Third Draft Statement on the effects of excess Vitamin A on maternal health
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
1. The Scientific Advisory Committee on Nutrition (SACN) last considered maternal diet and nutrition in relation to offspring health in its reports on ‘The influence of maternal, fetal and child nutrition on the development of chronic disease in later life’ (SACN, 2011) and on ‘Feeding in the first year of life’ (SACN, 2018). In the latter report, the impact of breastfeeding on maternal health was also considered.
2. In 2019, SACN agreed to conduct a risk assessment on nutrition and maternal health focusing on maternal outcomes during pregnancy, childbirth and up to 24 months after delivery; this would include the effects of chemical contaminants and excess nutrients in the diet.
3. SACN agreed that, where appropriate, other expert Committees would be consulted and asked to complete relevant risk assessments e.g. in the area of food safety advice. This subject was initially discussed during the horizon scanning item at the January 2020 meeting with a scoping paper being presented to the Committee in July 2020. This included background information on a provisional list of chemicals proposed by SACN. It was noted that the provisional list of chemicals was subject to change following discussion by COT who would be guiding the toxicological risk assessment process: candidate chemicals or chemical classes can be added or removed as the COT considered appropriate. The list was brought back to the COT with additional information in September 2020 TOX-2020-45 Maternal diet scoping paper (food.gov.uk). Following a discussion at the COT meeting in September 2020, it was agreed that papers on a number of components should be prioritised and to this end, papers on iodine, vitamin D and dietary supplements have been or will be presented to the Committee. The remaining list of compounds were to be triaged on the basis of toxicity and exposure. The current paper presents information intended to aid this process.
4. First and second draft Statements were prepared and presented to the COT in December 2021 and February 2022. A number of requests for clarification were made and the Committee requested a third draft Statement be presented to them in due course. Therefore, this current Statement seeks to address the points raised with new text being highlighted.
5. The Committee requested that additional studies (Piersma et al. (2006), EFSA (2006), Ritchie et al. (1998) and Wiegand et al. (1998)) should be addressed in the oral exposure section of the statement.
6. Re-wording of some paragraphs was requested for clarity and some references should be updated.
7. The Committee requested that more information on the Omenn et al. (1996) study and more clarification on the weight of evidence suggesting that beta carotene had not been responsible for the effects observed.
8. It was suggested that alternative text should be provided to explain the significance of ghee exposure in Asian populations.
9. It was suggested that more detail should be given on the methodology used to derive exposure calculations and more emphasis on the uncertainty of liver exposure in paragraph 118.
10. It was agreed that all aspects of dermal kinetics should be merged in one section with a focus on the aspects of systemic exposure via the dermal route.
Questions for the Committee
11. The Committee are asked to consider the following questions.
a) Does the Committee have any comment on the additional information in this draft Statement?
b) Does the Committee have any comments on the structure or content of the draft Statement?
Secretariat
March 2022
TOX/2022/18 Annex A
Introduction
1 The Scientific Advisory Committee on Nutrition (SACN) last considered
maternal diet and nutrition in relation to offspring health in its reports on ‘The influence of maternal, fetal and child nutrition on the development of chronic disease in later life’ (SACN, 2011) and on ‘Feeding in the first year of life’ (SACN, 2018). In the latter report, the impact of breastfeeding on maternal health was also considered.
2 In 2019, SACN agreed to conduct a risk assessment on nutrition and maternal health focusing on maternal outcomes during pregnancy, childbirth and up to 24 months after delivery; this would include the effects of chemical contaminants and excess nutrients in the diet.
3 SACN agreed that, where appropriate, other expert Committees would be consulted and asked to complete relevant risk assessments e.g. in the area of food safety advice. This subject was initially discussed by COT during the horizon scanning item at the January 2020 TOX-2020-45 Maternal diet scoping paper (food.gov.uk) meeting with a scoping paper being presented to the Committee in July 2020. This included background information on a provisional list of chemicals proposed by SACN. It was noted that the provisional list of chemicals was subject to change following discussion by COT who would be guiding the toxicological risk assessment process: candidate chemicals or chemical classes can be added or removed as the COT considered appropriate. The list was brought back to the COT with additional information in September 2020 Following a discussion at the COT meeting in September 2020, it was agreed that papers on a number of components should be prioritised and to this end, papers on iodine, vitamin D and dietary supplements have been or will be presented to the Committee. The remaining list of compounds were to be triaged on the basis of toxicity and exposure. The current paper presents information intended to aid this process.
4 Following discussion of the first prioritisation paper TOX-2020-45 Maternal diet scoping paper (food.gov.uk) on substances to be considered for risk assessment by the COT, the Committee decided that Vitamin A should be considered in a specific paper.
Current UK Government and International advice
5 UK Government advice as given on the NHS.uk website lists good sources of vitamin A as cheese, eggs, oily fish, fortified low-fat spreads, milk, yoghurt and liver and liver products such as pate (NHS, 2021). Good sources of β-carotene in addition to yellow, red or green (leafy) vegetables are carrots, sweet potatoes, red peppers and spinach and fruit such as mango, papaya and apricots.
6 UK Government dietary advice, as given on the NHS website, recommends that pregnant women, or women thinking about having a baby, should not consume liver or liver products such as pate, or supplements that contain vitamin A, including fish liver oil, unless advised by a GP, to avoid potential harm to the unborn child.
7 The World Health Organisation (WHO, 2021) recommend that vitamin A supplementation be given to pregnant women only in areas where vitamin A deficiency is a severe public health problem, to prevent night blindness. WHO’s recommendations are considered in more detail in paragraph 16 below.
Background
Structure and sources
8 Vitamin A is a retinoid, consisting of an alicyclic b-ionone ring and a 9-carbon-long isoprenoid side chain. Retinoids are derived from a family of pro-vitamin A carotenoids, the major one being b-carotene. On cleavage, one molecule of b-carotene yields two molecules of retinol. Retinol (vitamin A1 or vitamin A alcohol) and 3-dehydroretinol (vitamin A2, which has 40% of the biological activity of vitamin A1) occur in foods of animal origin and b-carotene is found in red and yellow and leafy green vegetables. (Bowman and Rand, 1982) (Figure 1, Merck Index, 1996).
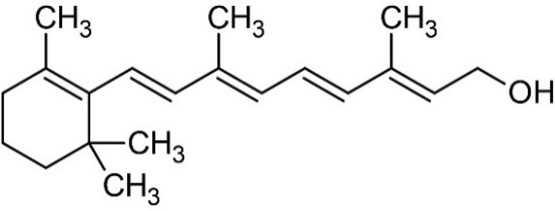
Retinol
b-carotene
Figure 1: Structure of retinol and b-carotene.
9 Other carotenoids, for example, b-cryptoxanthin, a-carotene, lycopene, lutein and zeaxanthin are also present in plants. The first two of these carotenoids have a b-ionone ring at only one end of the molecule and hence yield only one molecule of retinol upon hydrolysis but the latter three are not metabolised to retinol and are therefore not classified as pro-vitamin A carotenoids (Collins and Mao, 1999).
10 Therapeutically useful retinol analogues include the naturally occurring 13-cis-retinoic acid (also known as isotretinoin, used orally to treat severe acne), and the synthetic aromatic retinoids such as etretinate (the ethyl ester of the trimethylmethoxyphenyl homologue of all-trans-retinoic acid) and its de-esterified metabolite etretin.
11 The Expert Committee on Vitamins and Minerals (EVM) published a report in 2003 that included a review of vitamin A. Vitamin A was defined as “… a group of fat-soluble compounds known as ‘the retinoids’. Generally, their structure consists of a b-ionone ring, a conjugated isoprenoid side chain and a polar terminal group.” Vitamin A can be expressed on a weight basis as Retinol Equivalents (1 RE = 1 μg retinol = 1.78 mg retinyl palmitate = 6 mg b-carotene = 12 mg other carotenoids with provitamin A activity = 3.33 International Units (IU) vitamin A activity from retinol”. In this Statement, in quoting or describing literature where IU are used, the original values have been multiplied by 0.3 to convert them into RE for consistency.
12 The EVM statement was based on an original detailed review, published in 2002.
Previous evaluations
13 Previous evaluations of vitamin A have been carried out by EFSA (2002, 2015) and EVM (2002, 2003). EFSA set a Tolerable Upper Intake Level (TUL) for preformed vitamin A of 3,000 µg RE/day for women of childbearing age and men, based on the risk of hepatotoxicity and teratogenicity, which was proposed to also apply during pregnancy and lactation. This was based on the work of Rothman et al. (1995). EVM (2002, 2003) did not recommend a maximum level of intake but, in the context of bone health, considered that an intake greater that 1,500 µg/day was “inappropriate”.
14 The US Institute of Medicine Panel on Micronutrients (2001) also derived a UL of 3,000 µg RE/day for women of childbearing age, using a no-observed-adverse effect level (NOAEL) of 4,500 µg RE/day and an uncertainty factor of 1.5, also based upon the work of Rothman et al. (1995), Martinez-Frias & Salvador (1990), Dudas & Czeizel (1992), Khoury et al. (1996), Shaw et al. (1996), Mills et al. (1997) and Czeizel & Rockenbauer (1997). The uncertainty factor (UF) of 1.5 was selected on the basis of inter-individual variability in susceptibility. A higher UF was not justified as data showed no adverse effects up to 3,000 µg/day of vitamin A supplements in human studies.
15 The COT last considered vitamin A in relation to the diet of infants and children aged 1 – 5 years, in 2017. The Committee concluded that the Tolerable Upper Level (TUL) of 200 µg RE/kg bw/day derived by themselves, based on a LOAEL of 800 µg RE/kg bw/day for an endpoint of bulging fontanelles, “…was appropriate to evaluate the effect of vitamin A exposures in infants. However, no TULs could be established for the ages 12 - 60 months on the basis of the available data. Comparisons were therefore made with a conservative TUL based on teratogenic effects in adults. High-level consumers are approaching or exceeding levels of vitamin A reported in the literature as causing toxicity and the possibility of adverse effects from these levels cannot be excluded. However, if effects did occur they would only be in a small proportion of consumers. Though the data on liver consumption are limited, frequent consumption could be a cause for concern and the current Government recommendation that infants over six months old should not have more than one portion of liver per week is appropriate.”
16 The World Health Organisation (WHO, 2021) recommend that vitamin A supplementation be given to pregnant women only in areas where vitamin A deficiency is a severe public health problem, to prevent night blindness, ie if ≥5 % of women in a population have a history of night blindness in their most recent pregnancy in the previous 3–5 years that ended in a live birth, or if ≥20 % of pregnant women have a serum retinol level <0.70 µmol/L. Vitamin A supplementation in HIV-positive pregnant women is not recommended as a public health intervention for reducing the risk of mother-to-child transmission of HIV as there is no clear indication of benefit. Vitamin A supplementation in postpartum women, for the prevention of maternal and infant morbidity and mortality, is also not recommended as there is little or no evidence for any benefit.
Functions
17 Retinol performs many important physiological functions in animals. It is involved with the synthesis of collagen and elastin fibres by fibroblasts, as well in cell division, and differentiation and the functioning of skin and mucous membranes. Vitamin A influences bone development by regulating the activities of osteoblasts and osteoclasts. It decreases the secretion of thyroxine from the thyroid gland by suppressing production of thyrotropin by the pituitary gland. Vitamin A also stimulates the immune system and hence improves resistance to infections. (Rutkowski and Grzegorczyk, 2012)
18 Vitamin A is an antioxidant: the conjugated C = C bonds in the side chain are oxidised by reactive oxygen species (ROS) and free radicals and thus protect those bonds in the polyunsaturated fatty acids in cell membrane lipids. This also protects against uncontrolled oxidation of the glycosyl residues of proteins in cell membranes, which can play a role in neoplastic transformation (promotion). The antioxidative function also stabilises thiol groups (–SH) of membrane proteins and suppresses oxidatively stimulated expression of the c-myc oncogene. Epithelial cells in particular are protected against oxidative damage by vitamin A, including tissues of the nasal and throat cavity, oesophagus, stomach, intestines, respiratory tract, bladder, and prostate. (Rutkowski and Grzegorczyk, 2012)
19 Retinol is oxidised to retinal (vitamin A aldehyde or retinaldehyde) which, as its 11-cis isomer, functions as an essential component in the process of visual signal transduction in the retina, in the pigment rhodopsin in the rods as well as the pigments in the cones. (EVM, 2002).
20 Retinal is further oxidised to retinoic acid (RA) and thence undergoes further side-chain isomerisation and oxidation into a range of different products. Retinoic acid has pleiotropic effects in development and is well documented in the literature as a teratogen (Collins and Mao, 1999).
21 Retinoids are used clinically to treat a range of disorders from skin lesions and cancers and a range of synthetic analogues with enhanced receptor specificities and pharmacokinetic profiles has been developed in order to maximise the benefits of treatment while ameliorating their toxicity. Barnard et al. (2009) reviewed the design and structure of a wide range of synthetic retinoids with modified beta ionone heads, isoprenoid chains and hydrophilic end groups to explore this pharmacological space.
Mechanism of action.
22 The majority of the effects of ingested vitamin A are thought to be mediated by the action of retinoic acid. Since retinoic acid is produced endogenously and combines with specific nuclear receptor proteins that bind to DNA and regulate the expression of various genes, it is classified as a hormone. It is a ligand for specific nuclear receptors, the most studied of which are retinoic acid receptor (RAR) and retinoid X receptor (RXR), that regulate the transcription of numerous target genes, as homo- or hetero-dimers. More than 500 genes are known to be regulated by retinoic acid, many of which control embryonic development. Retinoic acid signalling is turned off by ligand degradation by cytochrome P450 (CYP) enzymes, such as CYP26A1.
