Bisphenol A (BPA): Additional information
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Background
1. In April 2023, the European Food Safety Authority (EFSA) Panel on Food Contact Materials, Enzymes and Processing Aids (CEP) established a new tolerable daily intake (TDI) for BPA of 0.2ng /kg body weight (bw) per day. This new TDI would mean that both mean and high-level consumers of all age groups would exceed the new TDI by 2-3 orders of magnitude.
2. The final EFSA opinion on BPA and diverging opinions from the European Medical Agency (EMA) and the Bundesinstitut fuer Risikobewertung (BfR) were discussed at their May 2023 meeting.
3. A draft interim position statement was presented to the Committee in May 2023 and following discussion, again at the September 2023 meeting. At the September COT meeting, Members enquired about health-based guidance values (HBGVs) established by European or international authorities. The available HBGVs and other relevant information were presented at the October 2023 meeting. Following discussions at the October meeting the current paper provides additional information on the derivation of a TDI by EFSA (2023) and by the BfR (2023), including detail of the BMD modelling and derivation of the human equivalent dose (HED), as well as brief detail on the study selection. However, a direct comparison of the studies assessed by EFSA and BfR has not been included.
Introduction
4. In 2023 EFSA established a new tolerable daily intake (TDI) of 0.2 ng BPA/kg bw per day. Both, the EMA and the BfR provided comments to EFSA, highlighting diverging views. As the diverging views could not be resolved, according to the respective founding regulations, EFSA and the EMA/BfR are obliged to present a joint document to the European Commission (EC) clarifying the contentious scientific issue and identifying relevant uncertainties in the data. Following the divergence, the BfR published their own assessment of BPA.
5. The link to the EFSA assessment and related documents can be found at Annex A to this paper.
EFSA 2023 Re-evaluation of BPA
6. EFSA’s 2023 re-evaluation was performed using a systematic approach and in accordance with a pre-established protocol, which underwent public consultation. Studies from 1 January 2013 to 15 October 2018 were included in the evaluation, and EFSA launched a call for evidence to obtain human or animal data relevant to the risk assessment. Although some studies were published after the cut-off date, the NTP CLARITY study and its associated grantee studies were also included in the evaluation. For genotoxicity the literature search was extended until 21 July 2021 and the studies in the 2015 evaluation were re-assessed.
7. EFSA used a health outcome category (HOC) cluster approach to assess the data. For example, the HOC General Toxicity would have a cluster liver toxicity and within that the endpoints alanine amino transferase (ALT) and aspartate amino transferase and (AST), and gamma-glutamyl transpeptidase (γ-GTP). The HOC approach worked through the database by endpoint rather than by study (so a particular study could occur in several endpoint clusters).
Benchmark dose (BMD) modelling
8. EFSA performed benchmark dose (BMD) analysis for dose-response modelling on all endpoints that were considered ‘very likely’ or ‘likely’ in accordance with the EFSA guidance. A cut-off value of maximum 10 was applied for the ratio between the lowest dose tested (> 0) and the BMD lower confidence interval (BMDL) for selection of the RP. Studies with a ratio ≥ 10 were considered inadequate for BMD analysis but were considered in the uncertainty analysis, as were studies that were considered ‘as likely as not (ALAN)’. BMD analysis was performed with the administered dose, without conversion to human equivalent dose (HED). HED converted values were however used to compare the different modelling outcomes.
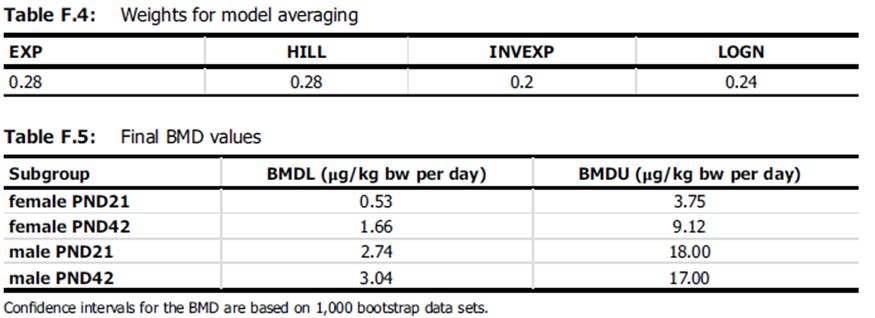
9. More than one BPA induced effect was identified by EFSA, with adverse effects seen in a similar dose range for other endpoints as for the increase of Th17 cells. Reproductive and developmental effects, i.e. the ratio of primordial and total ovarian follicles, sperm motility, and metabolic effects, i.e. uric acid, had BMDLs up to 7-fold higher than the BMDL for Th17 cells. However, the increase in the percentage of Th17 cells in the immune system was considered the most sensitive and hence the critical effect. EFSA highlighted that the selection of the pivotal study (Luo et al., 2016) was based on a risk of bias scrutiny of scientific papers. The conclusions from the present opinion were not solely based on one study but drawn from the WoE of the entire data set. The effect on Th17 cells was the most sensitive observed, even if the differences in doses with the other effects were relatively small. Furthermore, the effects described in the Luo et al. study were confirmed by more recently published studies. Potential bias through background contamination was taken into account during the appraisal of the study’s internal validity and the uncertainty of the dose at which the effect occurs was taken into account in the uncertainty analysis (UA).
