Background - Statement on the effects of excess Vitamin A on maternal health
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.Background
Structure and sources
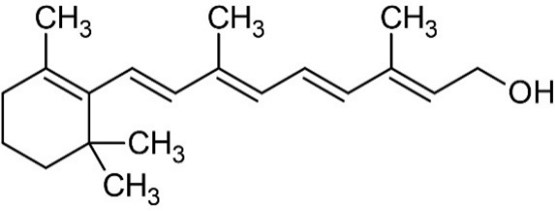
8 Vitamin A is a retinoid, consisting of an alicyclic b-ionone ring and a 9-carbon-long isoprenoid side chain. Retinoids are derived from a family of pro-vitamin A carotenoids, the major one being b-carotene. On cleavage, one molecule of b-carotene yields two molecules of retinol. Retinol (vitamin A1 or vitamin A alcohol) and 3-dehydroretinol (vitamin A2, which has 40% of the biological activity of vitamin A1) occur in foods of animal origin and b-carotene is found in red and yellow and leafy green vegetables (Bowman and Rand, 1982) (Figure 1, Merck Index, 1996).
Retinol
b-carotene
Figure 1: Structure of retinol and b-carotene.
9 Other carotenoids, for example, b-cryptoxanthin, a-carotene, lycopene, lutein and zeaxanthin are also present in plants. The first two of these carotenoids have a b-ionone ring at only one end of the molecule and hence yield only one molecule of retinol upon hydrolysis but the latter three are not metabolised to retinol and are therefore not classified as pro-vitamin A carotenoids (Collins and Mao, 1999).
10 Therapeutically useful retinol analogues include the naturally occurring 13-cis- retinoic acid (RA) (also known as isotretinoin, used orally to treat severe acne), and the synthetic aromatic retinoids such as etretinate (the ethyl ester of the trimethylmethoxyphenyl homologue of all-trans-RA) and its de-esterified metabolite etretin.
11 The Expert Committee on Vitamins and Minerals (EVM) published a report in 2003 that included a review of vitamin A. Vitamin A was defined as “… a group of fat-soluble compounds known as ‘the retinoids’. Generally, their structure consists of a b-ionone ring, a conjugated isoprenoid side chain and a polar terminal group.” Vitamin A can be expressed on a weight basis “as Retinol Equivalents (1 RE = 1 μg retinol = 1.78 mg retinyl palmitate = 6 mg b-carotene = 12 mg other carotenoids with provitamin A activity = 3.33 International Units (IU) vitamin A activity from retinol”. In this Statement, in quoting or describing literature where IU are used, the original values have been multiplied by 0.3 to convert them into RE for consistency.
12 The EVM statement was based on an original detailed review, published in 2002.
Previous evaluations
13 Previous evaluations of vitamin A have been carried out by EFSA (2002; 2015) and EVM (2002; 2003). EFSA set a Tolerable Upper Intake Level (TUL) for preformed vitamin A of 3,000 µg RE/day for women of childbearing age and men, based on the risk of hepatotoxicity and teratogenicity, which was proposed to also apply during pregnancy and lactation. This was based on the work of Rothman et al. (1995). EVM (2002, 2003) did not recommend a maximum level of intake but, in the context of bone health, considered that an intake greater that 1,500 µg/day was “inappropriate”.
14 The US Institute of Medicine Panel on Micronutrients (2001) also derived a UL, of 3,000 µg RE/day for women of childbearing age, using a no-observed-adverse effect level (NOAEL) of 4,500 µg RE/day and an uncertainty factor of 1.5, also based upon the work of Rothman et al. (1995), Martinez-Frias & Salvador (1990), Dudas & Czeizel (1992), Khoury et al. (1996), Shaw et al. (1996), Mills et al. (1997) and Czeizel & Rockenbauer (1997). The uncertainty factor (UF) of 1.5 was selected on the basis of inter-individual variability in susceptibility. A higher UF was not considered justified as data showed no adverse effects up to 3,000 µg/day of vitamin A supplements in human studies.
15 The COT last considered vitamin A, in relation to the diet of infants and children aged 1 – 5 years, in 2017. The Committee concluded that the Tolerable Upper Level (TUL) of 200 µg RE/kg bw/day derived by themselves, based on a LOAEL of 800 µg RE/kg bw/day for an endpoint of bulging fontanelles, “…was appropriate to evaluate the effect of vitamin A exposures in infants. However, no TULs could be established for the ages 12 - 60 months on the basis of the available data. Comparisons were therefore made with a conservative TUL based on teratogenic effects in adults. High-level consumers are approaching, or exceeding levels of vitamin A reported in the literature as causing toxicity and the possibility of adverse effects from these levels cannot be excluded. However, if effects did occur, they would only be in a small proportion of consumers. Though the data on liver consumption are limited, frequent consumption could be a cause for concern and the current Government recommendation that infants over six months old should not have more than one portion of liver per week is appropriate.”
16 The World Health Organisation (WHO, 2021) recommend that vitamin A supplementation be given to pregnant women only in areas where vitamin A deficiency is a severe public health problem, to prevent night blindness, i.e. if ≥5 % of women in a population have a history of night blindness in their most recent pregnancy in the previous 3–5 years that ended in a live birth, or if ≥20 % of pregnant women have a serum retinol level <0.70 µmol/L. Vitamin A supplementation in HIV-positive pregnant women is not recommended as a public health intervention for reducing the risk of mother-to-child transmission of HIV as there is no clear indication of benefit. Vitamin A supplementation in postpartum women, for the prevention of maternal and infant morbidity and mortality, is also not recommended as there is little or no evidence for any benefit.