23 Das et al. (2013) and Huang et al. (2014) detail the molecular biology and functions of the retinoid receptors. Studies have shown that RA plays a crucial role during skeletal development and in ensuring the suppression of left-right asymmetries during developmental pattern formation in embryos. RA is not produced by all cells of the body at all stages of development but is produced in a unique spatiotemporal pattern which orchestrates development. Further molecular detail is provided in paper TOX-2021-44
24 In preparation for implantation of the fertilised ovum, progesterone released from the corpus luteum causes the cells of the superficial layer of the endometrium to enlarge and compact. These cells are known as decidual cells because they are shed after birth and the process is decidual transformation (Bowman and Rand, 1982). Ozaki et al (2017) showed that decidual transformation of human endometrial stromal cells (HESCs) resulted in reprogramming of the retinoic acid signalling and metabolic pathways. The authors concluded that the data showed that decidualizing HESCs silence retinoic acid signalling by downregulating key cytoplasmic binding proteins and by increasing retinoid metabolism. However, excessive retinoic acid exposure is toxic for decidual cells and triggers a response that may lead to pregnancy failure. Further molecular detail is provided in TOX-2021-44
25 Both deficiency and excess of retinoic acid causes the ectopic induction and the down regulation of many genes as a prelude to changing the morphology of the embryo. For example, excess retinoic acid causes the chick limb bud to develop six digits instead of the normal three (Tamura et al 1997). Conversely, quail embryos deficient in retinoic acid have down-regulated genes, but also express ectopically induced genes, probably by the down-regulation of a repressor, also leading to limb malformations Stratford et al. (1999).
26 The hindbrain of embryos is also profoundly affected by vitamin A. Retinoic acid administration to mouse embryos induced the hindbrain Hox genes in an altered expression pattern which resulted in an altered morphology, with the seven structures in this tissue known as rhabdomeres developing in the wrong order, leading to malfunction of the whole brain region. Marshall et al. (1992).
27 Carazo et al. (2021) reviewed the forms, sources, detection, kinetics, function, deficiency, therapeutic use and toxicity of vitamin A. They concluded that “Given the importance of vitamin A in multiple crucial physiological processes, its deficiency can pose a serious health challenge, even leading to death in the most serious cases. At the same time, it can lead to serious health issues in high-dose situations.”
Absorption, distribution, metabolism and excretion
EFSA (2015) state that preformed vitamin A is efficiently absorbed in humans (70 – 90 % in children). The absorption of β-carotene appears to be highly variable (5 – 65 %), depending on food- and diet-related factors, genetic characteristics and the health status of the subject. Carazo et al. (2021), in their review of retinoid kinetics, found that all-trans RA had low bioavailability after oral administration and its high affinity to plasma proteins led to it being transported in the blood bound to albumin. Isotretinoin had a bioavailability of around 20 % and was also extensively bound to albumin in plasma. Tissue concentrations were usually lower than that in plasma. Etretinate and acitretin (etretin) had a bioavailability of approximately 50%.
28 Goodman et al. (1984) studied the human plasma kinetics of both retinol and its metabolites, retinyl palmitate and retinyl stearate following oral administration of retinol. Retinoid plasma kinetics were studied on the first day of treatment, at weeks 2 and 4, and every 2-3 months thereafter as long as the patient remained on therapy. Plasma retinol concentration did not change significantly up to 24 hours after a single oral dose of retinol (p > 0.05). The plasma concentration of retinyl palmitate and retinyl stearate markedly increased with a mean time to peak plasma concentration of 4.3 ± 0.7 hours. Retinyl palmitate disappearance from the plasma had an initial phase half-life of 2.2 ± 0.9 hours. The terminal phase half-life appeared prolonged and could not be accurately determined. Retinyl stearate was detected in the plasma of all patients with plasma concentrations paralleling and ranging from 20 % to 40 % of those of retinyl palmitate. With prolonged retinol administration, peak plasma retinyl palmitate concentrations increased with both increasing retinol dose (p <0.001) and increasing duration of treatment (p = 0.001).
29 Buss et al. (1994) dosed 10 healthy female volunteers with 5 different doses of vitamin A and studied the effects on plasma vitamin A and its metabolites. The single supplements were provided as either retinyl palmitate (15,000 and 45,000 mg RE) or an equivalent dose in fried calf liver. Blood was collected at intervals over the first 12 hours of dosing and thereafter for 6 days. The results showed substantial increases in plasma retinyl palmitate, 13-cis- and all-trans-retinoic acid, and 13-cis and all-trans-4-oxoretinoic acid. Women who received the supplement had significantly higher concentrations of retinoids than those who received the liver, possibly because the food matrix may have ameliorated the absorption rate or altered the circulating forms of vitamin A. However, plasma retinol changed only slightly, which supported the view that this method was not an appropriate means by which to evaluate a vitamin A supplementation trial.
30 Hartmann et al. (2005) evaluated plasma concentration-time curves of retinyl esters, retinol and their metabolites at increasing doses of vitamin A in 3 groups (12 per group) of non-pregnant women aged 18 - 40 years. received once daily oral doses of vitamin A palmitate up to 30,000 IU (9,000 mg RE)/day over 21 days. The area under the plasma concentration-time curve (AUC (24 hours)) served as indicator for exposure. The AUC (24 hours) of retinyl esters increased linearly with dose. Retinol concentrations were unaffected. All-trans RA exhibited a diurnal-like concentration-time profile (Maximum blood concentration (Cmax) at 3 hours; minimum blood concentration (Cmin) at 8 hours), concentrations decreasing below pre-dose levels at 5 hours and regaining pre-dose levels at 16 hours. The maximum temporary increase in exposure was 33 % (single dose) and 19 % (repeated doses) above baseline, but the AUC (24h) remained unaltered. The AUC (24h) increased linearly with dose for 13-cis RA and 13-cis-4-oxo RA. Repeated doses caused a 25 % increase in exposure with the highest vitamin A intake. Accumulation of 13-cis-4-oxo RA at 30,000 IU (9,000 mg RE)/day doubled compared to the 4,000 IU (1,200 mgRE)/day intake.
31 Spiegler et al. (2012) reviewed the absorption, distribution, metabolism and excretion of vitamin A in animals. Nearly all retinyl esters in the diet are hydrolysed to retinol in the intestinal lumen. Retinol is absorbed by intestinal epithelial cells, where it is re-esterified to long-chain fatty acids, primarily by the enzyme lecithin:retinol acyltransferase (LRAT), which is widely expressed in tissues, and is incorporated into chylomicra, which circulate in the intestinal lymph before moving into the general circulation. Once in the general circulation, lipoprotein lipase (LPL), which is bound to the luminal surface of the vascular endothelium, catalyses the lipolysis of triglycerides to generate free fatty acids and chylomicron remnants. Chylomicron remnants are cleared mainly by the liver, but extrahepatic uptake of the remnants may be important in the delivery of vitamin A to mammary tissue, bone marrow, adipose tissue, and spleen. Retinyl esters in serum are normally below 0.2 mmol/l in the fasting state but they increase significantly after a large influx of vitamin A, such as occurs after a vitamin A–rich meal.
32 In the liver, retinyl esters are again hydrolysed to retinol to be transferred to hepatic stellate cells and then re-esterified by LRAT for storage. Alternatively, retinol can bind to retinol-binding protein (RBP) and be secreted into the bloodstream as a 1:1 molar complex with the serum protein transthyretin. RBP thus functions to mobilise hepatic retinoid stores and deliver retinol to peripheral tissues and developing embryos. In fasting conditions, retinol-RBP accounts for approximately 95 – 99 % of all serum retinoids. Upon vitamin A intake, the concentration of retinoids in chylomicrons and chylomicron remnants can greatly exceed that of plasma retinol. Blood levels of retinol-RBP in both humans and animals are tightly controlled, except in extreme cases of insufficient intake of vitamin A, protein, calories and zinc; or in response to hormonal factors, stress; and in certain disease states.
33 Spiegler et al. (2012) also stated that the mechanisms that regulate the secretion of the complex retinol-RBP from the liver have yet to be fully elucidated. Even in the fasting state there are low concentrations of RE that are associated with circulating lipoproteins (in VLDL and LDL) and small amounts of circulating retinoic acid bound to albumin. Within cells, retinol is reversibly oxidized to retinal by members of the alcohol dehydrogenases, medium-chain dehydrogenase/reductases, retinol dehydrogenases and short-chain dehydrogenase/reductases. Retinal is further oxidized to retinoic acid by retinal dehydrogenases. Several intracellular binding proteins for retinol, retinal and retinoic acid have been identified and characterised, including cellular retinol-binding proteins I, II and III, cellular retinaldehyde binding protein and cellular retinoic acid-binding proteins I and II. Each of these retinoid-binding proteins has a distinct expression pattern and plays a specific role in vitamin A transport and metabolism.
34 The metabolism of pre-formed vitamin A in well-nourished people has been studied by several research groups. To control vitamin A deficiency, large therapeutic doses are administered in developing countries to women and children, who are often undernourished. Nevertheless, little attention has been given to the short-term kinetics (ie, after absorption but before storage) of a large dose of vitamin A or to the short- and long-term effects of such a dose given to lactating women on serum and breastmilk concentrations of retinol and its metabolites. Moreover, appropriate dosing regimens have not been systematically evaluated to ascertain the quantitative improvement in vitamin A status of the women and children who receive these supplements. The authors concluded that further research was needed to ascertain the areas of the world in which subclinical toxicity exists and to evaluate its effects on overall health and well-being. (Spiegler et al, 2012).
35 Nau (1995), in review, found that activation (oxidation of retinoic acids: hydrolysis of glycoconjugates) and deactivation reactions (isomerisation from trans- into cis- configuration; b-glucuronidation) appeared to relate to retinoid-induced teratogenesis. The b-glucuronides of retinoic acids showed poor placental transfer and prolonged presence in maternal animals. The observed low teratogenic potency of 13-cis-retinoic acid in the rat and mouse may have been explained by limited placental transfer, rapid plasma clearance and extensive metabolic detoxification; conversely, the high teratogenic activity of this retinoid in the monkey (and possibly humans) could be the result of more extensive placental transfer, slower plasma clearance and extensive metabolism to the active 4-oxo-metabolite. There is evidence that non-retinoid compounds such as antiepileptic agents appeared to exert some of their teratogenicity via alteration of endogenous retinoid levels.
36 Söderlund et al. (2005) measured serum concentrations of all-trans retinoic acid and 13-cis retinoic acid in newborns and their mothers and in women in the first trimester of pregnancy. The newborns had significantly lower retinol concentrations (1.0 mmol/L) than did their mothers (1.7 mmol/L; p = 0.013). Serum all-trans retinoic acid was also significantly lower in the newborns (3.4 nmol/L) than in their mothers (5.8 nmol/L; p = 0.008). Serum concentrations of 13-cis retinoic acid were significantly lower in the newborns (2.0 nmol/L) than in their mothers (2.6 nmol/L; p = 0.005). The serum concentrations of retinol did not accurately reflect the concentrations of the biologically active derivative all-trans retinoic acid. Pregnant women and those in childbirth had significantly lower serum concentrations of retinol than control subjects. The concentration of all-trans retinoic acid was higher in the parturient mothers than in the control subjects, but concentrations of 13-cis retinoic acid were lower than in the controls or pregnant women. No difference was observed in the concentrations of all-trans and 13-cis retinoic acid between pregnant women and control women.
37 Brazzell and Colburn (1982) studied the pharmacokinetics of orally administered isotretinoin and etretinate. The pharmacokinetic profile of isotretinoin exhibited linear pharmacokinetics. The drug was rapidly absorbed, highly bound to plasma protein, and metabolized to 4-oxo-isotretinoin. The apparent half-lives of elimination of isotretinoin and 4-oxo-isotretinoin following the oral administration of isotretinoin were 10 to 20 hours and 24 to 29 hours, respectively. Steady-state pharmacokinetic profiles in patients were consistent with the single-dose pharmacokinetics in normal subjects. Oral etretinate underwent first-pass biodegradation to its corresponding carboxylic acid, which appeared rapidly in the circulation, often earlier than the parent drug, and its plasma concentration was usually comparable to, or greater than, that of the parent drug. The apparent elimination rates of drug and metabolite were similar (6 to 13 hours) following a single dose, suggesting that metabolite elimination may be formation-rate limited. During multiple dosing of etretinate, a very slow terminal elimination phase was observed which was not seen after single-dose administration. The prolonged half-life of this phase suggested accumulation in a deep tissue compartment. Differences between the two retinoids were thought to reflect their differing physicochemical properties.
38 In addition to retinoid intake via the oral route, women of childbearing age may also be exposed via dermal application of medication for the treatment of skin conditions such as acne. Although not dietary, topical treatments have the potential to contribute to overall exposure to vitamin A and its derivatives, therefore, topical absorption data are provided below.
39 Willhite et al. (1990, from abstract) found that a single application of 17 µg/kg or 8.7 mg/kg of radiolabelled all-trans-[10,11-3H2]-retinoic acid dissolved in acetone to shaved dorsal hamster skin was rapidly absorbed and showed a dose-dependent rate of elimination. An equation describing a two-compartment open model with a very brief lag time and first-order uptake and elimination was used to describe the central plasma compartment kinetics. Unchanged all-trans-retinoic acid represented up to 4 % of the total circulating radioactivity. Peak circulating concentrations of parent all-trans-retinoic acid were less than those observed after an equivalent oral dose, but prolonged absorption from the skin contributed to high total bioavailability of retinoid applied topically.
40 Latriano et al. (1997) dosed 28 subjects in four treatment groups with a single dermal dose of tritiated tretinoin (all-trans retinoic acid) in a 0.05 % formulation of emollient cream or cream alone or with 28 days of repeated nonradioactive doses. In a second study, subjects received single topical doses of tritiated tretinoin cream alone (n = 5) or after 1 year of nonradioactive applications (n = 4). Plasma, urine, and faecal samples were analysed to determine absorption. Plasma samples in study 1 were also analysed for concentrations of tretinoin and its metabolites. Percutaneous absorption of tretinoin was approximately 2 % after a single dose and after 28 days of daily application. In patients receiving long-term therapy (>1 year), absorption averaged 1.1 %. Mean plasma concentrations of tretinoin after 28 days of treatment with either tretinoin emollient cream or tretinoin cream were not significantly changed when compared with the corresponding endogenous concentrations before treatment. Neither single dose nor long-term treatment with topical tretinoin formulations appeared to affect the endogenous levels of tretinoin or its metabolites.
