techniques. Dairy Science & Technology 87, 349–360. https://doi.org/10.1051/lait:200701
Ting, Y., Jiang, Y., Ho, C.-T., Huang, Q., 2014. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. Journal of Functional Foods 7, 112–128. https://doi.org/10.1016/j.jff.2013.12.010
Tiwari, R., Takhistov, P., 2012. Nanotechnology-Enabled Delivery Systems for Food Functionalization and Fortification, in: Nanotechnology Research Methods for Foods and Bioproducts. John Wiley & Sons, Ltd, pp. 55–101. https://doi.org/10.1002/9781118229347.ch5 [Abstract only]
Torchilin, V.P., 2007. Micellar Nanocarriers: Pharmaceutical Perspectives. Pharm Res 24, 1–16. https://doi.org/10.1007/s11095-006-9132-0 [Abstract only]
Trevaskis, N.L., Charman, W.N., Porter, C.J.H., 2008. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev 60, 702–716. https://doi.org/10.1016/j.addr.2007.09.007
Turánek, J., Mašek, J., Raška, M., Ledvina, M., Paulovičová, E., Hubatka, F., Kotouček, J., 2019. 13 - Modification of liposomal surface by polysaccharides: Preparation, characterization, and application for drug targeting, in: Maiti, S., Jana, S. (Eds.), Functional Polysaccharides for Biomedical Applications. Woodhead Publishing, pp. 433–467. https://doi.org/10.1016/B978-0-08-102555-0.00013-3 [Abstract only]
Uekaji, Y., Terao, K., 2019. Bioavailability enhancement of hydrophobic nutraceuticals using γ-cyclodextrin. J Incl Phenom Macrocycl Chem 93, 3–15. https://doi.org/10.1007/s10847-018-0856-3 [Abstract only]
Wang, P., Liu, H.-J., Mei, X.-Y., Nakajima, M., Yin, L.-J., 2012. Preliminary study into the factors modulating β-carotene micelle formation in dispersions using an in vitro digestion model. Food Hydrocolloids, 10th International Hydrocolloids Conference 26, 427–433. https://doi.org/10.1016/j.foodhyd.2010.11.018
Weiss, J., Decker, E.A., McClements, D.J., Kristbergsson, K., Helgason, T., Awad, T., 2008. Solid Lipid Nanoparticles as Delivery Systems for Bioactive Food Components. Food Biophysics 3, 146–154. https://doi.org/10.1007/s11483-008-9065-8 [Abstract only]
Williams, N.N.B., Ewell, T.R., Abbotts, K.S.S., Harms, K.J., Woelfel, K.A., Dooley, G.P., Weir, T.L., Bell, C., 2021. Comparison of Five Oral Cannabidiol Preparations in Adult Humans: Pharmacokinetics, Body Composition, and Heart Rate Variability. Pharmaceuticals 14, 35. https://doi.org/10.3390/ph14010035
Xing, L., Tan, Z.-R., Cheng, J.-L., Huang, W.-H., Zhang, W., Deng, W., Yuan, C.-S., Zhou, H.-H., 2017. Bioavailability and pharmacokinetic comparison of tanshinones between two formulations of Salvia miltiorrhiza in healthy volunteers. Sci Rep 7, 4709. https://doi.org/10.1038/s41598-017-02747-4
Xing, M., Yan, F., Yu, S., Shen, P., 2015. Efficacy and Cardiotoxicity of Liposomal Doxorubicin-Based Chemotherapy in Advanced Breast Cancer: A Meta-Analysis of Ten Randomized Controlled Trials. PLoS One 10, e0133569. https://doi.org/10.1371/journal.pone.0133569
Yáñez, J.A., Wang, S.W.J., Knemeyer, I.W., Wirth, M.A., Alton, K.B., 2011. Intestinal lymphatic transport for drug delivery. Advanced Drug Delivery Reviews, Lymphatic drug delivery: therapy, imaging and nanotechnology 63, 923–942. https://doi.org/10.1016/j.addr.2011.05.019