10. For the analysis of the dose response of the effect of BPA on Th17 cells, using the benchmark dose approach, a benchmark response (BMR) needed to be selected. In case of an absence of data on which a BMR can be selected, the EFSA guidance (2017) recommends a BMR of 5% as default deviation from controls. However, deviating from this default is possible, if justified. For Th17 cells, there is currently insufficient information available on the normal variability of this measure, either in the mouse strain used in the study, any other strain or in humans. Hence, while the effect is clear, EFSA consider a BMR of 5% would be too stringent as the variability in the study are larger than that. Based on the control variance in the selected study a BMR of 20% was considered adequate. A human study published in 2016 (Sorrenti et al.) reported mean values of Th17 cells in peripheral blood of 221.6 ± 90.2 cells/uL (10.5 ± 4.4%). Registered cases of lymphocyte associated diseases were excluded, as well as samples with values of total erythrocytes, lymphocytes, leucocytes and major lymphocytes outside the normal range, according to guidelines. However, EFSA considered that if the population at large showed a 40% increment in Th17 cells, individuals at the higher end of the range of Th17 cells will be put out of the normal range and as a consequence, the number of Th17-associated pathological conditions would be expected to go up. For that reason, EFSA considered the 40% change as adverse and applied this as the BMR.
Human equivalent dose
11. The extrapolation from the reference point (RP) to the TDI was performed using an approach by which the toxicokinetic standard subfactor for interspecies extrapolation was substituted by a BPA-specific human equivalent dose factor (HEDF). The HEDF is calculated by dividing the area under the curve (AUCs) of unmetabolized (parent) BPA of animals by the AUCs of humans, both AUC values being corrected for the dose (AUC [corrected for dose] animals / AUC [corrected for dose] human).
12. The concentrations used for the calculation of the AUCs are those in the blood of the systemic circulation that is the relevant metric for the exposure of organs/tissues other than the liver. Because of the high metabolic rate of the enterocytes and liver cells, most of the BPA is metabolised and the amount of unmetabolized BPA systemically available is roughly 0.45% (mice) and 2.5% (rats). Human data on i.v. administration to calculate systemic availability are lacking. No data were available on the proportion of the pre-systemic elimination of BPA from enterocytes in the gut wall and from the liver cells. This would be needed for parameterising a PBPK model allowing the prediction of the concentration in the liver and the related AUC. In the absence of such data, the AUC in the peripheral blood was used also for the liver to substitute the toxicokinetic part of the uncertainty factor (UF) by an HEDF. For all species this is a conservative assumption, because the concentration in the liver and the related AUC are higher than in the systemic circulation, i.e. when using the AUCs in the systemic circulation, effects are assumed to occur at lower concentrations then in reality.
13. EFSA considered the studies by Teeguarden et al. (2015) and Thayer et al. (2015) to represent realistic behaviour of humans eating food. When selecting the AUC for calculating the HEDF, EFSA decided to select the median value and to combine data from both studies, due to the small number of participants. The difference of the resulting AUC-value of 15.7 nM × h per 100 μg/kg bw compared to the AUC-value of 3.6 nM × h per 100 μg/kg bw from 2015, was based on the fact that the human AUC used in 2015 was the result of PBPK modelling, whereas repeated measures after a single dose in humans were used to estimate the AUC in the current assessment. The mean observed AUC per 100 μg/kg bw in the study by Teeguarden et al. (2015) was close to the result of PBPK modelling. However, the AUC of 15.7 nM × h per 100 μg/kg bw is the median value of two studies in which the observed lowest and highest AUC, both dose-corrected, differ by a factor of 7. EFSA also considered that the kinetic metric to be selected is the AUC. Therefore, HEDFs were calculated using the median AUCs from animal studies. The resulting HEDF-values are 0.0155 for mice and 0.1656 for rats.
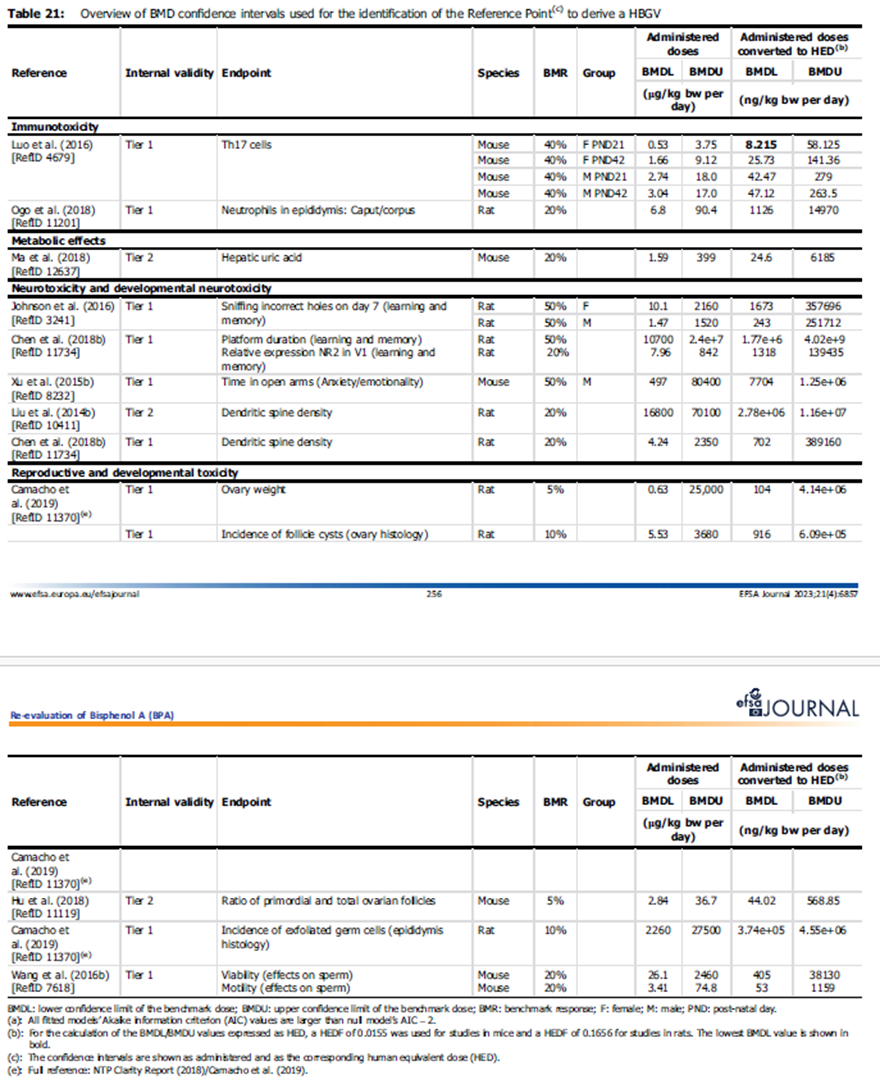
14. All BMDL values identified for the selection of the RP were converted to a HED. For more information, please see EFSA (2023), Table 21, pages 256-7.