41 Nohynek et al. (2005) investigated the effect of topical vitamin A on human endogenous plasma levels of Vitamin A and its metabolites. Two groups of 14 female volunteers of child-bearing age were kept on a vitamin A-poor diet and treated topically for 21 days with creams containing 0.30 % retinol or 0.55 % retinyl palmitate on approximately 3,000 cm2 of their body surface area. This gave a total dose of approximately 30,000 IU (9,000 mg RE) vitamin A/subject/day. After a 12-day wash-out period, the study groups received single oral doses of 5,600 and 16,800 mg retinyl palmitate (RP), corresponding to the maximal EU allowance during pregnancy or three-times higher (3000 and 9000 mg RE), respectively. Blood samples were collected over 24 hours on study days -3 (pre-study), 1, 21 (first and last days of topical treatment) and 34 (oral administration) at 0, 1, 2, 4, 6, 8, 12, 14-16 hours and 24 hours after treatment. Plasma concentrations of retinol (REL), retinyl palmitate (RP), retinol oleate (RO) and retinol stearate (RS), 9-cis-, 13-cis-, all-trans- (AT), 13-cis-4-oxo- or AT-4-oxo-retinoic acids (RAs) were analysed. With the exception of transient mild (RP-group) to moderate (REL-group) local irritation on the treatment sites, no adverse local or systemic effects were noted. On days 1 or 21 of topical treatment, no changes were measured in individual or group mean plasma Cmax, AUC (0 - 24 hours) or other pharmacokinetic parameters of REL, retinyl esters or RAs relative to pre-study data. In contrast, single oral doses of RP at 3,000 or 9,000 mg RE produced dose-related and sustained increases in Cmax and AUC (0 - 24 hours) values of plasma RP, RO, RS, 13-cis- and 13-cis-4-oxo-RAs, as well as a transient increase in AT-RA. Topical exposure to retinol- or retinyl ester-containing cosmetic creams at 9,000 mg RE /day and maximal use concentrations were therefore found to not affect plasma levels of retinol, retinyl esters or RAs, whereas single oral doses at 3,000 or 9,000 mg RE produced significant increases in plasma retinyl esters and RAs.
42 Retinol metabolites are excreted mainly in the urine (38 to 60 %), but also in faeces (18 to 37 %) and breath (18 to 30 %) in humans after 400 days on a vitamin A-deficient diet. Retinol is metabolised in the liver to numerous products, some of which are conjugated with glucuronic acid or taurine for excretion in bile and the amount of retinol metabolites excreted in bile increases as the liver retinol exceeds a critical concentration. Excretion of labelled retinol metabolites in bile of rats fed increasing amounts of retinol traced by [3H]-retinyl acetate was constant when hepatic retinol concentrations were low (≤ 32 µg/g (112 nmol/g) and increased rapidly (by eight-fold) as liver retinol concentration increased, up to a plateau at hepatic retinol concentration ≥ 140 µg/g (490 nmol/g) This increased biliary excretion may serve as a protective mechanism for reducing the risk of excess storage of vitamin A. (EFSA 2015)
Beta carotene
43 Allen and Heskell (2002) found no reports of high-carotene intakes from foods ever having caused vitamin A toxicity. It had been assumed that about one-third of a dose of dietary carotenoids was absorbed and half that amount was converted to retinol, resulting in a bioconversion factor of 6:1 for b-carotene to retinol. This bioconversion factor had been used in most food composition tables to convert carotenoids to retinol equivalents. However, in the early 1990’s it become apparent that absorption of carotene from plant sources, especially from vegetables, was substantially less than one-third of that absorbed from a dose given in oil. More recent estimates of b-carotene absorption from a diet consisting mainly of vegetables showed that absorption was about one half what was previously assumed. Based on such studies, the Institute of Medicine estimated that 1 retinoic acid equivalent was equal to 12 mg of b-carotene instead of the 6 mg of b-carotene estimate used previously.
Acute and chronic toxicity
44 Acute clinical features of vitamin A toxicity in age groups other than infants are lethargy, pain in the joints, dry skin, headache and nausea and vomiting, although these vary in severity. More severe signs that can diagnose hypervitaminosis A clinically, include alopecia, drowsiness, liver and bone damage and visual problems (Loughrill, 2016; SCF, 2002). In infants, the major symptom of toxicity is bulging fontanelles.
45 Symptoms of chronic toxicity include dry thickening of the skin, cracking of lips, conjunctivitis, erythematous eruption, alopecia, reduced bone mineral density, bone joint pain, chronic headache, intracranial hypertension and hepatotoxicity. Some adverse effects, for example hepatotoxicity, are regarded as reversible with withdrawal of the vitamin but others, such as deficits in the eyes and bone, are not. Kamm, 1982).
46 Penniston and Tamuihardjo (2006) found that few human studies had looked at the acute effects of a large dose of vitamin A on circulating vitamin A concentrations. Evidence suggested that intermediate effects without clinical signs of toxicity may be a growing concern, because intake from preformed sources of vitamin A often exceeded the recommended dietary allowances (RDA) for adults, especially in developed countries. Osteoporosis and hip fracture have been associated with preformed vitamin A intakes of only twice the current RDA. Assessing vitamin A status in cases of intermediate effects or frank toxicity is complicated because serum retinol concentrations are non-sensitive indicators in this range of liver vitamin A reserves.
Reproductive effects of vitamin A
47 It is now generally believed that all-trans retinoic acid (ATRA) supports both male and female reproduction as well as embryonic development. (Zile, 1998; Clagett-Dame and DeLuca, 2002; and Clagett-Dame and Knutson, 2011). This conclusion is based on the ability of RA to reverse most reproductive and developmental blocks found in vitamin A deficiency induced in experimental animals either by nutritional or genetic means, and the fact that the majority of embryonic defects arising from vitamin A deficiency are also observed in retinoic acid receptor null mutants. The differential activity of CYP26 enzymes in tissues is a key regulatory mechanism. If severely vitamin A-deficient pregnant rats are given small amounts of carotene or limiting quantities of RA early in organogenesis, embryos form but show a collection of defects called the vitamin A deficiency syndrome or late vitamin A deficiency. Vitamin A is essential for the maintenance of the male genital tract and spermatogenesis and participates in a signalling mechanism that initiates meiosis in the female gonad during embryogenesis, and in the male gonad postnatally. Both nutritional and genetic approaches have been used to elucidate the vitamin A-dependent pathways upon which these processes depend.
48 The teratogenic effects of retinoic acid have been documented both in animals and in humans (Zile, 1998). Retinoic acid induces differential patterns of malformations in mammalian embryos based on the different stages of embryonic development. Children exposed in utero to isotretinoin ingested by their mother have been found to exhibit congenital malformations, known as “the retinoic acid syndrome” (Collins and Mao, 1999). The malformations include effects on the central nervous system (hydrocephalus, anencephaly, exencephaly, spina bifida), eyes (anophthalmia, microphthalmia, defects of the retina), face (harelip, cleft palate, brachygnathia, hypoplastic maxilla), dentition, ear (absent or deformed), limb (phocomelia), urinogenital system (hypoplastic kidney, polycystic kidney, absent/hypoplastic genitalia), heart (incomplete ventricular septation, transposition of the great vessels, double aortic arch, hypoplastic aortic valves), thyroid gland (hypoplasia), and the axial skeleton (vertebral and rib fusions, extra vertebrae and ribs, hypoplastic tail). (Maden, 2001)
Animal studies
49 Within embryos of experimental animals, both too little or too much vitamin A/RA causes malformations. Rat fetuses in mothers reared on vitamin A-deficient diets show a range of anomalies known as “fetal vitamin A deficiency” (VAD) syndrome, which comprises defects of hind-brain, eye, ear, heart, lung, diaphragm, kidney, testis, limbs, and skeleton. Mice with compound null mutations of RA nuclear receptors and RA-synthesizing enzymes also have malformations resembling the VAD syndrome. Excess vitamin A/RA in humans and animal models causes malformations resembling the fetal VAD syndrome. Lee et al. (2012) investigated the effect of excess retinoic acid on the development of rodent embryonic kidneys. Both vitamin A excess and deficiency lead to lack of kidney development (bilateral renal agenesis) in the hamster and the mouse before any morphologically identifiable precursor of the organ is present. Paradoxically, the malformations observed following maternal high dose (100 mg/kg bw) RA may have been due to RA deficiency at a crucial stage in development. The mechanism appeared to be RA-induced inhibition of its own endogenous synthesis and increased expression of RA-metabolising CYP enzymes. Pleiotropic mutations resulted, many of which were ameliorated by supplementation with a lower dose of RA given to the mother after fetal clearance of the original high dose.
50 The potential adverse effects of retinoids have been assessed in animal studies using both oral and dermal routes of exposure.
Oral exposure
51 Piersma et al (1996) tested the teratogenicity of a single dose of retinyl palmitate in rats. Pregnant rats were treated at gestation day 10 by gavage with 100, 300 or 1000 mg/kg body weight retinyl palmitate on a dietary background level of 5 mg/kg feed. By gestation day 11 the number of embryos with an open cranial neural tube had increased with the dose. At gestation day 21, the high dose group showed an increase in late resorptions, whereas both the high and the medium dose groups had a high incidence of foetuses with malformations typical of retinoid embryopathy. The data suggested that delayed neural tube closure had occurred in a large proportion of the embryos. In a second experiment, the high oral dose was applied on gestation day 10 in pregnant rats receiving retinyl palmitate at 1.5, 5, 15, or 50 mg/kg feed for 6 weeks. Delayed neural tube closure, post-implantation loss and the nature and incidence of malformations were similar between diet groups, as well as being reminiscent of the high dose group in the first experiment. Thus the dietary status of the animals did not seem to influence the teratogenic potential of a single high dose of retinyl palmitate.
52 As reviewed by EFSA (2006), Ritchie et al (1998) quantified the teratogenic potencies of retinoids on cultured rat embryos, and compared them with circulating concentrations of the same metabolites in vivo after administration of a teratogenic dose of vitamin A. Their conclusion was that plasma retinol was the best predictor of teratogenicity, and that an intake of 7500 μg RE/day of vitamin A would be unlikely to generate teratogenic plasma concentrations of retinoids. However, species differences, protein binding and transfer to the embryo were not taken into account, preventing the recommendation of this method to predict the teratogenicity of vitamin A in humans. Work by Wiegand et al, (1998) on Cynomolgus monkeys indicated that a dose of 2250 μg RE/kg bw/day (as retinyl palmitate) from the 16th to the 27th day of gestation did not produce any malformations of the pups compared with controls fed a diet providing 300 μg RE/kg bw/day. Extrapolating these data to humans on the basis that the dose-responses for the teratogenicity of isotretinoin (13 cis RA) and the conversion of cis RA to trans RA appeared similar in monkeys and humans, led to the conclusion that a daily intake of 9000 μg RE should be considered non-teratogenic in humans.
53 Schnorr et al. (2011) dosed rats with vitamin A at 750, 375 and 7500 mg RE/kg and observed an increase of oxidative damage markers in the reproductive tissues and plasma of dams. The activity of glutathione-S-transferase was modulated by vitamin A supplementation, increasing in the liver of dams and decreasing in the kidneys of mothers and offspring. In pups, supplementation decreased the total antioxidant potential of the liver as well as the superoxide dismutase/catalase activity ratio in the kidney. Lipoperoxidation increased in male offspring but decreased in female pups. Although no clear explanation was given for the sex difference in response, the authors suggested that male offspring were more susceptible to free radical injury than were females. The results suggested that excessive vitamin A intake during gestation and lactation might be toxic for mothers with adverse effects for the developing offspring.
Dermal exposure
54 Willhite et al. (1990, from abstract) found that topical administration to intact skin of up to three consecutive doses of 10.5 mg/kg/d all-trans-retinoic acid or a single 5 mg/kg dose of etretinate (Ro 10-9359) during a critical stage of embryogenesis in hamsters caused erythema and/or dose-dependent epidermal hyperplasia at the site of application but failed to induce a significant teratogenic response. Topical application of 0.01-1.0 mg/kg of artificial carotenoid Ro 13-6298 resulted in dose-dependent mucocutaneous toxicity and an increase in the numbers of dead embryos and malformed offspring. The marked skin toxicity and attenuated concentrations in maternal blood, compared to the oral route, limited the amounts of retinoid that reached the hamster embryo. Therefore, it was considered more important to compare the absorbed dose than the applied dose, when interpreting the bioassays. The data suggested that in human skin, toxicity limits the amounts of retinoid that can be applied during pregnancy and subsequently reaches the embryo whereas in the rodent, overt skin toxicity under continued dosing could increase the penetration.
55 A technical report by the US National Toxicology Program (NTP, 2012) quotes a study by Seegmiller et al. (1990) in which, time-mated Sprague-Dawley rats were administered retinoic acid topically to clipped intact dorsal skin on gestational days 11 to 14 at 12, 100, or 250 mg/kg bw. Maternal weight gain, pup weight, number of resorptions, number of fetuses with gross malformations, and skeletal and organ anomalies were determined. Dams treated dermally with retinoic acid exhibited skin lesions at the site of application from gestational day 15, and most dams showed vaginal bleeding by day 16. Approximately 20% did not survive to day 19. Maternal weight gains in the treated groups were decreased by approximately 50% relative to control animals at the lowest dose, with essentially no weight gain at the intermediate- and high-dose levels. Decreases in fetal weights at the two higher dose levels were significant, but there were no differences from controls in the number of resorptions or malformation frequencies.
Human studies
Teratogenicity- Food and food supplements.
56 Werler et al. (1990) used data from a case-control study to assess the maternal use of vitamin A supplements alone and vitamin A-containing multivitamin supplements in relation to the occurrence of certain birth defects involving structures derived, at least in part, from cranial neural crest cells. The cases were 2,658 infants with such defects (primarily craniofacial and cardiac malformations) with the controls being 2,609 infants with other malformations. Vitamin A supplementation was defined as daily use for at least 7 days of retinol alone or with vitamin D, or of fish oils. Information on vitamin A dose and nutrition was not available. The mothers of six controls used vitamin A supplements in each of the first trimester of pregnancy in comparison to the mothers of 15, 14, and 10 cases in months 1, 2, and 3, respectively. Relative risk estimates and (95 % confidence intervals) were 2.5 (1.0 - 6.2) for month 1, 2.3 (0.9 - 5.8) for month 2, and 1.6 (0.6 - 4.5) for month 3. The findings were considered tentative because no dose information was available, only small numbers of cases and controls were exposed to vitamin A supplements, and relative risk estimates were not statistically significant.