Yeap, Y.Y., Trevaskis, N.L., Porter, C.J.H., 2013. Lipid Absorption Triggers Drug Supersaturation at the Intestinal Unstirred Water Layer and Promotes Drug Absorption from Mixed Micelles. Pharm Res 30, 3045–3058. https://doi.org/10.1007/s11095-013-1104-6
Yao, M., Xiao, H., McClements, D.J., 2014. Delivery of lipophilic bioactives: assembly, disassembly, and reassembly of lipid nanoparticles. Annu Rev Food Sci Technol 5, 53–81. https://doi.org/10.1146/annurev-food-072913-100350
Yuan, H., Chen, J., Du, Y.-Z., Hu, F.-Q., Zeng, S., Zhao, H.-L., 2007. Studies on oral absorption of stearic acid SLN by a novel fluorometric method. Colloids and Surfaces B: Biointerfaces 58, 157–164. https://doi.org/10.1016/j.colsurfb.2007.03.002
Zordan-Nudo, T., Ling, V., Liu, Z., Georges, E., 1993. Effects of nonionic detergents on P-glycoprotein drug binding and reversal of multidrug resistance. Cancer Res 53, 5994–6000.
Zou, L., Zheng, B., Liu, W., Liu, C., Xiao, H., McClements, D.J., 2015. Enhancing nutraceutical bioavailability using excipient emulsions: Influence of lipid droplet size on solubility and bioaccessibility of powdered curcumin. Journal of Functional Foods 15, 72–83. https://doi.org/10.1016/j.jff.2015.02.044
Zur Mühlen, A., Mehnert, W., 1998. Drug release and release mechanism of prednisolone loaded Solid Lipid Nanoparticles. Pharmazie 53, 552–555.
Appendix A: Literature search for specific toxicology studies with novel Supplement formulations
Searches for studies investigating the toxicity of vitamin C, CBD, and curcumin in novel formulations were conducted in PubMed using the search strings listed in table 1.
Table 1. Search strings and number of total and relevant results.
|
Search string |
Results |
Relevant |
|
“Vitamin C” AND “toxicity” AND “encapsulated” |
7 |
0 |
|
“Vitamin C” AND “toxicity” AND “liposomal” |
5 |
0 |
|
“Vitamin C” AND “toxicity” AND “micelles” |
6 |
0 |
|
“Vitamin C” AND “toxicity” AND “emulsion” |
3 |
0 |
|
“CBD” AND “toxicity” AND “encapsulated” |
1 |
0 |
|
“CBD” AND “toxicity” AND “liposomal” |
0 |
0 |
|
“CBD” AND “toxicity” AND “micelles” |
0 |
0 |
|
“CBD” AND “toxicity” AND “emulsion” |
0 |
0 |
|
“Curcumin” AND “toxicity” AND “encapsulated” |
121 |
7 |
|
“Curcumin” AND “toxicity” AND “liposomal” |
33 |
5 |
|
“Curcumin” AND “toxicity” AND “micelles” |
84 |
8 |
|
“Curcumin” AND “toxicity” AND “emulsion” |
27 |
3 |
PM = PubMed; WoS = Web of Science.
Relevant results were only retrieved for novel formulations of curcumin. A total of 23 studies were identified, 11 of which were in vitro studies and 11 of which were in vivo studies. Three studies were performed in human subjects and are reviewed in the main paper. A few of the relevant hits were retrieved by more than one search string and in these cases such results were omitted from the ‘relevant’ count in the subsequent strings such that all ‘relevant’ counts are unique. Publications investigating the toxicology of novel curcumin formulations in vitro and in vivo are briefly summarised table 2 and the full references are listed below table 2.