15. After conversion of the doses from the Luo et al. (2016) study, the lowest BMDL40 was identified as a HED of 8.2 ng/kg bw per day and used as RP for the derivation of a health based guidance value (HBGV).
Derivation of the TDI
16. EFSA did not apply an UF for inter-species variability in toxicokinetics as this was already accounted for in the conversion to HED. The default UFs of 2.5 and 10 were applied for inter-species toxicodynamic differences and intra-species variability in toxicokinetics and toxicodynamics, respectively.
17. The increase in Th17 cells is considered an intermediate endpoint and for it to be considered in risk assessment it needs to have a causal relationship with an adverse outcome. EFSA noted that while this endpoint does not have an established relevant quantitative adverse outcome pathway (AOP), the information reviewed indicated that an increment in Th17 cell percentage and their cytokine IL17 was linked to inflammation. Hence, it meets the definition of adversity set by EFSA and the WHO. EFSA did not consider it necessary to apply an additional UF to account for the use of an intermediate rather than apical endpoint as there was a lack of relevant quantitative data or specific guidance on risk assessments based on RPs which are considered intermediate endpoints.
18. Averaging across experts, the probability that the lowest BMD for endpoints occurring in animals which were relevant to humans was below the RP of 8.2 ng/kg bw per day (HED) was 57 – 73%, the overall range of probabilities given by individual experts was even wider (44 – 98%), with the lowest probability being 27 – 43%. As there was sufficient uncertainty in the hazard assessment, EFSA considered it justifiable to include an additional UF of 2 when setting the TDI to account for the uncertainties affecting the RP and the possibility that other endpoints are more sensitive. The additional UF needed to be large enough to cover its median estimate for the lowest estimated BMD, such that it is equally probable (50%) that the lowest estimated BMD is higher or lower.
19. Applying an overall UF of 50 to the RP, EFSA established a TDI of 0.2 ng/kg bw per day.
Uncertainty analysis
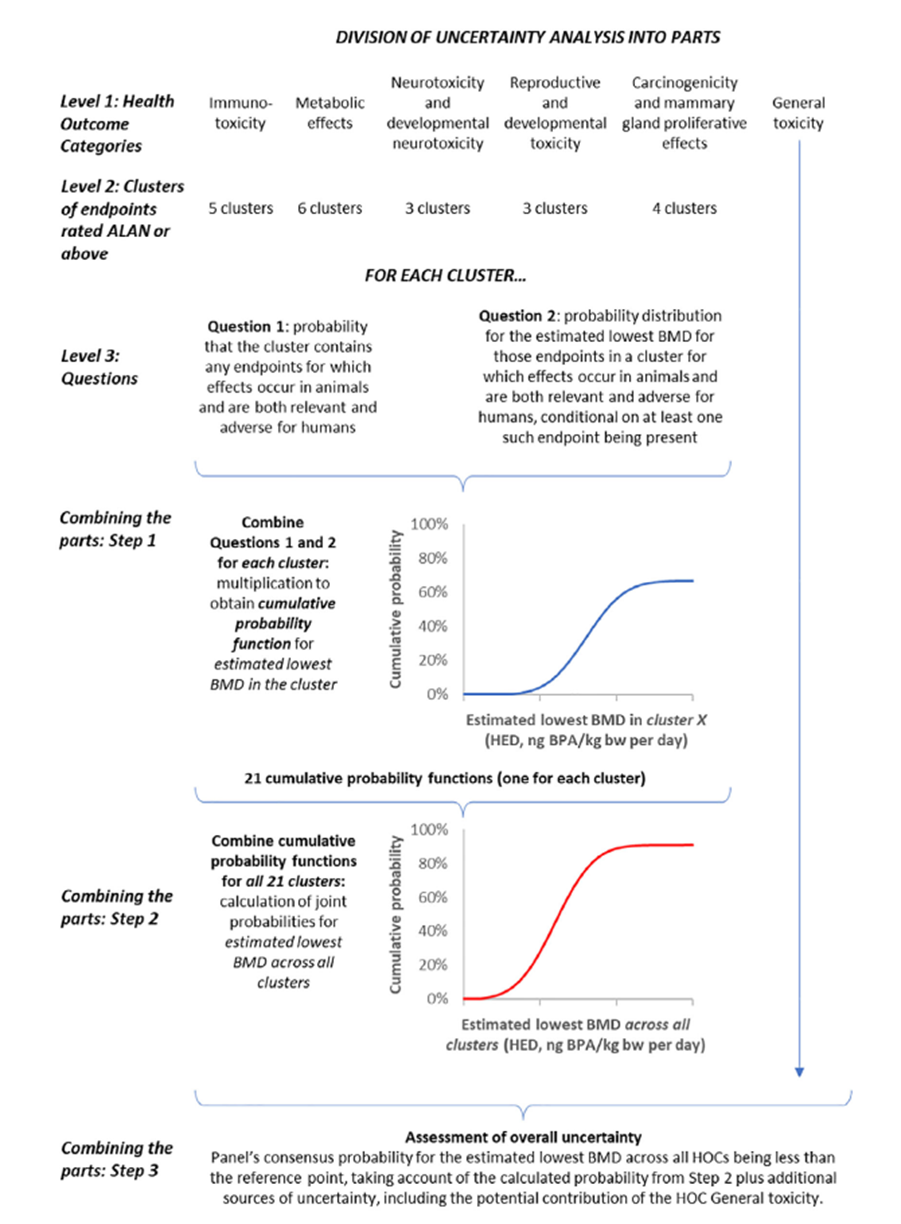
20. EFSA undertook an uncertainty analysis to identify sources of uncertainty affecting the hazard assessment and to quantify their combined impact on estimation of the lowest BMD for effect in animals that are relevant and adverse for humans (Figure 1). The uncertainty analysis followed the case-specific assessment, as described in Section 4 of EFSA’s guidance on uncertainty analysis (2018), as the assessment involved some refined approaches, e.g. conversion of animal doses to HED and many endpoints and study designs beyond those normally considered routine regulatory assessments.
Figure 1: Diagram of the conceptual model for the uncertainty analysis of the hazard assessment for HOCs other than genotoxicity; uncertainty analysis was conducted separately and described elsewhere.
21. EFSA undertook a structured uncertainty analysis using expert knowledge elicitation (EKE) to identify and quantify (by expert judgement) the impact of the uncertainties on the hazard assessment.