57 Rothman et al. (1995) obtained vitamin A supplement data on 22,748 pregnant women when they had screening for maternal serum alpha-fetoprotein or underwent amniocentesis. Information on the outcomes of pregnancy was obtained from the obstetricians who delivered the babies or from the women themselves. Of the 22,748 women, 339 had babies with birth defects; 121 of these babies had defects occurring in sites that originated in the cranial neural crest. For defects associated with cranial-neural crest tissue, the ratio of the prevalence among the babies born to women who consumed more than 4,500 mg RE of preformed vitamin A per day from food and supplements to the prevalence among the babies whose mothers consumed 1,500 mg RE or less per day was 3.5 (95 % confidence interval, 1.7 to 7.3). For vitamin A from supplements alone, the ratio of the prevalence among the babies born to women who consumed more than 3,000 mg RE per day to that among the babies whose mothers consumed 4,500 mg RE or less per day was 4.8 (95 percent confidence interval, 2.2 to 10.5). Using a smoothed regression curve, an apparent threshold was identified near 3,000 mg RE per day of supplemental vitamin A. The increased frequency of defects was concentrated among the babies born to women who had consumed high levels of vitamin A before the seventh week of gestation. The authors concluded that among the babies born to women who took more than 3,000 mg RE of preformed vitamin A per day in the form of supplements, about 1 infant in 57 had a malformation attributable to the supplement.
58 Azaïs-Braesco and Pascal (2000) reviewed reported cases of teratogenicity associated with high intakes of vitamin A in pregnancy. Up to 20 case reports of the relationship between high vitamin A intake and an adverse pregnancy outcome in humans had been published over the preceding 30 years; however, these were of limited use for establishing a quantitative link between vitamin A intake and teratogenic events. The malformations observed were not always consistent with the retinoic acid syndrome, thus calling their true origin into question. Five case-control studies since 1990 retrospectively estimated the intake of vitamin A in control subjects and mothers of malformed babies (see Table 1), but these varied in the classification of malformations, statistical power, and vitamin A consumption data. In most cases, no association was found between moderate doses of vitamin A (<3,000 mg RE) and fetal malformations. Moreover, too few women consumed high amounts of vitamin A markedly limiting the power of the study. Only one prospective study, that of Rothman (1995) had been conducted and the results were inconsistent with the retrospective studies, showing that an intake exceeding 3,000 mg RE was associated with increased risk of malformations (prevalence ratio: 4.8; 95 % CI: 2.2,10.5). However, the latter paper had been largely criticised because of suspected misclassification of the malformations, but the authors felt it should not be ignored. Another clinical trial had been carried out in Hungary in which a supplement of 1,800 mg RE vitamin A did not increase the incidence of fetal malformations, but since folic acid was administered simultaneously with vitamin A only limited conclusions could be drawn regarding the incidence of neural tube defects.
Table 1. Case-controlled studies comparing the intake of vitamin A in control subjects and in mothers of malformed babies, as identified by Azaïs-Braesco and Pascal (2000)
|
Cases |
Controls |
Exposure (mg RE/day) |
Odds ratio for defects (95 % confidence interval) |
Reference |
|
11,193 |
11,293 |
> 3,000 > 13,000 |
2.7 (0.8, 11.7) 1.1 (0.5, 2.5) |
Biesalski HK, 1989 |
|
2,658 |
2,609 |
No information on the vitamin A doses |
2.3 (0.9, 5.8) 1.6 (0.6, 4.5) 2.5 (1.0, 6.2) NTDs |
Martines-Frias et al., 1990 |
|
158 |
3,026 |
Multivitamin supplements |
0.57 (0.33, 1.00) Conotruncal defects |
Werler et al., (1990) |
|
548 (NTDs) |
573 |
> 2,400
>3,000 |
NTDs: 0.91 (0.31, 3.68) Other defects: 1.05 (0.51, 2.18)
NTDs: 0.73 (0.40, 1.53) Other defects: 0.92 (0.40, 2.11) |
Botto et al., 1996 |
|
426
16
6 |
432
12
7 |
0 – 3,333
3,000 – 4,500
>4,500 |
1.0
1.4 (0.6, 2.8)
NTDs only |
Mills et al., 1997 |
NTD, neural tube defect. Conotruncal refers to the outflow region of the developing heart.
59 The pharmacokinetics of vitamin A have been investigated in the context of the reported adverse effects.
60 Buss et al. (1994) found that, based on the formation of all-trans-retinoic acid, consuming liver and taking supplements were not of equivalent teratogenic potential. The authors suggested that advice to pregnant women on the consumption of liver based on the reported teratogenicity of vitamin A supplements should be reconsidered.
61 Hartmann et al. (2005) reported that repeated oral doses of up to 30,000 IU (9,000 mg RE) of vitamin A in addition to dietary vitamin A were without safety concern. Safe doses were probably higher, since plasma concentrations and exposure to RA remained at levels earlier shown to be without increased risk of teratogenicity in pregnant women.
62 Piersma et al. (2017) reviewed the central role of RA in embryo development and how the biomarkers of its actions may be used in developmental toxicity testing. This included the enzymes of RA anabolism and catabolism, as well as related morphogenetic factors and their genes, the expression of which may be affected by changes in RA balance. The authors noted that a preliminary adverse outcome pathway for RA mediated malformations had been published and that expansion of this framework and its application in developmental toxicity testing may allow the detection of a large variety of embryotoxicants with diverse modes of action. RA homeostasis could provide a set of molecular tools to be used in mode of action driven animal-free developmental toxicity testing.
Teratogenicity- oral medications
63 The effects of exposure of pregnant women to oral isotretinoin (also known by the Hoffman-La Roche trade name of Accutane) was investigated by a number of groups (for example Lammer et al. (1985, from abstract), Willhite et al. (1986) Howard et al. (1986), reviewed by Kizer et al. (1990)). Various outcomes were observed and their frequencies (shown in brackets) reported: spontaneous abortions (8 %), elective abortions (47 – 62 %), malformed infants (14 – 53 %), normal infants (17 %). Women who become pregnant while using isotretinoin are advised to discuss with their physicians the advisability of continuing the pregnancy. The guidelines from the manufacturer for the use of Accutane by women stressed the necessity of obtaining a negative pregnancy test two weeks before initiating therapy and the importance of using an effective form of contraception a month before, during, and for a month after taking it. Despite these restrictions and warnings to physicians and consumers, women taking Accutane continued to become pregnant and produce malformed infants. Overall, the risk for serious birth defects in infants of pregnant women with exposure to isotretinoin was about 25 %. (Kizer et al., 1990).
64 Zomerdijk et al. (2014) estimated isotretinoin exposure in 203,962 Dutch pregnant women and analysed the occurrence of adverse fetal or neonatal outcomes in these pregnancies. Proportions of adverse fetal or neonatal outcomes, defined as intrauterine deaths at ≥16 weeks of gestation and neonates with major congenital anomalies were measured in relation to isotretinoin exposure in the 30 days before or during pregnancy. Isotretinoin prescriptions dispensed on the same day were assumed to be used simultaneously and therefore these were pooled and considered as one dispensing, so the prescriptions of 10 and 20 mg tablets dispensed at the same time to reach a daily dosage of 30 mg. Odds Ratios (ORs) with 95 % confidence intervals (CIs) adjusted for maternal age were calculated to estimate the risk of adverse fetal or neonatal outcome of 51 pregnancies, 2.5 (95% CI 1.9 to 3.3) per 10,000 pregnancies, were exposed to isotretinoin despite a pregnancy prevention programme being in place (for women of child-bearing age on oral retinoids) in the EU since 1988. Forty-five of these pregnancies were exposed to isotretinoin and six women became pregnant within 30 days of discontinuing treatment. In five out of the 51 isotretinoin exposed pregnancies (53 fetuses), 9.4 % (95 % CI: 1.3 % to 17.6 %), had an adverse fetal or neonatal outcome (OR: 2.3; 95 % CI: 0.9 to 5.7 after adjustment for maternal age). At the time, isotretinoin exposed pregnancies and adverse fetal and neonatal events potentially related to the exposure still occurred, and in the Netherlands at least, there was no full compliance to the isotretinoin exposure prevention programme.
65 MacDonald et al. (2019) used the 2011 – 2015 Truven Health MarketScan® Database to identify pregnancies, including losses and terminations, in a cohort of non-pregnant women filling a prescription for isotretinoin or tretinoin (all-trans-retinoic acid) and a second group of women without either prescription. Women were followed for 365 days or until conception, medication discontinuation, or enrolment discontinuation (“prescription episode”). Rates of pregnancy, risks of pregnancy losses, and prevalence of infant malformations at birth were assessed by exposure. The authors identified 2,179,192 livebirths, 8,434 stillbirths, 2,521 mixed births, 415,110 spontaneous abortions, 124,556 elective terminations, and 8,974 unspecified abortions. There were 86,834 isotretinoin and 973,587 tretinoin episodes, matched to 5,302,105 unexposed women. Pregnancy rates were 3 (isotretinoin), 19 (tretinoin), and 34 (unexposed) per 1,000 person-years. Risk of pregnancy losses were similar, but terminations were more common in the women exposed to isotretinoin (28 % [95 % CI: 21 – 36 %]), than those exposed to tretinoin (10 % [95 % CI: 9 – 11 %]) or unexposed (6 %). Malformations occurred in 4.5 % (95 % CI: 3.5 – 5.6 %) of the tretinoin-exposed pregnancies and 4.2 % of the unexposed pregnancies (adjusted OR: 1.16 [95 % CI: 0.85 – 1.58]); isotretinoin-exposed births were too few to assess malformations.
66 Robson et al. (2020) state: “It is estimated that annually 1 in 500 pregnant women are exposed to oral isotretinoin. Although the UK Teratology Information Service maintains a list of teratogenic medicines, an agreed list of common teratogens with similar interventions to reduce pregnancy exposure in general practice remains an outstanding task for regulatory and professional bodies.”
67 Etretinate is currently approved for oral use in the treatment of psoriasis. In Europe, seven cases of fetal malformations due to etretinate exposure during pregnancy had been reported: these included meningomyeloceles, craniofacial and skeletal abnormalities, severe brain defects with anophthalmia, and low-set ears. A case of congenital malformation was reported in a child born to a woman from Brazil who had discontinued etretinate therapy almost a year before she conceived (Lammer et al, 1988). There had been no reports of birth defects associated with its use in the United States, but it was approved only in late 1986. The lowest human teratogenic doses for the two retinoids, isotretinoin and etretinate, are estimated to be 0.4 and 0.2 mg per kg bw per day, respectively (Ross, 1983). Vitamin A is metabolized to the all-trans-retinoic acid, which differs from isotretinoin only in the conformation of the isoprenoid side chain.
68 The UK Teratology Information Service UKTIS states that: “Acitretin (etretin, a metabolite of etretinate) is a second-generation oral retinoid, licensed for the treatment of severe psoriasis, congenital ichthyosis and keratosis follicularis (Darier’s disease). Concurrent exposure to alcohol may induce reverse metabolism to etretinate, which is stored in the liver and has a much longer half-life. Effective contraception (ideally two complimentary forms) is therefore recommended for four weeks prior to commencing treatment, during and for three years after treatment with acitretin. Multiple malformations, including facial dysmorphia, cleft palate, cardiovascular malformations, and limb and skeletal defects have been reported following in utero exposure to acitretin. The available data are, however, limited and the risk of malformation following acitretin exposure in utero remains unquantified, although experience from other retinoids suggests that it is likely to be high. An increased risk of spontaneous abortion and impaired neurodevelopment in the absence of malformation have been observed following in utero exposure to isotretinoin and exposure to acitretin may carry similar risks.”
69 Choi et al. (2021) performed a systematic review and meta-analysis on the rates of major malformations after gestational exposure to isotretinoin covering the period from 1982 – 2011, in the USA, Germany, the Netherlands, Canada, Italy and Israel. The review covered 2,783 isotretinoin-exposed women from ten studies. Of the studies that report a dose rate, it ranged from 0.5 to 80 mg/kg bw/day but individual rates of malformations did not relate directly to dose rates. Overall, the rate of major malformations weighted for the sample size was 15 %. Pooled odds ratio of major malformations for isotretinoin-exposed women before 2006 was 3.76. After 2006, the pooled odds ratio of major malformations for isotretinoin exposure was significantly lower at 1.04, probably due to lower doses being prescribed. Of the studies that include a non-exposed group (3), the rate of major malformations varied between 0.7 and 4.3 % of the pregnancies. The authors acknowledged various study limitations: only three studies had both exposed and unexposed groups; the included individual studies had limited sample sizes and inconsistent characteristics; they may have underestimated the malformation rates due to numerous abortions; not all the studies were of high quality, which may have affected the accuracy of the results; the only evaluated fetal outcome was malformation and no longer-term evaluations were carried out.
Teratogenicity – topical medications
70 The potential teratogenicity of topically applied retinoic acid prescribed clinically for treatment of acne has long been recognised and has been the subject of debate in the past. For example, Wilkinson, (1975, 1976) and Morrison (1976) debated in letters the efficacy and safety of RA for the treatment of acne in Canada, where its use in women of childbearing age was contraindicated. Wilkinson’s opinion was that the treatment was a “new, highly effective modality” and should be available there as it was in other countries. However, the opinion of Morrison and the Health Protection Branch of Health and Welfare Canada was that retinoic acid had the potential for deleterious effects on the human fetus. The Branch recommended that contraindication for women of childbearing potential should be written into product monographs and package inserts and that indications should be restricted to two types of acne. No pharmaceutical manufacturer had at that time agreed to market retinoic acid under those conditions.
71 Panchard et al. (2012) performed a prospective, controlled, multicentre, observational study that involved 11 teratology information services and pregnant women exposed to topical retinoids during the first trimester. These women or their doctors had contacted an information service to seek advice between 1992 and 2006. Patients were asked for consent to further gather follow-up information. The women were exposed to adapalene, tretinoin, isotretinoin, motretinide, retinol, or tazarotene; if more than 1 topical retinoid was used, exposure was classified as a combination. A control group of women had used drugs considered as nonteratogenic during pregnancy (eg, paracetamol, labetalol, meclozine, loratadine, salbutamol, ranitidine, amoxicillin, omeprazol, budesonide inhalation). No significant differences in infants exposed to topical retinoids compared with controls on any outcome measured, except for elective pregnancy termination. There was no evidence of an increase in anomalies consistent with retinoic acid embryopathy. The findings were consistent with the known limited systemic bioavailability of retinoids applied by the transdermal route.