Table 2. Summary of studies investigating the toxicity of novel curcumin formulations.
|
Formulation: In Vitro |
System |
Key findings |
Study |
|
Curcumin nano-blisomes |
Non-cancer cell line (Wi-38l) |
Lower cytotoxicity vs unformulated curcumin |
Abbas et al. 2022 |
|
Micellar curcumin |
Immortalised fibroblasts. Glioblastoma LN229. Human endothelial cell line. Primary vascular endothelial cells. Primary smooth muscle cells. Primary pericytes. |
Reduced cell viability. Reversible genotoxicity (comet assay) Similar efficacy of native vs. micellar curcumin. |
Beltzig et al. 2021 |
|
Liposomal curcumin |
Human lymphocytes. EBV-transformed B-cells (LCL) |
Empty DMPC liposomes toxic. Liposomal curcumin inhibited LCL proliferation. |
Chen et al. 2009 |
|
Micellar curcumin |
Breast tumor cell line. Human stromal cells. Zebrafish embryotoxicity assay. |
Induced apoptosis in tumour cells and spheroids. Reduced viability in stromal cells. Toxicity to zebrafish embryo development. Micellar curcumin more toxic. |
Do et al. 2022 |
|
Curcumin chitosan nanoparticles |
Cervical tumour cells. VERO cells. |
Cytotoxicity to tumour cells. Biocompatible with VERO cells. |
Facchi et al. 2019 |
|
Liposomal curcumin |
Human synovial fibroblasts. Mouse macrophages. |
Liposomal curcumin less toxic to cells. |
Kloesch et al. 2016 |
|
Curcumin microemulsion |
HepG2 cells. |
Cytotoxicity to HepG2 cells, greater with smaller emulsion droplet size. |
Lin et al. 2014 |
|
Micellar curcumin |
HepG2 cells. |
Cytotoxicity to HepG2 cells. |
Phan et al. 2016 |
|
Solid lipid curcumin nanoparticles |
3T3 fibroblasts. |
Reduction in cell viability and alteration of lipid profile (dependent upon particle composition) |
Rosa et al. 2022 |
|
Liposomal curcumin |
Red blood cells in vitro. |
Dose-dependent echinocyte formation and increases in mean cellular volume. |
Storka et al. 2013 |
|
Liposomal solid curcumin gels |
Huh7it cell line. |
Non-cytotoxic |
Yusuf et al. 2022 |
|
Formulation: In Vivo |
System |
Key findings |
Study |
|
Curcumin PLGA nanoparticles |
Mice RAW 264.7 cell line. |
Increased lymphocytes . No changes in hepatotoxic biomarkers. Higher toxicity in RAW 264.7 cells at higher concentrations, but not at lower concentrations. |
Busari et al. 2017 |
|
Curcumin-loaded hydrogel nanoparticles |
“in vivo” [abstract only] |
Low toxicity. No genotoxicity observed. |
Dandekar et al. 2010 |
|
Hydrogenated curcumin |
Sprague Dawley rats. |
No treatment related toxicity. |
Gopi et al. 2016 |
|
Alginate-curcumin conjugate; micelle forming |
Mouse tumour models. |
No toxicity observed in blood parameters, histology, comet assay, or cytokine levels. |
Karabasz et al. 2019 |
|
Nano-micelle curcumin |
Male Wistar rats |
Testicular toxicity observed; DNA damage.
|
Moshari et al. 2017 |
|
Nano-micelle curcumin |
Male Wistar rats. |
Testicular toxicity observed; suppression of spermatogenesis; DNA damage.