22. One major source of uncertainty was the large number of non-standard studies and endpoints, and the possibility that some endpoints had no observed adverse effect levels (NOAELs) and lowest observed adverse effect levels (LOAELs) lower than the RP and hence could be more sensitive. However, EFSA was unable to calculate BMDLs for these endpoints. The uncertainty analysis included any endpoints considered ‘ALAN’, ‘likely’ or ‘very likely’. The overall uncertainty was expressed as the probability that the estimated lowest BMD for effects in animals which were relevant/adverse to humans were below any given dose. Sensitivity analysis showed that the probability for lower doses was predominantly driven by allergic lung inflammation, followed by cellular immunity, which included increased percentage of Th17 cells. EFSA noted that the main impact on the low TDI was the new evidence available and the resulting RP, with the UA confirming that a RP in this range was reasonable when taking into account all evidence and uncertainties.
23. For more detail of the uncertainty analysis, please see Section 2.3.4 or Appendix D in the EFSA opinion.
BfR assessment
24. The BfR does not support the new EFSA opinion due to several scientific and methodologic divergencies and therefore undertook its own assessment in 2023. The link to the BfR assessment can be found at Annex B to this paper.
25. A comprehensive systematic literature search was undertaken, and the reliability of the studies was assessed based on pre-defined parameters, e.g. statistical power, reporting, blinding/possible background exposure, and grouped into three Tiers reflecting the respective weight of evidence (WoE). It should however be noted that the literature evaluation was limited to the areas of reproductive toxicity, immunological effects, increased serum uric acid and toxicokinetics. For their assessment the BfR considered all relevant literature and data, including literature/data from the EFSA 2015 and 2023 assessments.
26. The retrieved epidemiological studies and rodent study covering the metabolic endpoint were not considered for the TDI derivation due to methodological weaknesses or biological implausibilities. Since the BfR criticised the factor used for extrapolation for the critical dose from animal studies to the HED, the BfR also undertook a review of the available literature on the toxicokinetics of BPA.
Benchmark dose (BMD) modelling
27. The BfR considered all available studies in mice that which reported immunological effects. However, upon evaluation, the data were found to be differing between studies regarding effect size and dose, lacked standardisation and suffered from shortcomings in design and reporting. Given that the effect only represents an intermediate endpoint, for which a causal link to apical effects in a dose range relevant to human exposure is unclear, the BfR concluded that adverse immunological effects in humans, if they occur, are unlikely to result from BPA exposure in the range of the TDI of 0.2 ug/kg bw per day.
28. In 2015, EFSA concluded that BPA causes toxic effects to reproduction in experimental animals at high doses (above a HED of 3.6 mg/kg bw per day). However, there was a high variability in incidence and magnitude of adverse effects and hence the likelihood of the effect was considered ALAN. Since 2013, numerous studies have emerged, however the variability of results and dose-response relationships is still considerable.
29. The BfR focused their evaluation on parameters related to fertility and sexual function in males and females and on endpoints identified by EFSA as likely in the reproductive toxicity cluster such as sperm motility, ovary and uterus histology, and testis and epididymis histology. The following organs/tissues/parameters were also evaluated: testes, epididymis, prostate, sperm parameters, time to balano-preputial separation, ano-genital distance, ovary, uterus, vagina, oestrous cycle, time to vaginal opening, implantations. Effects judged as likely/relevant were then considered for TDI determination.