72 More recently, Williams et al. (2020, from abstract) stated: “…rational drug design has been applied to create today's third-generation retinoids (adapalene, tazarotene, and bexarotene). These compounds include aromatic rings within their molecular cores to provide structural rigidity that contrasts with the flexible aliphatic backbone of vitamin A and the earlier generations of retinoids, and thus limits their off-target activity. As a result of these design features, the teratogenic potential in animals of the third-generation retinoids and those reformulated for topical use is generally less than seen with oral administration of earlier generations of retinoids. The available, but limited, epidemiologic data further show little-to-no teratogenic potential associated with real-life use of these compounds in humans. Given the paucity of epidemiologic data available at this time, however, it is recommended that the use of topical retinoids during pregnancy be avoided. However, in circumstances when inadvertent exposure in pregnancy may occur, the available data provide some reassurance that adverse pregnancy outcomes are unlikely.”
73 Regarding tretinoin, the UKTIS states: “Although sporadic case reports have described malformations, including cardiovascular defects, limb defects, ear defects and CNS defects following maternal use of topical tretinoin during the first trimester of pregnancy, no increased risk of congenital malformation has been shown in subsequent larger cohort studies of topical first trimester tretinoin exposure. These data are, however, too limited to definitively exclude a fetal risk and use during pregnancy is therefore not generally recommended. An individual risk assessment is advised where exposure to supratherapeutic doses of topical tretinoin has occurred, or risk factors which increase absorption of the drug are present in association with pregnancy. There are insufficient data (particularly relating to first trimester exposure) to quantify the risks posed to a developing fetus following oral exposure to tretinoin. The risk-benefit balance of maternal vs. fetal wellbeing must be addressed on an individual basis. Other retinoids are known to be teratogenic at therapeutic doses and the likelihood of an increased risk of structural malformation and neurodevelopmental impairment with tretinoin use in the first trimester should therefore be considered and discussed with the patient. The manufacturer advises that there is a high risk of severe malformations and that effective contraception (progesterone-only pills are not considered to be an effective measure of contraception during treatment with tretinoin) must be used for the duration of oral treatment and for one month afterwards.”
Other reproductive and developmental endpoints
74 The pleiotropic nature of the actions of retinoids leads to the possibility that doses of vitamin A given to counter deficiency in pregnancy, at a level that would not be expected to carry a significant risk of fetal malformation, could still lead to negative effects. Cox et al. (2006) dosed a group of 89 pregnant Ghanaian women with either 3,000 mg RE weekly of retinyl palmitate, or placebo (groundnut oil plus tocopherol) until 6 weeks postpartum. While this appeared to improve maternal response to opportunistic viral, bacterial and protozoal infections, it also potentiated Th1-mediated pro-inflammatory responses which carried the risk of placental damage and could threaten the mother and the viability of the fetus, potentially leading to spontaneous abortion (Raghupathy et al. 1999, Raghupathy et al. 2000, Kwak-Kim et al., 2003).
75 Cohen et al. (2015) performed a systemic review and meta-analysis on observational studies that measured maternal blood levels of vitamins A, C, E, and carotenoids during pregnancy or within 72 hours of delivery, and related maternal antioxidant levels during pregnancy with preeclampsia or small-for-gestational-age (SGA) offspring. The studies were heterogeneous with regard to the trimester in which blood retinol was measured, the presence and severity of preeclampsia and the levels of retinol that were correlated with SGA versus appropriate for gestational age (AGA) birth. One study suggested that intrauterine growth restriction pregnancies may be partially due to reduced placental transfer of vitamin A, leading to higher-than-expected maternal blood levels, but two other studies measured retinol levels shortly after delivery and found no significant differences for mothers who delivered SGA compared to AGA babies.
A paper by Mawson and Croft (2019) is included in this statement as it explores the possibility that alterations in the hepatic metabolism of vitamin A may underlie signs and symptoms seen in rubella infection. However, this paper also discusses a discredited relationship between vaccines and autism, a hypothesis which the Committee wishes to emphasise it does not in any way support. The authors provide evidence that rubella can induce alterations in the metabolism of vitamin A and its accumulation in the liver. It is proposed that this would lead to mild toxicity due to hepatic inflammation and dysfunction and to the spillage of stored vitamin A compounds into the circulation in low concentrations. The authors hypothesise that these effects in the early weeks of pregnancy with maternal liver dysfunction could lead to exposure of the fetus to excess endogenous vitamin A, leading to predisposition to long-term metabolic and neurodevelopmental disorders.
Vitamin A and bone
76 Yee et al. (2021) reviewed the effects of vitamin A on bone health. While the majority of the papers they cited related to effects in males and post-menopausal women, they referred to the paper of Händel et al. (2016), which documents the associations between maternal serum retinol and β-carotene concentrations during late pregnancy and offspring bone mineralization assessed at birth, observed in the Southampton Womens’ Survey. In this survey, the maternal health, lifestyle, and diet of a mother-offspring birth cohort were assessed pre-pregnancy and at 11 and 34 weeks of gestation. In late pregnancy, maternal serum retinol and β-carotene concentrations were measured. In total, 520 and 446 mother-offspring pairs had measurements of maternal serum retinol and β-carotene, respectively. Offspring total body bone mineral density (BMD), bone mineral content (BMC), and bone area (BA) were measured within 2 weeks after birth.
77 The results of the Southampton Womens’ Survey were that higher maternal serum retinol in late pregnancy was associated with lower offspring total body BMC (β = −0.10 SD/SD [standardised beta coefficients]; 95 % CI: −0.19, −0.02; P = 0.020) and BA (β = −0.12 SD/SD; 95 % CI: −0.20, −0.03; P = 0.009) but not BMD. Conversely, higher maternal serum β-carotene concentrations in late pregnancy were associated with greater total body BMC (β = 0.12 SD/SD; 95 % CI: 0.02, 0.21; P = 0.016) and BA (β = 0.12 SD/SD; 95 % CI: 0.03, 0.22; P = 0.010) but not BMD. The authors concluded that maternal serum retinol and β-carotene concentrations had different associations with offspring bone size and growth at birth: retinol was negatively associated with these measurements, whereas β-carotene was positively associated. These findings highlighted the need for further investigation of the effects of maternal retinol and carotenoid status on offspring bone development.
Isotretinoin and depression
78 Masgin et al. (2005) reviewed the evidence for a link between isotretinoin use and depression and suicide in acne patients. There had been case reports linking isotretinoin to depression or suicide in the medical and psychological literature since 1982. Between 1982 and 2000 the FDA had received reports of 394 cases of depression, and 37 suicides occurring in patients exposed to isotretinoin. Isotretinoin was recorded as the fifth most common drug reported to the US Adverse Event Reporting System (AERS) in association with depression, and the tenth most common (and the only non-psychotropic drug) in suicide reports. In Canada, fifty six events of psychiatric adverse effects in patients taking isotretinoin had been reported to Health Canada between 1983 and 2003 and forty-two psychiatric reactions, including a small number of suicides, had been reported to the British Medicines Control Agency between 1982 and 1998. In Australia from 1995 to 1998 the Adverse Drug Reactions Advisory Committee received 12 reports of depression in patients taking isotretinoin. Two cases were described as severe, in four there were psychotic features, in three there were suicidal thoughts and there were three suicide attempts (with one completed suicide). The authors also found that many of the studies relating isotretinoin and depression were subject to confounders such as other drug use and methodological problems, such as inappropriate controls. They concluded that GPs should be aware of the possibility of such problems when prescribing but that actual cases are probably rare.
79 Huang and Cheng (2017) conducted a systematic review and meta-analysis of the literature published up to September 30, 2016, including controlled or prospective non-controlled trials on ≥15 acne patients receiving isotretinoin treatment. The prevalence of depression and change in depression scores were calculated. Thirty-one studies met the inclusion criteria. In the controlled studies, the change in depression scores from baseline was not significantly different between patients receiving isotretinoin treatment and those receiving an alternative treatment (standardized mean difference -0.334, 95% CI -0.680 to 0.011). The prevalence of depression after isotretinoin treatment significantly declined (relative risk [RR] 0.588, 95% CI 0.382-0.904). The mean depression scores significantly decreased from baseline (SMD -0.335, 95% CI -0.498 to -0.172). However, as a limitation, no randomized controlled trials were reviewed and large inter-study variation was observed. Overall, isotretinoin treatment for acne did not appear to be associated with an increased risk for depression and the treatment of acne appeared to ameliorate depressive symptoms.
80 Li et al. (2019) performed a systematic review and meta-analysis on the use of isotretinoin and risk of depression in patients with acne. Twenty studies were identified via electronic searches of PubMed, Embase and the Cochrane Library up to 28 December 2017, comparing isotretinoin with other interventions in patients with acne. Seventeen studies showed a significant association of the use of isotretinoin with improved acne symptoms compared with the baseline before treatment (SMD = −0.33, 95% CI −0.51 to −0.15, p<0.05; I 2=76.6%, p<0.05)). Four studies were related to the analysis of the risk of depression. The pooled data showed no association of the use of isotretinoin with the risk of depressive disorders (RR=1.15, 95% CI 0.60 to 2.21, p=0.14). The association of the use of isotretinoin with the risk of depressive disorders was statistically significant on pooling retrospective studies (RR=1.39, 95% CI 1.05 to 1.84, p=0.02), but this association was not evident on pooling prospective studies (RR=0.85, 95% CI 0.60 to 2.21, p=0.86). Overall, there appeared to be an association of the use of isotretinoin in patients with acne with significantly improved depression symptoms but further randomised controlled trials were recommended to verify these findings.
81 Luvizetto and Schmitt (2020) performed a prospective study on patients treated with isotretinoin. Patients were evaluated before the start of isotretinoin and in months 1, 3, 6, 9, and 12, until the final date of use of the medication, for demographic data; severity of acne, severity of scars, and depression. The vast majority of patients (6/7) who presented clinically significant depression did so at the start of treatment and most of these patients showed improvement at subsequent evaluations. There was no correlation with the clinical severity of acne at the outset, indicating that underlying factors, such as socioeconomic level may have had a psychological impact. In the first months there was significant reduction in depression scores, suggesting that the expectation and perception of continued improvement may have had a psychological effect. There appeared to be an association between the intensity of mucocutaneous adverse effects and depressive symptoms, so the importance of being attentive to these factors, applying measures to effectively mitigate them and guiding the patient in advance, especially at the beginning of treatment, was highlighted for physicians.
82 A review of isotretinoin use in acne treatment (Bagatin and Costa, 2020) found only one study correlated treatment with depression and that in the majority of studies conducted on this endpoint, the psychological state of adolescents improved with reduction of the symptoms of acne brought about by the treatment. There is currently no psychological indication for isotretinoin. The authors concluded that: “This drug is effective, despite common, controllable, and reversible mucocutaneous side effects. Serious adverse events are rare and represent individual reactions. Teratogenicity is the most severe, requiring rigorous control. We believe that no other therapeutic option, even topicals combined to oral antibiotics accomplish same results. Recurrence after treatments other than isotretinoin is the rule, prolonging risk of scars, compromising skin appearance, and causing emotional distress in teenagers. If there is no absolute contraindication, isotretinoin should be the first line treatment for moderate to severe inflammatory acne.”
Interactions
Ethanol
83 Zachmann and Gummer (2006) reviewed the literature on interactions between ethanol and retinoic acid as a possible mechanism for birth defects described as fetal alcohol syndrome. Different models have been proposed:
- the synthesis of retinoic acid from retinol, catalysed by alcohol dehydrogenase, might be competitively inhibited by ethanol leading to retinoic acid deficiency;
- ethanol consumption might affect maternal retinol, retinyl ester, or retinoic acid levels, RAR binding, and the levels of RAR expression in developing fetal organs, as has been seen in rats, although specific defects resulting from specific RAR changes have not yet been identified;
- ethanol exposure might mimic vitamin A deficiency, since retinoic acid appears to prevent the adverse effects of ethanol in a quail model;
- retinoic acid and ethanol might reverse or block each other's effects, as has been seen in neuroblastoma cells in vitro.
84 The authors suggested that these experiments showed definite interactions between ethanol and vitamin A, but further studies would be needed to determine if any of these mechanisms significantly contributed to prenatal ethanol consumption embryopathy.
Vitamin D
85 An early paper on the interaction between vitamins A and D (Cruess & Clark, 1964) indicated that an interaction occurred between toxic amounts of vitamins A and D in rats, which prevented, to a large extent, the alterations in bone lipids (increased triglycerides, esterified cholesterol and phospholipids) that were seen to occur in hypervitaminosis D.
86 Metz et al. (1985) investigated the effect of vitamins A and D individually and in combination on the bone growth of turkey poults. Excessive levels of vitamin A in the diet resulted in a rachitic condition characterized by lower growth rate, greater thickness of the proximal tibial epiphyseal plates and marked reduction in bone mineral mass compared to birds fed a diet containing the estimated required level of vitamin A. In addition, high dietary levels of vitamin A were effective in preventing the renal tubular mineralization and growth depression associated with hypervitaminosis D.
87 Rohde et al. (1999) investigated the hypothesis that vitamin A intensifies the severity of rickets, and inhibits the ability of vitamin D to cure this disease. Increasing exposure of weanling rats to retinyl acetate in the presence of dietary calcium, phosphorus and ergocalciferol (vitamin D2) led to a progressive and significant decrease in total bone ash (p = 0.001) and an increase in epiphyseal plate width (p = 0.001). Repeating the experiment with increasing amounts of vitamin D2 (0 to 645 ng/d) indicated that retinyl acetate antagonised all vitamin D2 dosages. Increasing the dose of retinyl acetate eliminated the ability of vitamin D2 to elevate the level of serum calcium. The mechanism proposed to explain the observed antagonistic effects was competition for the RXR receptor by the vitamins.