|
Radmanesh et al. 2021 |
|
Nano-liposome curcumin with tetrandrine |
Zebrafish |
No developmental toxicity. |
Song et al. 2022 |
|
Chitosan solid lipid nanoparticle curcumin with sulforaphane |
BALB/c mice. |
No toxicity in acute, subacute, or chronic tests. |
Thakkar et al. 2016 |
|
Micellar curcumin |
Male Wistar rats. |
No haematopoietic or liver tissue toxicity. |
Tzankova et al. 2016 |
Full references
In vitro
Abbas, H., El-Feky, Y.A., Al-Sawahli, M.M., El-Deeb, N.M., El-Nassan, H.B., Zewail, M., 2022. Development and optimization of curcumin analog nano-bilosomes using 21.31 full factorial design for anti-tumor profiles improvement in human hepatocellular carcinoma: in-vitro evaluation, in-vivo safety assay. Drug Deliv 29, 714–727. https://doi.org/10.1080/10717544.2022.2044938
Beltzig, L., Frumkina, A., Schwarzenbach, C., Kaina, B., 2021. Cytotoxic, Genotoxic and Senolytic Potential of Native and Micellar Curcumin. Nutrients 13, 2385. https://doi.org/10.3390/nu13072385
Chen, C., Johnston, T.D., Jeon, H., Gedaly, R., McHugh, P.P., Burke, T.G., Ranjan, D., 2009. An in vitro study of liposomal curcumin: stability, toxicity and biological activity in human lymphocytes and Epstein-Barr virus-transformed human B-cells. Int J Pharm 366, 133–139. https://doi.org/10.1016/j.ijpharm.2008.09.009
Do, X.-H., Hoang, M.H.T., Vu, A.-T., Nguyen, L.-T., Bui, D.T.T., Dinh, D.-T., Nguyen, X.-H., Than, U.T.T., Mai, H.T., To, T.T., Nguyen, T.N.H., Hoang, N.T.M., 2022. Differential Cytotoxicity of Curcumin-Loaded Micelles on Human Tumor and Stromal Cells. Int J Mol Sci 23, 12362. https://doi.org/10.3390/ijms232012362
Facchi, S.P., Scariot, D.B., Bueno, P.V.A., Souza, P.R., Figueiredo, L.C., Follmann, H.D.M., Nunes, C.S., Monteiro, J.P., Bonafé, E.G., Nakamura, C.V., Muniz, E.C., Martins, A.F., 2016. Preparation and cytotoxicity of N-modified chitosan nanoparticles applied in curcumin delivery. Int J Biol Macromol 87, 237–245. https://doi.org/10.1016/j.ijbiomac.2016.02.063
Kloesch, B., Gober, L., Loebsch, S., Vcelar, B., Helson, L., Steiner, G., 2016. In Vitro Study of a Liposomal Curcumin Formulation (Lipocurc™): Toxicity and Biological Activity in Synovial Fibroblasts and Macrophages. In Vivo 30, 413–419.
Lin, C.-C., Lin, H.-Y., Chi, M.-H., Shen, C.-M., Chen, H.-W., Yang, W.-J., Lee, M.-H., 2014. Preparation of curcumin microemulsions with food-grade soybean oil/lecithin and their cytotoxicity on the HepG2 cell line. Food Chem 154, 282–290. https://doi.org/10.1016/j.foodchem.2014.01.012
Phan, Q.T., Le, M.H., Le, T.T.H., Tran, T.H.H., Xuan, P.N., Ha, P.T., 2016. Characteristics and cytotoxicity of folate-modified curcumin-loaded PLA-PEG micellar nano systems with various PLA:PEG ratios. Int J Pharm 507, 32–40. https://doi.org/10.1016/j.ijpharm.2016.05.003
Rosa, A., Nieddu, M., Pitzanti, G., Pireddu, R., Lai, F., Cardia, M.C., 2023. Impact of solid lipid nanoparticles on 3T3 fibroblasts viability and lipid profile: The effect of curcumin and resveratrol loading. J Appl Toxicol 43, 272–286. https://doi.org/10.1002/jat.4379
Storka, A., Vcelar, B., Klickovic, U., Gouya, G., Weisshaar, S., Aschauer, S., Helson, L., Wolzt, M., 2013. Effect of liposomal curcumin on red blood cells in vitro. Anticancer Res 33, 3629–3634.