30. Please see Section 3.1.4, 3.1.4.2 (male) and 3.1.4.3 (female) for more detail on reproductive toxicity.
31. Studies in Tier 1 and Tier 2 were included in the dose-response analysis.
32. For each endpoint the BMR for adversity was defined upfront, i.e. 10% for all but the immunological effects; the latter was set to 100%. BMD analysis was carried out in line with EFSA recommendations (2022), the BMD, BMDL and BMDU were calculated with Bayesian Model Averaging (BMA) with the default settings (except critical effect size (CES)/BMR which was set to the predefined value). In parallel, calculations were also done with the standard model averaging. If either or both of the approaches failed to produce a single model-averaged BMDL/BMD/BMDU triplet or BMDL/BMDU value, but gave two or more individual model results, the mean BMD, lowest BMDL and highest BMDU were used for further calculations. If, for a given study and endpoint, after this procedure still BMDLs/BMDs/BMDUs from both the standard and the Bayesian modelling approach were available and acceptable, the result after BMA was chosen, as this approach reflects current EFSA recommendations. If an acceptable BMDL/BMD/BMDU triplet (or a BMDL/BMDU-pair, resp.) was not available, this was generally resolved by using the NOAEL or (if unavailable) LOAEL from the respective study as PoD for TDI derivation.
33. The immunological endpoint and studies identified by EFSA in 2023 were not considered suitable for TDI derivation by the BfR, however, the respective studies were still submitted to BMD modelling in order to evaluate to which extend the derived TDI would also be protective for these immunological effects.
34. The BfR based their TDI derivation on two studies showing reduced sperm count after subchronic BPA exposure of adult Wistar rats (Liu et al., 2013; Srivastava and Gupta et al., 2018). Dose response analysis was performed on these two studies by means of BMD modelling, resulting in a BMDL10 of 26 ug/kg bw per day and a NOAEL of 50 ug/kg bw per day, respectively.
35. Please see Section 3.1.7 for more detail.
Human equivalent dose
36. In accordance with EFSA, the BfR used toxicokinetic data for oral application of BPA in animals and humans to derive species specific HEDFs. The respective AUCs were linearly dose-adjusted to an external dose of 100 ug/kg bw as there is a linear relationship between external oral exposure and serum concentration of free BPA in blood. The BfR noted that there are limitations in the HED approach as described here, mainly due to the fact that only single (bolus) dosing of BPA was considered.
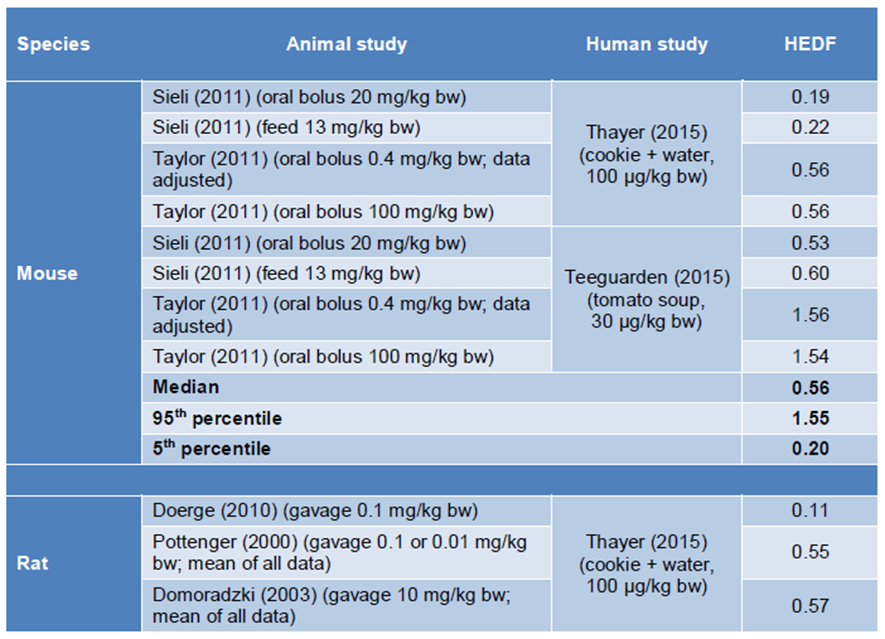
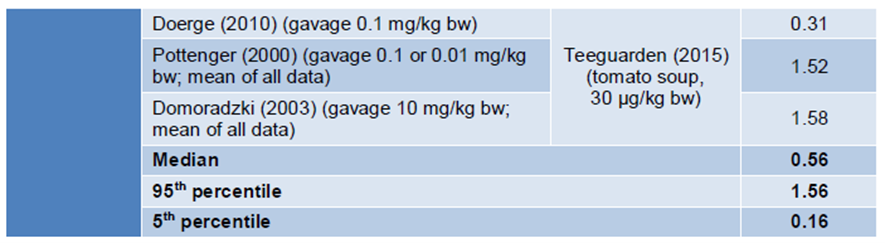
37. In order to not give too much weight to the studies of Pottenger et al. (2000) and Domoradzki et al. (2003), compared to Doerge et al. (2010), the results from the first two studies were averaged for each study. No further information could be identified by the Secretariat as to why the average of two studies was applied.
38. The BfR derived HEDFs in the range of 0.11 – 1.58 (median 0.56) for rats and 0.19 – 1.56 (median 0.56) for mice, calculated from free BPA blood concentrations in animals and humans.