88 Parr et al. (2018) studied 61,676 school-age Norwegian children, considering data on maternal food and supplement intake in pregnancy and infant supplement use at age 6 months. Maternal subjects were controlled for age at delivery, parity, pre-pregnancy BMI, education, history of asthma and atopy and smoking in pregnancy. Interactions were observed between vitamin A and various dietary components and pharmaceuticals, including vitamin D, which appeared to ameliorate vitamin A toxicity to some extent and vice versa. Asthma in offspring increased with maternal intake of total RE. A diet naturally high in vitamin A combined with the use of supplements containing retinol during pregnancy placed women at risk of vitamin A excess, which was associated with increased susceptibility to asthma in their children by the time they reached school age. This effect was observed for maternal intakes of ≥2.5 times the recommended dose, below the EFSA UL for retinol of 3,000 µg/day.
Conjugated linoleic acid
89 Dietary conjugated linoleic acid (CLA) has been found to increase tissue levels of retinol (vitamin A alcohol) and its sole specific circulating carrier protein retinol-binding protein (RBP or RBP4). However, the precise mechanism of this action has not been elucidated. Carta et al. (2014) suggested that retinol and CLA may compete for catabolic pathways modulated by the activity of peroxisome proliferator-activated receptor- α (PPAR-α) and RXR heterodimer and may position PPAR-α at the crossroads between the metabolism of lipids and vitamin A.
Zinc
90 Christian and West (1998) reviewed how zinc status has been purported to influence several aspects of vitamin A metabolism, including its absorption, transport, and utilization. Postulated mechanisms relate to either the regulatory role of zinc in vitamin A transport, mediated through protein synthesis, and /or the oxidative conversion of retinol to retinal by a zinc-dependent retinol dehydrogenase. A curvilinear relationship appeared to describe an effect of plasma zinc on vitamin A transport but clear evidence of synergy and its public health significance in humans was lacking.
Vitamin K
91 The EVM (2003) stated that vitamin A may antagonise the action of vitamin K in blood clotting function and may potentiate the development of intracranial hypertension when taken in combination with tetracycline and minocycline type antibiotics. Drugs such as ketoconazole, which inhibit CYP enzymes, can significantly increase the half-life of retinoic acid. Hypervitaminosis A may decrease vitamin C tissue storage and may have an anti-thyroid effect.
Folate and folic acid
92 Folate is a naturally occurring vitamin, found in vegetables such as beans, peanuts and whole grains and animal products such as liver and eggs. Folic acid is the unionised form of the vitamin that is used in supplements.
93 Qi and Ratnam (2006) found that folate receptor FR-b selectively mediated growth inhibition in human AML cells by dideazatetrahydrofolate, and this was greatly potentiated by all-trans retinoic acid (ATRA) and the enzyme inducers of retinoic acid metabolism trichostatin A (TSA), valproic acid (VPA), and FK228. This effect was also observed by Blaser et al. (2007) and Lynn et al. (2015).
94 Excess treatment of 13-cis-RA on pregnant women induces craniofacial malformation found in infants, although the effect of folic acid on 13-cis-RA-induced cellular damages of developing midfacial processes is unknown. Therefore Kriangkrai et al. (2017) investigated the pre-treatment effect of folic acid (FA) on 13-cis-RA-induced cellular damage in developing midfacial processes in rat embryos. Rat embryos in vitro were exposed to 13-cis-RA (20 µM) with or without pre-treatment of FA (100 µM). Midfacial morphogenesis, cell proliferation and apoptosis of the midfacial processes were evaluated. The 13-cis-RA-treated embryos at 24 hours showed atrophy of midfacial processes and significantly decreased morphogenesis and cell proliferation, with increased apoptotic cell death. In contrast, embryos pre-treated with FA for 18 hours, followed by 13-cis-RA treatment for 24 hours showed significantly greater morphogenesis, increased cell proliferation and lower apoptotic cell death in comparison with the 13-cis-RA-treated group. Thus, the FA reduced the teratogenic effects of 13-cis-RA on midfacial process tissue. However, it is important to note that the doses of FA selected in this study were particularly high. Future investigations into the anti-teratogenic mechanism of FA on midface malformations induced by 13-cis-RA in pregnant woman were recommended.
95 Piersma et al. (2017) also highlighted interactions of RA with diverse classes of xenobiotics, such as anticonvulsants (valproic acid, an enzyme inducer of retinoic acid metabolism, alone and in combination with the CYP inducers phenytoin, phenobarbital, carbamazepine, or ethosuximide) ; triazole antifungals, through interaction with CYP26 expression, TGFb (transforming growth factor beta) and CRABP (cellular RA binding protein); methylmercury, through alterations in RA-related gene expression; tributyl tin chloride causing a decrease in RARa and sonic hedgehog expression (in fish embryos); flame retardants (monosubstituted isopropylated triaryl phosphate (mITP) and triphenylphosphate) affecting RA homeostasis and Hox family gene expression. Dioxin-induced cleft palate had also been shown to depend on RA signalling that controls AhR expression and polybrominated biphenyl ethers (PBDEs) to affect RA homeostasis and lead to embryotoxicity under marginal vitamin A status in rats.
Beta-carotene
96 The Joint FAO/WHO Expert Committee on Food Additives (JECFA) considered b-carotene in 1974 and concluded that hypercarotenaemia per se was not toxicologically significant and caused no adverse symptoms or hypervitaminosis A; the condition disappears if excess intake of beta-carotene is discontinued. JECFA quoted a study from 1959, (Greenberg et al., 1959) where fifteen subjects received 60 mg beta-carotene daily for three months. Serum carotene levels rose from 128 µg/100 ml to a maximum of 308 µg/100 ml after one month while vitamin A levels remained unchanged. No clinical signs of hypervitaminosis A were seen. Other subjects ate several pounds of raw carrots daily, resulting in some skin discoloration. Beta-carotene appeared in breast milk. High doses of beta-carotene were found to reduce liver storage of labelled dl-gamma-tocopherol acetate (vitamin E) to 70%. In their evaluation, JECFA (FAO/WHO,1974) identified a NOAEL in rat of 50 mg/kg bw and established an ADI of 0 – 5 mg/kg bw based on a four-generation study at dietary levels of 0 ppm and 1,000 ppm of beta-carotene for 110 weeks that showed no adverse effects in any of the generations.
97 In 2017, at the 84th meeting of JECFA, the Committee noted that new data showed large differences in absorption of β-carotene between rodents and humans and therefore considered that rodents were an inappropriate animal model for establishing an ADI for β-carotene. At the 87th meeting in 2019, the Committee considered that “…no adverse health effects were observed in the general population in large, well-conducted human intervention studies in which healthy participants were administered 20 – 50 mg β-carotene per day for up to 12 years, in addition to background exposure from the diet. …For the general population, the Committee concluded that the estimated high exposure to β-carotene of 9 mg/day for a 30 kg child and 6 mg/day for a 60 kg adult from its current uses as a food additive, in addition to background exposure from the diet, would not be expected to be a safety concern,” (FAO/WHO, 2019).
98 Omenn et al. (1996) reported the commencement and study design of the b-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high-risk populations: smokers and asbestos-exposed workers. CARET was a multicentre, two-armed, double-blinded randomized chemoprevention trial in Seattle, Portland, San Francisco, Baltimore, Connecticut, and Irvine, to test whether oral administration of b-carotene (30 mg/day) plus retinyl palmitate (25,000 ID/day) could decrease the incidence of lung cancer in high-risk populations. The outcomes of this trial are discussed below.
99 Goodman et al. (2004) reported that CARET was stopped ahead of schedule because participants who were randomly assigned to receive the active treatment were found to have a 28 % increase in incidence of lung cancer (RR RR =1.28, 95% CI =1.04 to 1.57; P=0.02), a 17 % increase in incidence of death (RR = 1.17, 95% CI = 1.03 to 1.33; P=0.02) and a higher rate of cardiovascular disease mortality compared with participants in the placebo group. With follow-up through December 31, 2001, the post-intervention relative risks of lung cancer and all-cause mortality for the treatment group compared with the placebo group were 1.12 (95 % [CI] 0.97 to 1.31) and 1.08 (95 % CI 0.99 to 1.17), respectively. Relative risks remained above 1.0 throughout the post-intervention follow-up but conversely, the relative risk of cardiovascular disease mortality decreased rapidly to 1.0 after the intervention was stopped. During the post-intervention phase, females had larger relative risks of cardiovascular disease mortality (1.44 versus 0.93; p=0.03), and all-cause mortality (1.37 versus 0.98; p=.001) than males.
100 Despite b-carotene being widely considered as protective against cancer and cardiovascular diseases, researchers of the CARET concluded that “supplements containing β-carotene were harmful to cigarette smokers, causing increases in the incidence of lung cancer and in overall mortality”. The reported adverse effects of b-carotene and retinyl palmitate on lung cancer incidence and all-cause mortality in cigarette smokers and individuals with occupational exposure to asbestos persisted after drug administration was stopped, although they were no longer statistically significant. b-carotene was considered to be responsible for influencing lung cancer incidence as similar effects were seen in the treatment group of the Alpha-Tocopherol Beta-Carotene Cancer Prevention (ATBC) Trial, and that a skin cancer trial that administered higher doses of retinol than that administered in the CARET study reported no adverse effects (although the trial was not powered to study lung cancer incidence”. Additionally, subgroup analyses planned at the time suggested that the excess risks of lung cancer were restricted primarily to females, and cardiovascular disease mortality primarily to females and to former smokers. However, the low statistical power of this study led to wide confidence intervals, leaving the results on lung cancer difficult to interpret clearly (Goodman et al., 2004).
101 Tayyem et al. (2019) conducted a case-controlled study on 400 Jordanian women aged 20 - 65. Two hundred women recently diagnosed with breast cancer were matched in age, income, and marital status to 200 breast cancer-free women. A food frequency questionnaire was used to assess nutrient intake patterns. A significant increase in breast cancer risk was associated with high vitamin C and β-carotene intake (the highest for the fourth quartile; odds ratio [OR], 5.42; 95 % CI, 2.11 to 13.91; ptrend = 0.001). Conversely, a significant inverse trend was detected for the risk of breast cancer and high calcium, phosphorus, and vitamin D intake. A high-fat nutrient intake also showed a significant direct association with breast cancer risk in the third (OR, 3.88; 95 % CI, 1.58 to 9.51) and fourth (OR, 3.87; 95 % CI, 1.53 to 9.77) quartiles (ptrend = 0.001).
102 Evidence was somewhat weaker for a link between vitamin C and β-carotene with hormone-sensitive breast cancer in the study of Bakker et al. (2016). Moreover, Nagel et al. (2010) demonstrated no associations of breast cancer with high dietary intake of vitamin C and β-carotene. For their study, Tayyem et al. offered the explanation of Salganik et al. (2001), who reported that reactive oxygen species in moderate concentrations act as mediators of apoptosis and phagocytosis, and that in people with a low level of reactive oxygen species, an excess of antioxidants could block these mechanisms and promote cancer.
Exposure
Population estimates
In many regions of the world, for example, regions of Africa and south-west Asia (Harika et al., 2017) the issue with vitamin A is deficiency and the deleterious effects this has upon the health of unborn children. However, in developed countries, many people regularly have an intake that exceeds EFSA’s dietary reference value, with a range of values across European countries being reported, although values did not exceed the tolerable upper limit of 3,000 mg RE/day (Jenab et al., 2009). Allen and Haskell (2002) found that in the United States, the 95th percentile of RE intake from foods and supplements for non-pregnant, non-lactating women aged 19 – 30 years exceeded the UL but was below the NOAEL of 4,500 µg/day (adopted by the US Institute of Medicine) for women of reproductive age. Specifically, for non-pregnant, non-lactating women aged 19 – 30 years, the median intake of vitamin A (retinol and provitamin A carotenoids) from food was 530 mg of RE/day, and the 95th percentile was 1,112 mg of RE/day. Reportedly, 17 % of this group took supplements. For these women, the median level of vitamin A in the supplements was about 1,422 mg of RE/day with a 95th percentile of 2,543 mg/day. A woman who consumed the 95th percentile of vitamin A from both diet and supplements would consume about 3,655 mg of RE/day, exceeding the UL of 3,000 mg/day.
103 Van den Berg et al. (1996) assessed the distribution of dietary vitamin A intake among Dutch women aged 16 - 50 and among pregnant women, and to evaluate the effect of the use of a vitamin A (1,200 RE) containing multivitamin supplement in terms of nutritional and teratogenic risk. Data from the 2nd Dutch national food consumption survey (1992) were used to calculate the vitamin A intake among 1,725 16 - 50 year old women and 58 pregnant women with and without simulation of the use of a supplement containing 1,200 RE vitamin A. Average vitamin A intake, based on a two-day dietary record method, was 850 RE for the 16 - 50 year old non-pregnant (NP) women (RDA: 800 RE), and 990 RE for the pregnant (P) women (RDA: 1,000 RE), respectively. Consuming liver on one of the survey days resulted in 60 % of the women in this subgroup exceeded 3,000 RE, and in 23 % of the cases intakes were >7,500 RE (Van den Berg et al., 1996). Those not consuming liver or liver products on the survey days had average intakes (NP: 540 RE; P: 720 RE]. About 70 % of the non-liver consumers had intakes below the RDA. Including the daily use of a vitamin A containing multivitamin supplement with 1,200 RE resulted in intakes >RDA, while only in 2 % (NP), and 3 % (P) of the cases the intake exceeded the 3,000 RE but was less than 7,500 RE/day (the lowest dose found by EFSA (2006) to be hepatotoxic). The authors concluded that the use of a vitamin A-containing multivitamin supplement (maximum 1,200 RE) could contribute to a controlled and adequate vitamin A intake and be considered as safe for pregnant women or women who wish to become pregnant, if the consumption of liver was completely avoided and the consumption of liver products is limited to a daily maximum of one.
104 EFSA (2015) estimated the average dietary intake in adults as being between 816 and 1,498 μg RE/day (retinol and provitamin A carotenoids). Average daily intakes were in most cases slightly higher in males than in females, mainly owing to the larger quantities of food consumed per day.
105 UK Government dietary advice, as communicated via the NHS.uk website recommends a daily vitamin A intake for adults aged 19 to 64 of 700 µg for men and 600 µg a day for women and that the diet should provide this. Pregnant women are warned about eating liver or liver products such as pate, or supplements that contain vitamin A to avoid potential harm to the unborn baby.