In vivo
Busari, Z.A., Dauda, K.A., Morenikeji, O.A., Afolayan, F., Oyeyemi, O.T., Meena, J., Sahu, D., Panda, A.K., 2017. Antiplasmodial Activity and Toxicological Assessment of Curcumin PLGA-Encapsulated Nanoparticles. Front Pharmacol 8, 622. https://doi.org/10.3389/fphar.2017.00622
Dandekar, P.P., Jain, R., Patil, S., Dhumal, R., Tiwari, D., Sharma, S., Vanage, G., Patravale, V., 2010. Curcumin-loaded hydrogel nanoparticles: application in anti-malarial therapy and toxicological evaluation. J Pharm Sci 99, 4992–5010. https://doi.org/10.1002/jps.22191
Gopi, S., Jacob, J., Mathur, K.Y., 2016. Acute and subchronic oral toxicity studies of hydrogenated curcuminoid formulation “CuroWhite” in rats. Toxicol Rep 3, 817–825. https://doi.org/10.1016/j.toxrep.2016.10.007
Karabasz, A., Lachowicz, D., Karewicz, A., Mezyk-Kopec, R., Stalińska, K., Werner, E., Cierniak, A., Dyduch, G., Bereta, J., Bzowska, M., 2019. Analysis of toxicity and anticancer activity of micelles of sodium alginate-curcumin. Int J Nanomedicine 14, 7249–7262. https://doi.org/10.2147/IJN.S213942
Moshari, S., Nejati, V., Najafi, G., Razi, M., 2017. Nanomicelle curcumin-induced DNA fragmentation in testicular tissue; Correlation between mitochondria dependent apoptosis and failed PCNA-related hemostasis. Acta Histochem 119, 372–381. https://doi.org/10.1016/j.acthis.2017.03.007
Radmanesh, F., Razi, M., Shalizar-Jalali, A., 2021. Curcumin nano-micelle induced testicular toxicity in healthy rats; evidence for oxidative stress and failed homeostatic response by heat shock proteins 70-2a and 90. Biomed Pharmacother 142, 111945. https://doi.org/10.1016/j.biopha.2021.111945
Song, J.-W., Liu, Y.-S., Guo, Y.-R., Zhong, W.-X., Guo, Y.-P., Guo, L., 2022. Nano-Liposomes Double Loaded with Curcumin and Tetrandrine: Preparation, Characterization, Hepatotoxicity and Anti-Tumor Effects. Int J Mol Sci 23, 6858. https://doi.org/10.3390/ijms23126858
Thakkar, A., Chenreddy, S., Thio, A., Khamas, W., Wang, J., Prabhu, S., 2016. Preclinical systemic toxicity evaluation of chitosan-solid lipid nanoparticle-encapsulated aspirin and curcumin in combination with free sulforaphane in BALB/c mice. Int J Nanomedicine 11, 3265–3276. https://doi.org/10.2147/IJN.S106736
Tzankova, V., Gorinova, C., Kondeva-Burdina, M., Simeonova, R., Philipov, S., Konstantinov, S., Petrov, P., Galabov, D., Yoncheva, K., 2016. In vitro and in vivo toxicity evaluation of cationic PDMAEMA-PCL-PDMAEMA micelles as a carrier of curcumin. Food Chem Toxicol 97, 1–10. https://doi.org/10.1016/j.fct.2016.08.026
Yusuf, H., Novitasari, E.K.D.D., Purnami, N.L.W., Mahbub, A.W., Sari, R., Setyawan, D., 2022. Formulation Design and Cell Cytotoxicity of Curcumin-Loaded Liposomal Solid Gels for Anti-Hepatitis C Virus. Adv Pharmacol Pharm Sci 2022, 3336837. https://doi.org/10.1155/2022/3336837
[1] The details of this report were retrieved via a quote from the company and are not publicly advertised.
[2] The details of this report were retrieved via a quote from the company and are not publicly advertised.