Figure 2: HEDF for mice and rats calculated as ratio of the AUC at 100 ug/kg bw in the respective species and the AUC in humans at 100 ug/kg bw.
39. A recent review by Poet and Hays (2018) concluded that a HEDF of 0.9 would be scientifically sound for both rats and mice. As shown above, this value is within the range of possible HEDF for mice and rats derived by the BfR and the BfR concluded it seemed plausible also with respect to the effect of repeated-dose administration. However, the BfR decided not to use a single HEDF value, but the calculated range for derivation of a TDI, within the uncertainty assessment.
40. The 5th and 95th percentile, and median HEDFs were applied to the probabilistic uncertainty assessment for the derivation of the TDI. Toxicokinetic studies were not available for rabbits, hence the standard allometric scaling according to REACH guidance was applied.
41. For more information see Section 3.1.2
Uncertainty analysis and derivation of the TDI
42. After weighing the evidence, the BfR, in a conservative approach, identified effects on the male reproductive system (e.g. sperm count and mobility, testis histology) as the most sensitive endpoint and based its TDI derivation on the effect dose for reduced sperm count observed in two studies in rats (Lui et al., 2013; Srivastava and Gupta, 2018).
43. The results of the dose-response analysis and BMD modelling were subjected to a probabilistic uncertainty assessment according to the approach by the WHO IPCS (2018)/Approximate probabilistic analysis (APROBA). The distribution of possible HEDFs resulting from the evaluation of the literature on toxicokinetics was combined with typical distributions for other uncertainties, e.g. interhuman variability, study duration.
44. Applying the WHO ICPS/APROBA approach, the BfR considered that the TDI derivation and uncertainty analysis were not separated but rather the TDI was determined as the result of the uncertainty analysis in an integrated way. In addition, rather than adding up worst-case assumptions, combining individual uncertainties in a statistically sound way results in a more realistic overall uncertainty estimate.
45. Please see Section 3.1.8. for more detail.
46. The overall TDI was determined as the lowest TDI of the TDIs calculated, based on the point of departure (POD) from all studies included in the quantitative risk assessment. Studies for which the POD was the NOAEL obtained as the highest dose tested were excluded; of the PODs which was either a BMDL, LOAEL or NOAEL that was not the highest dose tested, two studies investigated the effects of BPA on sperm counts in rats following sub chronic exposure. As stated above, the BfR considered these the most relevant for TDI setting.
47. When using the more conservative approach, as proposed by the WHO IPCS, the 90% confidence interval for the TDI was 0.14 – 39 ug/kg bw per day and 0.2 – 78 ug/kg bw per day for the two studies. Again, in following the conservative approach, the BfR decided to select the lower confidence intervals as the TDI (the mean of the two P05). Hence, a TDI of 0.2 ug/kg bw per day was derived, which is protective of 99% of the population, with 95% confidence. The TDI is also protective for any other relevant effects/toxicological endpoints, including a 100% increase in the respective intermediate endpoints.
Comparison of the different approaches by EFSA and the BfR
48. In their published statement, both EFSA and the BfR acknowledged that the interpretation of available information and risk assessment are linked to the tools and methodologies applied, resulting in the divergence of opinion. The key points of divergence were the adverse effect definition, the inclusion/exclusion of scientific information, apical versus intermediate endpoint (reference point acceptability, adversity, relevance), reproductive toxicity endpoints, uncertainty analysis and the HEDF.
Adverse effect definition
49. The BfR agreed that there is evidence that BPA has an effect on Th17 cell counts and other effects on the immune system, however they did not consider the evidence convincing on the relationship between BPA mediated increase in Th17 cells and adverse outcomes in animals and humans. No adverse apical effects were reported in either the study from which the endpoint was derived (Luo et al., 2016), other long-term studies (CLARITY report) or epidemiological studies. Furthermore, no endorsed adverse outcome pathway exists for this endpoint. Hence, the BfR concluded that the increase in Th17 cells in the spleen does not seem sufficiently justified and is therefore not suitable for the derivation of a HBGV.
50. The BfR considered that EFSA had used conservative worst-case assumptions in every step of the risk assessment process, resulting in an over-conservative HBGV. For a data rich assessment, like BPA, the WHO/IPCS (2018) provide a suitable methodology for uncertainty characterisation, which the BfR applied on their assessment as described above.
Adverse effect definition
51. EFSA concluded that the new data confirmed the immune system as a target for BPA and considered the increase of Th17 cells in mice as the critical endpoint to derive a TDI.
52. While the BfR did not question the use of an intermediate endpoint as such, they did expressed concern for the use of the selected intermediate endpoint for setting a HBGV, when not accompanied by the observation of corresponding apical effects in the relevant in vivo data. The role of Th17 cells is context dependent and not yet fully understood in mice and humans, with a genetic link between increased IL-17A levels and disease in humans still missing (Li et al., 2018; Zwicky et al., 2020). The BfR would not have classified the study by Luo et al. (2016) as Tier 1 and would not have included it in the WoE.
53. After weighing the available evidence, the BfR concluded, in a conservative approach, that effects on the male reproductive system was the most sensitive endpoint and based its derivation of a HBGV on the effect dose for reduced sperm count in two studies of Wistar rats. However, the BfR noted, as previously EFSA had, that while the effects on the male reproductive system (e.g. sperm count, motility, testis histology) were observed in a range of experimental animals (mice, rats and rabbits), there was a high variability in incidence and effect dose in the database. In some studies effects were seen from approximately ≥ 100 µg/kg bw per day, while in other comparable studies an effect was not reported until doses of up to 450,000 µg/kg bw per day.