UK retinoid intake
106 Consumption data for the assessment of vitamin A intake were obtained from NDNS years 1-8 (Bates et al., 2014, 2016; Roberts et al., 2018). The NDNS is designed to collect detailed information on the diet, food consumption patterns and nutrient intake of the UK population on a rolling basis. The vitamin A content of foods consumed by NDNS respondents has been published (McCance and Widdowson’s ‘The Composition of Foods’ – the UK food composition tables (FSA, 2019) . The FSA uses CRÈME software to interrogate these dietary dataset to derive vitamin A intake. The software derives a mean and high-level intake value from a distribution of vitamin A intakes among consumers of foods that have been reported in Appendix 1. The following data were extracted from the Appendix. Table 2 gives the daily intake of RE in women aged 16 – 49.
Table 2. Chronic exposure of Vitamin A (retinol equivalents) in women from food sources only (Bates et al., 2014; 2016; 2018)**.
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Age group |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
16 – 49 yrs |
760 |
2,600 |
11 |
39 |
|
19 – 64 yrs |
830 |
2,800 |
12 |
43 |
*Rounded to 2 significant figures
**Based on total population
107 Liver and liver products in the diet constitute a major source of dietary pre-formed RE. As shown in Table 3, only a small number of such consumers was recorded in the NDNS. The small number of liver consumers creates uncertainty surrounding the data. However, the Exposure Assessment Team cross referenced the data from NDNS with online sources of intake (from supermarkets and recipes) and found that the amount consumed from these sources were similar to those in the Survey.
Table 3. Chronic exposure of Vitamin A from Liver (with recipes) in women aged 16 -49 (Bates et al., 2014; 2016; 2018)^.
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
25 |
3500 |
7500 |
50 |
97 |
*Rounded to 2 significant figures
**Consumption or exposure estimates made with a small number of consumers may not be accurate. The number of consumers is less than 60, this should be treated with caution and may not be representative for a large number of consumers.
^Based on food consumers on all types of liver.
108 Heat-clarified butter, known as ghee, also contains appreciable amounts of vitamin A. Ghee forms a staple part of the cuisine of some Asian cultures and thus contributes to vitamin A intake in these population groups. The FSA Analytics Team investigated whether this potentially ethnicity-based dietary component might lead to a small “hot spot” of the population being exposed to a disproportionately high intake of vitamin A. The ethnicity of ghee consumers are presented in Table 4 below. The total number of ghee consumers were 123. Only eight and two of these consumers were exposed to vitamin A from ghee above the mean of 9.5 µg /day and p97.5 of 120 µg /day respectively (Table 4).
Table 4: Ethnicity and number (percentage) of ghee consumers
|
Ethnicity |
Total ghee consumers (n=123)* |
Consumers above the mean (n=8)* |
Consumers above the 97.5th percentile (n=2)* |
|
White
|
103 (84) |
3 (38) |
0 (0) |
|
Black or Black British |
4 (3.3) |
1 (13) |
0 (0) |
|
Asian or Asian British |
15 (12) |
4 (50) |
2 (100) |
|
Mixed ethnic group |
1 (0.8) |
0 (0) |
0 (0) |
*Rounded to 2 significant figures
109 Although Asian and Asian British women of childbearing age are more likely to consume ghee than those in other ethnic groups, the majority of ghee consumers were found to be White (84%). This results mainly from White people accounting for the majority of the survey population. However, the highest consumers of ghee are more likely to be Asian or Asian British (Table 4). Given that Asian/Asian British, Black/Black British and white respondents represent 5.4%, 2.7% and 88%, of the total NDNS respondents respectively. It is assumed that ethnic groups thought to consume above average amounts of ghee are represented. However, the low numbers of consumers of ghee within ethnic groups in NDNS is acknowledged as an important source of uncertainty.
110 Appendix A, Table 16 gives a list of food products fortified with vitamin A. On the basis of one of these products being consumed once daily, the highest contribution that any one of them would make would be an extra 432 µg RE/day (Dr Witt Multivitamin drink).
111 Appendix A Table 17 gives a list of food supplements containing b-carotene or vitamin A. The supplements containing 1 – 7 mg of b-carotene do not have warnings against their use by pregnant women because of the accepted low risk of this provitamin, but the supplements containing various esters of preformed vitamin A (300 – 906 mg RE/serving) are not recommended in pregnancy.
112 An internet search reveals that in Norway at least, the processing of raw cod liver oil to produce the refined product for sale to the public leads to a reduction in the vitamin A and D content and these vitamins are then added back to the product so that the recommended dose to the consumer is 1,100 to 4,600 IU (330 – 1,380 mg RE) vitamin A per teaspoon. At the maximum level, 2 teaspoons of the oil would result in an intake of 2,760 mg RE per day, which is below the maximum level set by EFSA but exceeds the “appropriate” level set by EVM. Cod Liver Oil and Vitamin A: Notes | Acubalance Wellness Centre
Risk Characterisation
113 As noted in paragraph 13, EFSA (2006) derived a UL for vitamin A of 3,000 mg of RE per day for women of childbearing age. This was based upon a study by Rothman et al. (1995). In Paragraph 55 Azaïs-Braesco and Pascal (2000) noted that The Rothman et al. (1995) study was inconsistent with previous retrospective studies and had been widely criticised on the grounds of possible misclassification of deformities but could not be ruled out in the consideration of the teratogenic effects of the vitamin. Conversely, the EVM (2003) was unable to reach a firm conclusion on an upper intake limit but considered that an intake greater that 1,500 µg/day was “inappropriate”.
114 Based upon the EFSA UL and taking women of childbearing age as a whole, the intake of retinol equivalents from food at the 97.5th percentile of consumption is close to, but still below, the UL and therefore would not be a concern for their health or for the development of a fetus borne by these women. However, the small group in the food surveys who consume liver at the mean level have an intake that marginally exceeds the UL (117 %) and those who are in the highest consumer group have an intake of 250 % of the UL. If the latter level of consumption were continued into a pregnancy, then this may lead to an increased risk of the fetus suffering a neural tube defect or other developmental lesion that may lead to deformity. Despite the caveat that the data on liver consumption are only recorded in a small number of women of childbearing age and thus bear a greater level of uncertainty, this explains the rationale behind the UK Government’s advice for pregnant women to abstain from consuming liver and products containing liver during pregnancy.
115 Likewise, vitamin A-fortified food products if eaten to excess, may contribute to an exceedance of the UL, although this would only be marginal.
116 Moreover, although the consumption of vitamin A rich food supplements on their own do not provide enough RE to exceed the UL, the nature of their consumption, in addition to a normal diet, especially in the case of cod liver oil, could push RE intake over the UL. This is consistent with the current UK Government advice that supplements containing vitamin A are not recommended for pregnant women.
117 Conversely, taking the EVM maximum “appropriate” consumption level of 1,500 mg RE per day, then although the mean consumption in the diet is within the acceptable range, it is exceeded by the 97.5th percentile consumption. Even the mean consumption of liver would result in exceedance of the suggested appropriate intake. On this basis, current Government advice for pregnant women to limit their consumption of liver and other foodstuffs containing high concentrations of preformed vitamin A is still appropriate.
Discussion and conclusions
118 Vitamin A in the diet, either as pro-vitamin A carotenoids from plants or as preformed retinol from animal sources is essential for health in general and for fetal development and vision in particular.
119 The functions of vitamin A are mediated by various isomeric forms of retinaldehyde and retinoic acid. Retinaldehyde has a central function in vision that is not specific to any section of the population. Retinoic acid is involved in multiple aspects of embryogenesis but in excess, is a known teratogen.
120 There is evidence that retinol may have detrimental effects on fetal bone development. If anything, β-carotene may be beneficial on fetal bone development, but further work is required to clarify these relationships.
121 Teratogenicity and embryotoxicity have been observed in animals exposed to high doses of vitamin A as retinol or retinyl esters and to isotretinoin and etretinate. Oral Isotretinoin exposure has been observed in human case control studies to be associated with an increased risk of spontaneous abortions and birth defects. However, in general, findings in humans, although suggestive of neural crest defects in development, have been mixed and in some cases their aetiology uncertain. Despite this ambiguity, pregnant women or those considering becoming pregnant are recommended to not consume foods, such as liver, or take supplements that are rich in pre-formed vitamin A.
122 Intake of vitamin A in developed countries may in some cases exceed the intake deemed acceptable by the EVM and the UL as set by EFSA.
123 Topical application of retinoids for the treatment of acne does not appear to contribute markedly to overall plasma levels but women are advised that they should not be used during pregnancy to avoid the risk of birth defects.
124 Oral supplements of vitamin A or synthetic analogues may lead to retinoic acid levels that could exert teratogenic or other effects in humans, although dietary levels generally do not.
125 Recent reviews of the data suggest that reported links between the use of oral isotretinoin for the treatment of acne and clinical depression, including increased risk of suicide in patients, may be complicated by pre-existing socioeconomic and psychological factors. Acne itself may lead to depression and relief of skin symptoms by treatment may ameliorate rather than exacerbate this effect.
126 Excess intake of b-carotene does not lead to increased plasma retinol concentrations because of its low conversion rate but may, for example in heavy smokers, increase the risk of cancer in specific circumstances. However, the Committee considers that the risks posed by smoking in pregnancy are in themselves unacceptable to mother and fetus, irrespective of any increase caused by concurrent consumption of beta carotene. Therefore, the smoking habit should continue to be discouraged since that in itself is a major health risk.
127 The current UK Government advice for pregnant women and those planning pregnancy to limit their consumption of preformed vitamin A remains appropriate.
Statement
03/22
Abbreviations
|
AGA |
Adequate (size) for Gestational Age |
|
AML |
Acute myelogenous leukaemia |
|
AUC |
Area under the dose-response curve |
|
BA |
Bone area |
|
BMC |
Bone mineral content |
|
BMD |
Bone mineral density |
|
Bw |
Body weight |
|
CARET |
Beta Carotene and Retinol Efficacy Trial |
|
CI |
Confidence Interval |
|
Cmax |
Maximum concentration in plasma |
|
COT |
Committee on the Toxicity of Chemicals in Food, the Environment and Consumer Products |
|
CYP |
Cytochrome P450 |
|
DNA |
Deoxyribonucleic Acid |
|
DRV |
Dietary Reference Value |
|
EFSA |
European Food Safety Authority |
|
EVM |
Expert committee on Vitamins and Minerals |
|
FA |
Folic acid |
|
FR |
Folic acid receptor |
|
FSA |
Food Standards Agency |
|
HIV |
Human Immunodeficiency Virus |
|
IFN-g |
Interferon-gamma |
|
IU |
International Units |
|
JECFA |
Joint FAO/WHO Committee on Food Additives |
|
LPL |
Lipoprotein Lipase |
|
LRAT |
Lecithin: Retinoic acid Acyltransferase |
|
mg |
milligram |
|
NOAELNo- |
Observed Adverse Effect Level |
|
NTD |
Neural tube defect |
|
OR |
Odds Ratio |
|
PPAR |
Peroxisomal Proliferator Activated Receptor |
|
PRI |
Population Reference Index |
|
RA |
Retinoic acid |
|
RAL |
Retinaldehyde |
|
RAR |
Retinoic Acid Receptor |
|
RBP |
Retinol Binding Protein |
|
RDA |
Recommended Daily Allowance |
|
RE |
Retinol equivalents |
|
RP |
Retinyl palmitate |
|
RXR |
Retinoid-X-Receptor |
|
SACN |
Scientific advisory Committee on Nutrition |
|
SGA |
Small for Gestational Age |
|
TSA |
Trichostatin A |
|
TUL/UL |
Tolerable Upper Limit |
|
VAD |
Vitamin A Deficiency |
|
VPA |
Valproic acid |
|
mg |
microgram |
References
Allen LH, Haskell M. Estimating the Potential for Vitamin A Toxicity in Women and Young Children. Journal of Nutrition. 2002; 132: 2907S–2919S,.
Azaïs-Braesco V, Pascal G. Vitamin A in pregnancy: requirements and safety limits The American Journal of Clinical Nutrition, 2000, 71(5): 1325S–1333S, https://doi.org/10.1093/ajcn/71.5.1325s
Bagatin E, Costa CS. The use of isotretinoin for acne – an update on optimal dosing, surveillance, and adverse effects Expert Review of Clinical Pharmacology 2020, 13(8) 885–897 https://doi.org/10.1080/17512433.2020.1796637
Bakker MF, Peeters PHM, Klaasen VM, et al. Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort. American Journal of Clinical Nutrition 2016;103:454–64.
Barnard JH, Collings JC,[, Whiting, A, Przyborski SA, Marder TB. Synthetic Retinoids: Structure–Activity Relationships. Chemistry: a European. Journal. 2009, 15, 11430 – 11442
Biesalski HK. Comparative assessment of the toxicology of vitamin A and retinoids in man. Toxicology 1989;57:117–61.
Blaser, B., Gonit, M., Qi, H. et al. Induction of folate receptor type β in a bone marrow engraftment model of acute myelogenous leukemia. Leukemia 21, 2233–2235 (2007). https://doi.org/10.1038/sj.leu.2404786
Botto LD, Khoury MJ, Miulinare J, Erickson JD. Periconceptional multivitamin use and the occurrence of conotruncal heart defects: results from a population-based, case-control study. Pediatrics 1996;98:911–7.
Bowman WC, Rand MJ Textbook of Pharmacology, second edition, 1982. Blackwell Scientific Publications, Oxford. ISBN 0-632 09990 9.
Brazzell RK, Colburn WA. Pharmacokinetics of the retinoids isotretinoin and etretinate: A comparative review Journal of the American Academy of Dermatology 1982 6,(4), Part 2, 643-651. https://doi.org/10.1016/S0190-9622(82)70053-2Get rights and content
Buss NE, Tembe EA, Prendergast BD, Renwick AG, George CF. The teratogenic metabolites of vitamin A in women following supplements and liver Human and Experimental Toxicology 1994 13(1):33-43. doi: 10.1177/096032719401300106.
