Assessment
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.Assessment
Identity and characterisation
14. The following information was provided on the substance:
-
Chemical Name: Calcium tert-butylphosphonate
-
Synonym: Calcium t-butylphosphonate
-
CAS Registry Number: 81607‐35‐4
-
Molecular Formula: C4H11CaO4P
-
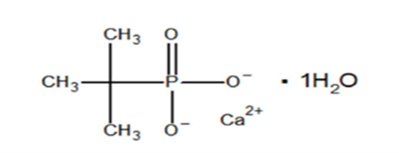
Structural Formula:
- Molecular Weight: 194.2 g/mol
Description and Intended Use
15. Members assessed the description and intended use of the product (in contact with all types of food (i.e. dry, aqueous, acidic, alcoholic, and fatty), including infant formula and human milk) and were satisfied with the additional information provided clarifying that the infant formula packaged in polyolefin-based food contact materials and articles containing calcium tert-butylphosphonate may be in both liquid (ready to be consumed as-is) or solid (intended to be reconstituted from a dry powder before consumption) state.
Spectroscopy data
16. The data (nuclear magnetic resonance (NMR) spectroscopy and attenuated total reflection infrared (ATR-IR) spectroscopy) provided by the Applicant to confirm the identity of the food contact substance was evaluated by the FCMJEG. They were content with the reasoning behind the selection of spectroscopic techniques and the data presented by the Applicant.
Manufacturing details
17. Calcium tert-butylphosphonate is made via a three-step manufacturing process. It has been agreed by the FSA to keep details of the raw materials used in the manufacturing process confidential.
18. The FCMJEG were satisfied with the manufacturing details provided by the Applicant.
19. The Applicant provided information on analysis of impurities present within the substance. The FCMJEG were satisfied with the information provided.
Purity Specifications
20. Internal specifications for calcium tert‐butylphosphonate, as sold, including purity and residual per cent water, plus known impurity concentrations, were submitted.
21. Overall, it was agreed that the information provided for specifications was satisfactory.
% Purity of the Product
22. Calcium tert‐butylphosphonate is manufactured to a minimum purity specification of 96%.
23. This specification was checked by gas chromatography (GC).
Test results for % purity of three production batches were provided by the Applicant. The FCMJEG reviewed and accepted the methods and purity data; a triplicate percentage purity measurement was provided. An average purity of 99.6% was reported in the Certificate of Analysis (CoA).
Physical and Chemical Properties of Substance
Melting Point
24. Not applicable – calcium tert-butylphosphonate decomposes at high temperatures.
Boiling Point
25. Not applicable – calcium tert-butylphosphonate decomposes at high temperatures.
Decomposition Temperature
26. Calcium tert-butylphosphonate is expected to remain stable under the intended conditions of use.
27. The decomposition temperature of calcium tert‐butylphosphonate is 468°C in air and 560°C in nitrogen (N2) atmosphere. One molecule of water of hydration is removed at 190°C.
Solubility (g/L)
28. The solubility of calcium tert‐butylphosphonate in water is approximately 0.411 g/L.
29. The Applicant confirmed that the solubility of calcium tert-butylphosphonate in water is very low but not zero. Any dissolved salt will consist of fully solvated Ca2+ cations and tert-butylphosphonate dianions. Its aqueous solubility was described by the Applicant. The determination was by reference to the concentration of phosphonate dianion in a saturated solution of calcium tert-butylphosphonate determined by liquid chromatography-mass spectrometry (LC/MS) in negative ionisation mode. External calibration was against standards of disodium tert-butylphosphonate, which is very soluble in water at room temperature. A stoichiometric correction from phosphonate dianion to calcium tert-butylphosphonate was made.
30. Calculations for determining the concentration of calcium tert-butylphosphonate from the chromatograms and calibration curve were provided by the Applicant.
31. The FCMJEG considered the analytical method used to determine the solubility of calcium tert-butylphosphonate in water. The Applicant provided additional information, which was found to be satisfactory.
Octanol/water partition (log Po/w)
32. The log Po/w of calcium tert-butylphosphonate is -2.5. Full analytical method parameters were provided.
33. The log Po/w provided by the Applicant would suggest that the compound is very water soluble, yet the Applicant goes on to state that its lack of aqueous solubility meant it would be unlikely to migrate. When requested to clarify whether the log Po/w was indeed -2.5, therefore indicating hydrophilicity the Applicant explained that when dehydrated, solubility of the compound decreases, it thereby becoming hydrophobic. The FCMJEG accepted the Applicant’s explanation.
Chemical Properties
34. Information on the following chemical properties was provided:
-
Nature: Calcium tert‐butylphosphonate is pH-neutral.
-
Reactivity: Calcium tert‐butylphosphonate is an additive, which does not react with the plastic in which it is incorporated. On this basis, no reaction products are anticipated by the Applicant to be present in the substance, per se, or when calcium tert‐butylphosphonate is used as intended. Furthermore, as this substance is not polymeric in nature, no oligomeric fraction would be present.
-
Stability: Calcium tert‐butylphosphonate is stable under the intended conditions of use.
-
Hydrolysis: Calcium tert‐butylphosphonate is not expected to hydrolyse under the intended conditions of use.
35. The Applicant stated that “phosphonate bonds are very stable chemically and will not be reactive under the conditions of food contact in polyolefins”.
36. It was also noted that “alkylphosphonic acids are typically 2-3 pKa units more acidic than the corresponding carboxylic acids. Thus, the metal salts from these acids cannot be dissociated by typical carboxylic acids in food simulants, i.e. acetic acid in water.” The Applicant cited Franz (2001) to support this assessment.
-
Intentional decomposition/transformation: Calcium tert‐butylphosphonate is not intended to decompose or transform under the proposed conditions of use.
-
Unintentional decomposition/transformation: Calcium tert‐butylphosphonate is not expected to unintentionally decompose or transform under the proposed conditions of use.
37. The Applicant stated that, in support of stability during use, a thermogravimetric analysis (TGA) of the substance had been provided in the original submission and the FCMJEG concurred with the conclusions.
-
Interaction with food substances: Calcium tert‐butylphosphonate is not expected to interact with food substances.
38. The Applicant stated that “The molecule is highly insoluble in most solvents, is stable chemically, and is not easily hydrolysed in the presence of food substances”.