Choice of HED
54. In the study by Doerge et al. (2011) levels of free BPA were only observed above the detection limit within the first three measurement points, and only in one or two of the twelve mice investigated per time point. This resulted in the AUC of free BPA and thus the HEDF to be very low compared to other studies. The ratio of total to free BPA in serum also differed significantly from other studies, EHR was not covered. In other studies, the ratio of/the concentration/time profile of free BPA in serum mirrored the concentration/time course of total BPA in numerous studies, including studies with intervenors application. The BfR therefore consider the Doeger et al. study to be inadequate to derive and select a realistic HEDF.
55. According to the BfR, the studies by Sieli et al. (2011) and Taylor et al. (2011) should have been considered instead. Tayler et al. and others have clearly shown a clear linearity of the concentration of unconjugated BPA in serum after 24 hours (oral application, wide dose range (2 – 1000,000 µg/kg bw)). The linear dose-adjusted concentration/time profiles at 400 and 1000,000 µg/kg bw, respectively, match, with the exception of the last time point where analytical problems may have occurred. The BfR considers the results plausible with respect to the low solubility of BPA in water. BPA administered in fat or rodent chow will only slowly change into the aqueous environment of the stomach and intestine and therefore saturation of enzymes in intestinal cells seen in vitro would be unlikely in vivo, even at comparable doses. EFSA has argued that these studies would not be suitable as the doses applied (up to 13,000 – 100,000 µg/kg bw) might be above linear dose range. Already in 2015 EFSA considered that the AUCs in those two studies were not increasingly proportional to the dose used and hence the observation pointed to a non-linear relationship. In addition, due to possible limitations of intestinal enzymes, the AUC of unconjugated BPA in the serum may be higher at higher doses, even if linearly dose adjusted.
56. In the view of the BfR the HEDF for mice should be corrected, with a realistic HEDF being 10 – 100 times higher. The BfR also did not think that this fact was sufficiently considered in the uncertainty assessment, partly due to the process of the uncertainty assessment used by EFSA (EKE) but also the date being used in the first step.
BMD modelling and TDI derivation
57. The BfR applied both EFSA’s preferred Bayesian Model Averaging approach following the updated guidance from 2022 and the previous PROAST standard model averaging approach. EFSA applied its older guidance in their own assessment. Appreciable differences between the BMDLs derived from the two methods can be seen in Tables 6 and 7 (pages 46-53 and 54-55, respectively) of the BfR assessment.
58. When deriving the TDI, EFSA applied a deterministic approach, while the BfR applied a probabilistic approach.
Question on which the views of the Committee are sought:
i. Do Members have any comments on the information/comparison of the EFSA and BfR assessment?
ii. Does the Committee consider either TDI, or an alternative HBGV by any other authority applicable as an interim measure until their own assessment of BPA has concluded?
iii. Does the Committee have any further comments?
Secretariat
December 2023
Abbreviations
|
ALAN |
As likely as not |
|
ALT |
Alanine amino transferase |
|
AOP |
Adverse outcome pathway |
|
APROBA |
Approximate probabilistic analysis |
|
AST |
aspartate amino transferase and |
|
AUC |
Area under curve |
|
BMD |
Benchmark dose response modelling |
|
BMDL |
BMD lower confidence interval |
|
BMA |
Bayesian model averaging |
|
BMR |
Benchmark response |
|
BPA |
Bisphenol A |
|
bw |
Body weight |
|
CES |
Critical effect size |
|
EKE |
Expert knowledge elicitation |
|
HBGV |
Health based guidance value |
|
HED |
Human equivalent dose |
|
HEDF |
Human equivalent dose factor |
|
HOC |
Health outcome cluster |
|
g-GTP |
Gamma-glutamyl transpeptidase |
|
LOAEL |
Lowest observed adverse effect level |
|
NOAEL |
No observed adverse effect level |
|
RP |
Reference point |
|
TDI |
Tolerable daily intake |
|
UA |
Uncertainty analysis |
|
UF |
Uncertainty factor |
|
WoE |
Weight of evidence |
Organisations
|
BfR |
Bundesamt fuer Risikobewertung |
|
COT |
Committee on Toxicity in Food, Consumer Products and the Environment |
|
CEP |
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids |
|
EC |
European Commission |
|
EFSA |
European Food Safety Authority |
|
EMA |
European Medical Agency |
|
IPCS |
International Programme on Chemical Safety |
|
WHO |
World Health Organisation |
TOX/2023/62 – Annex A
EFSAs 2023 re-evaluation of the risk to public health from bisphenol A (BPA) in foodstuffs
EFSA’s 2023 re‐evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Re‐evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs | EFSA (europa.eu)
Annex N – comments from public consultation: efsa.onlinelibrary.wiley.com/action/downloadSupplement?doi=10.2903%2Fj.efsa.2023.6857&file=efs26857-sup-0014-Annex-N.pdf
The report on divergent views between EFSA and EMA on EFSA’s updated bisphenol A assessment can be found at: ema-efsa-article-30.pdf (europa.eu)
The report on diverging views between EFSA and BfR on EFSA updated bisphenol A assessment can be found at: Report on diverging views between EFSA and BfR on EFSA bisphenol A (BPA) opinion (europa.eu)
The summaries of the draft opinion provided to the COT at the extraordinary meeting on 10th February 2022 can be found at: COT Meeting: 10th February 2022 | Committee on Toxicity (food.gov.uk).
Please note, EFSA has since amended the HBGV, so some of the information may be out of date.
TOX/2023/62 – Annex B
Bisphenol A: BfR proposes health based guidance value, current exposure data are needed for a full risk assessment
The BfR’s assessment of BPA can be found at:
TOX/2023/62 – Annex 3
Other references
Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW (2010). Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicology and Applied Pharmacology, 247, 158–165. https://doi.org/10.1016/j.taap.2010.06.008
Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW (2011). Pharmacokinetics of bisphenol A in neonatal and adult CD-1 mice: Inter-species comparisons with Sprague-Dawley rats and rhesus monkeys. Toxicology Letters, 207(3): 298–305. https://doi.org/10.1016/j.toxlet.2011.09.020
Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW (2012). Pharmacokinetics of bisphenol A in serum and adipose tissue following intravenous administration to adult female CD-1 mice. Toxicology Letters 211 (2), 114-119. https://doi.org/10.1016/j.toxlet.2012.03.008
Domoradski et al. (2003).
EFSA (2007). Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to 2,2-bis(4-hydroxyphenyl)propane. EFSA Journal 5(1), 428. https://doi.org/10.2903/j.efsa.2007.428
EFSA (2008). Toxicokinetics of Bisphenol A - Scientific Opinion of the Panel on Food additives, Flavourings, Processing aids and Materials in Contact with Food (AFC). EFSA Journal 6(7), 759. https://doi.org/10.2903/j.efsa.2008.759
EFSA (2015). Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal, 13(1): 3978, 1040 pp. https://doi.org/10.2903/j.efsa.2015.3978
EFSA (2022). Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal, 20(10), e07584. https://doi.org/10.2903/j.efsa.2022.7584
Liu C, Duan W, Li R, Xu S, Zhang L, Chen C, He M, Lu Y, Wu H, Pi H, Luo X, Zhang Y, Zhong M, Yu Z, Zhou Z (2013). Exposure to bisphenol A disrupts meiotic progression during spermatogenesis in adult rats through estrogen-like activity. Cell Death and Disease, 4(6), e676. https://doi.org/10.1038/cddis.2013.203
Li J, Casanova JL, Puel A (2018). Mucocutaneous IL-17 immunity in mice and humans: host defense vs. excessive inflammation. Mucosal Immunology, 11 (3), 581-589. https://doi.org/10.1038/mi.2017.97
Luo SM, Li Y, Li YP, Zhu QX, Jiang JH, Wu CH, Shen T (2016). Gestational and lactational exposure to low-dose bisphenol A increases Th17 cells in mice offspring. Environmental Toxicology and Pharmacology, 47: 149–158. https://doi.org/10.1016/j.etap.2016.09.017
Poet T and Hays S (2018). Extrapolation of plasma clearance to understand species differences in toxicokinetics of bisphenol A. Xenobiotica, 48(9), 891-897. https://doi.org/10.1080/00498254.2017.1379626
Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JM Jr. (2000). The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicological Sciences, 54(1), 3-18. https://doi.org/10.1093/toxsci/54.1.3
Sorrenti V, Marenda B, Fortinguerra S, Cecchetto C, Quartesan R, Zorzi G, Zusso M, Giusti P, Buriani A (2016). Reference values for a panel of cytokinergic and regulatory lymphocyte subpopulations. Immune Network, 16(6), 344–357. https://doi.org/10.4110/in.2016.16.6.344
Srivastava S and Gupta P (2018): Alteration in apoptotic rate of testicular cells and sperms following administration of Bisphenol A (BPA) in Wistar albino rats. Environmental Science and Pollution Research, 25(22), 21635-21643. https://doi.org/10.1007/s11356-018-2229-2
Sieli PT, Jašarević E, Warzak DA, Mao J, Ellersieck MR, Liao C, Kannan K, Collet SH, Toutain PL, Saal FSVS, Rosenfeld CS (2011). Comparison of serum bisphenol a concentrations in mice exposed to bisphenol a through the diet versus oral bolus exposure. Environmental Health Perspectives, 119(9): 1260-1265. https://doi.org/10.1289/ehp.1003385
Taylor JA, vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, Toutain PL, Laffont CM, VandeVoort CA (2011). Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: Relevance for human exposure. Environmental Health Perspectives, 119(4): 422-430. https://doi.org/10.1289%2Fehp.1002514
Teeguarden JG, Twaddle NC, Churchwell MI, Yang X, Fisher JW, Seryak LM, Doerge DR (2015). 24-hour human urine and serum profiles of bisphenol A: Evidence against sublingual absorption following ingestion in soup. Toxicology and Applied Pharmacology, 288(2), 131-142. https://doi.org/10.1016/j.taap.2015.01.009
Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, Garantziotis S, Kissling GE, Easterling MR, Bucher JR, Birnbaum LS (2015). Pharmacokinetics of bisphenol A in humans following a single oral administration. Environment International, 83, 107-115. https://doi.org/10.1016%2Fj.envint.2015.06.008
WHO/IPSC (2018). Guidance document on evaluating and expressing uncertainty in hazard characterization. 2nd ed. World Health Organization, Geneva. https://apps.who.int/iris/handle/10665/259858
Zwicky P, Unger S, Becher B (2020). Targeting interleukin-17 in chronic inflammatory disease: A clinical perspective. Journal of Experimental Medicine, 217(1). https://doi.org/10.1084%2Fjem.20191123