Carta G, Murru E, Cordeddu L , Ortiz B , Giordano E, Belury MA, Quadro L, Banni S. Metabolic Interactions between Vitamin A and Conjugated Linoleic Acid Nutrients 2014, 6, 1262-1272; doi:10.3390/nu6031262 nutrients ISSN 2072-6643 Nutrients | An Open Access Journal from MDPI
Choi EJ, Kim N, Kwak H-S, Han HJ, Chun KC, Kim Y-A, Koh JW, Han JY, Joo SH, Lee JS, Koren G. The rates of major malformations after gestational exposure to isotretinoin: a systematic review and meta-analysis. Obstetrics and Gynecological Sciences 2021;64(4):364-373 https://doi.org/10.5468/ogs.20373 eISSN 2287-8580
Christian P, West KP Jr. Interactions between zinc and vitamin A: an update. American Journal of Clinical Nutrition 1998,68(suppl):435S–41S.
Clagett-Dame M, Knutson D. Vitamin A in Reproduction and Development. Nutrients 2011, 3, 385-428; doi:10.3390/nu3040385
Cohen JM, Beddaoui M, Kramer MS, Platt RW, Basso O, Kahn SR Maternal Antioxidant Levels in Pregnancy and Risk of Preeclampsia and Small for Gestational Age Birth: A Systematic Review and Meta-Analysis. PLoS ONE 2015 10(8): e0135192. doi:10.1371/journal.pone.0135192
Collins MD, Mao GE. Teratology of retinoids. Annual Review of. Pharmacology and Toxicology 1999. 39, 399–430.
COT (2021) TOX-2021-44 Vitamin A in the maternal diet (food.gov.uk)
Cox SE, Arthur P, Kirkwood BR, Yeboah-Antwi K, Riley EM.Vitamin A supplementation increases ratios of proinflammatory to anti-inflammatory cytokine responses in pregnancy and lactation. Clinical and Experimental Immunology,2006 144: 392–400
Cruess RL, Clark I. Alterations in the lipids of bone caused by hypervitaminosis A and D. Biochemical Journal 1965 96(1):262-265.
Czeizel AE, Rockenbauer M. Prevention of congenital abnormalities by vitamin A. International Journal of Vitamin and Nutrient Research 1998 68:219–231.
Danby FW. Retinoic acid in acne therapy. Canadian Medical Association Journal 1978 119 854.
Das BC, Thapaa P, Karkia R, Dasa S, Mahapatraa S, Liuc T-C , Torregrozac I, Wallaced DP, Kambhampatia S, Van Veldhuizena P, Vermae A, Rayf SK, Evans T. Retinoic Acid Signaling Pathways in Development and Diseases. Bioorganic Medicinal Chemistry. 2014: 22(2): 673–683. doi:10.1016/j.bmc.2013.11.025.
Dudas I, Czeizel AE. Use of 6,000 IU vitamin A during early pregnancy without teratogenic effect. Teratology 1992 45:335–336.
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2006 Tolerable upper intake levels of vitamins and minerals. ISBN: 92-9199-014-0
EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015. Scientific opinion on dietary reference values for vitamin A. EFSA Journal 2015;13(3):4028, 84 pp. doi:10.2903/j.efsa.2015.4028.
EVM (2002) Expert Group on Vitamins and Minerals. Review of Vitamin A) [ARCHIVED CONTENT] (nationalarchives.gov.uk)
EVM (2003) Expert Group on Vitamins and Minerals. Safe Upper Levels for Vitamins and Minerals vitmin2003.pdf (food.gov.uk)
FAO/WHO ( 1975) Revision of the Directive on colouring matters authorized for use in foodstuffs intended for human consumption Reports of the Scientific Committee for Food. First Series p17 – 30,
FAO/WHO (1974) Eighteenth Report of the Joint FAO/WHO Expert Committee on Food Additives, Wld Hlth Org. techn. Rep. Ser., 1974, No. 557.FAO Nutrition Meetings Report Series, 1974, No. 54.
FAO/WHO (2019) Eighty- seventh meeting Rome, 4–13 June 2019 ca5270en.pdf (fao.org)
Goodman GE, Alberts DS, Peng YM, Beaudry J, Leigh SA, Moon TE Plasma kinetics of oral retinol in cancer patients Cancer Treatment Reports 1984 68(9):1125-1133.
Goodman GE, Thornquist MD, BalmesJ ,. Cullen MR, Meyskens FL Jr., Omenn GS, Valanis B, Williams JH Jr. The beta-carotene and retinol efficacy trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping b-carotene and retinol supplements. Journal of the National Cancer Institute 2004;96:1743–1750.
Greenberg R, Cornbleet T, Joffay A I. (1959) Accumulating and excretion of vitamin a-like fluorescent material by sebaceous glands after the oral feeding of various carotenoids. Journal of Investigational Dermatol., 32, 599.
Händel, MN, Moon RJ, Titcombe P, Abrahamsen B, Heitmann BL, Calder PC, Dennison EM, Robinson SM, Godfrey KM, Inskip HM, Cooper C, Harvey NC. Maternal serum retinol and β-carotene concentrations and neonatal bone mineralization: results from the Southampton Women’s Survey cohort. American Journal of Clinical Nutrition. 2016 104(4): 1183–1188.
Harika R, Faber M, Samuel F, Kimiywe J, Mulugeta A, Eilander A micronutrient status and dietary intake of iron, vitamin A, iodine, folate and zinc in women of reproductive age and pregnant women in Ethiopia, Kenya, Nigeria and South Africa: a systematic review of data from 2005 to 2015. Nutrients 2017, 9: 1096; doi:10.3390/nu9101096.
Hartmann S, Brørs O, Bock J, Blomhoff R, Bausch J, Wiegand U-W, Hartmann D, Hornig DH. Exposure to retinyl esters, retinol, and retinoic acids in non-pregnant women following increasing single and repeated oral doses of vitamin A Annals of Nutrition and Metabolism. 2005; 49(3):155-64 doi: 10.1159/000086879.
Howard WB, Willhite CC: Toxicity of retinoids in humans and animals. Journal of Toxicology Toxin Reviews 1986; 5:55-94
Huang P, Chandra V, Rastinejad F. Retinoic Acid Actions Through Mammalian Nuclear Receptors. Chemical Reviews 2014 114(1): 233–254. doi:10.1021/cr400161b.
Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001
Jenab M, Salvini S, van Gils CH, Brustad M, Shakya-Shrestha S, Buijsse B, Verhagen H, Touvier M, Biessy C, Wallstro¨m P, Bouckaert K, Lund E, Waaseth M, Roswall N, Joensen AM, Linseisen J, Boeing H, Vasilopoulou E, Dilis V, Sieri S, Sacerdote C, Ferrari P, Manjer J, Nilsson S, Welch AA, Travis R, Boutron-Ruault MC, Niravong M, Bueno-de-Mesquita HB, van der Schouw YT, TormoMJ, Barricarte A, Riboli E, Bingham S, Slimani N. Dietary intakes of retinol, b-carotene, vitamin D and vitamin E in the European Prospective Investigation into Cancer and Nutrition cohort. European Journal of Clinical Nutrition 2009 63, S150–S178
Kamm JJ, Toxicology, carcinogenicity, teratogenicity of some orally administered retinoids. Journal of the American Academy of Dermatology 19826(4), Part 2, 652-659.
Khoury MJ, Moore CA, Mulinare J. Vitamin A and birth defects. Lancet 1996 347:322.
Kizer KW, Fan AM, Bankowska J, Jackson RJ, Lyman DO. Vitamin A- a pregnancy hazard alert. Western Journal of Medicine 1990; 152:78-81).
Kriangkrai R, Chareonvit S, Iseki S, Limwongse V.Pretreatment Effect of Folic Acid on 13-Cis-RA-Induced Cellular Damage of Developing Midfacial Processes in Cultured Rat Embryos Open Dent J. 2017; 11: 200–212
Kwak-Kim JY, Chung-Bang HS, Ng SC, Ntrivalas EI, Mangubat CP, Beaman KD, Beer AE, Gilman-Scahs A., et al. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Human Reproduction 2003; 18:767–773.
Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JY, Curry CJ, Fernhoff PM, Grix Jr AW, Lott IT, et al. Retinoic acid embryopathy New England Journal of Medicine1985 313(14):837-41. doi: 10.1056/NEJM198510033131401
Lammer EJ: Embryopathy in infant conceived one year after termination of maternal etretinate (Letter). Lancet 1988; 2:1080-1081.
Latriano L, Tzimas G, Wong F, Wills RJ The percutaneous absorption of topically applied tretinoin and its effect on endogenous concentrations of tretinoin and its metabolites after single doses or long-term use Journal of the American Academy of Dermatology 1997 36(3 Pt 2):S37-46.
Lee LMY, Leunga C-Y, Tanga WWC, Choia HL, Leung YC, McCaffery PJ, Wanga C-C, Woolfe AS, Shuma ASW. A paradoxical teratogenic mechanism for retinoic acid. PNAS 2012 109(3), 13668–13673
Li C, Chen J,Wang W, Ai M, Zhang Q, Kuang L. Use of isotretinoin and risk of depression in patients with acne: a systematic review and meta-analysis BMJ Open. 2019; 9(1): e021549. doi: 10.1136/bmjopen-2018-021549
Loughrill E, Govinden P, Zand N. Vitamins A and E content of commercial infant foods in the UK: A cause for concern? Food Chemistry 2016 210:56-62. doi: 10.1016/j.foodchem.2016.04.014.
Luvizotto PP, Schmitt JV Depressive symptoms before and during treatment of acne with isotretinoin and its correlations: a prospective study Anais Brasilieros Dermatologias. 2020 95(6): 760–763.
Lynn RC, Poussin M, Kalota A, Feng Y, Low PS, DimitrovDS, Powell DJ Jr. Targeting of folate receptor β on acute myeloid leukemia blasts with chimeric antigen receptor–expressing T cells Blood 2015 125(22): 3466–3476.
MacDonald SC, Cohen JM, Panchaud A, McElrath TF, Huybrechts KF, Hernández-Díaz S. Identifying Pregnancies in Insurance Claims Data: Methods and Application to Retinoid Teratogenic Surveillance. Pharmacoepidemiological Drug Safety. 2019 28(9): 1211–1221. doi:10.1002/pds.4794.
Maden M. Vitamin A and the developing embryo. Postgraduate Medical Journal 2001;77:489-491.
Magin P, Pond D, Smith W. Isotretinoin, depression and suicide: a review of the evidence British Journal of General Practice 2005 55(511): 134–138.
Marshall H, Nonchev S, Sham MH, Muchamore I, Lumsden A, Krumlauf R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature. 1992 360(6406):737-41. doi: 10.1038/360737a0.
Martines-Frias ML, Salvador J. Epidemiological aspects of prenatal exposure to high doses of vitamin A in Spain. European Journal of Epidemiology 1990;6:118–23.
Mawson AR, Croft AM. Rubella Virus Infection, the Congenital Rubella Syndrome, and the Link to Autism. International Journal of Environmental Research and Public Health 2019, 16: 3543; doi:10.3390/ijerph16193543
Merck Index, 12th edition Merck & Co. Inc. Whitehouse Station NJ 1996 ISBN 0911910-12-3
Metz AL, Walser MM, Olsen WG. The interaction of dietary vitamin A and vitamin D related to skeletal development in the turkey poult. Journal of Nutrition 1985 115: 929-935.
Mills JL, Simpson JL, Cunningham GC, Conley MR, Rhoads GG. Vitamin A and birth defects. American Journal of Obstetrics and Gynecology 1997;177:31– 36.
Nagel G, Linseisen J, van Gils CH et al. Dietary β-carotene, vitamin C and E intake and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Breast Cancer Research and Treatment 2010 119, 753–765 https://doi.org/10.1007/s10549-009-0444-8.
(NTP (2012) National Toxicology Program. NTP technical report on the photo-cocarcinogenesis study of retinoic acid and retinyl PALMITATE TR-568: Photocarcinogenesis Studies of Retinoic Acid and Retinyl Palmitate [CASRNs. 302-79-4 (All-trans-retinoic acid) and 79-81-2 (All-trans-retinyl palmitate)] in SKH-1 Mice (simulated solar light and topical application study) (nih.gov)
Nau H. Chemical structure - teratogenicity relationships, toxicokinetics and metabolism in risk assessment of retinoids. Toxicology Letters 1995 ;82/83 : 975-979
NHS advice on vitamins and minerals: Vitamin A. Last reviewed, August 2021 Vitamins and minerals - Vitamin A - NHS (www.nhs.uk)
NHS Foods to avoid during pregnancy. Last reviewed August 2021 Foods to avoid in pregnancy - NHS (www.nhs.uk)
Nohynek GJ, Meuling WJA, Vaes WHJ, Lawrence RS, Shapiro S, Schulte S, Steiling W, Bausch J, Gerber E, Sasa H, Nau H. Repeated topical treatment, in contrast to single oral doses, with Vitamin A-containing preparations does not affect plasma concentrations of retinol, retinyl esters or retinoic acids in female subjects of child-bearing age Toxicology Letters 2006;163(1):65-76 doi: 10.1016/j.toxlet.2005.09.029. .
Omenn GS, Goodman G, Thornquist M, Grizzle J, Rosenstock L, Barnhart S, Balmes J, Cherniack MG, Cullen MR, Glass A, Keogh J, Meyskens FJr, Valanis B, Williams J Jr. The b-Carotene and Retinol Efficacy Trial (CARET) for Chemoprevention of Lung Cancer in High Risk Populations: Smokers and Asbestos-exposed Workers. Cancer Research Suppd 54. 2(B8s-2ll43s, April 1. IW4]
O’Reilly KC, Shumake J, Gonzalez-Lima F, Lane,MA, Bailey SJ. Chronic Administration of 13-Cis-Retinoic Acid Increases Depression-Related Behavior in Mice Neuropsychopharmacology (2006) 31, 1919–1927. doi:10.1038/sj.npp.1300998
Ortega-Senovilla H, Alvino G, Taricco E, Cetin I, Herrera E. Enhanced circulating retinol and non-esterified fatty acids in pregnancies complicated with intrauterine growth restriction. Clin Sci. 2010; 118(5):351–8.
Ozaki R, Kuroda K, Ikemoto Y, Ochiai A, Matsumoto A, Kumakiri J, et al. Reprogramming of the retinoic acid pathway in decidualizing human endometrial stromal cells. PLoS ONE 2017; 12(3): e0173035. doi:10.1371/journal. pone.0173035.
Panchaud A, Csajka C, Merlob P, Schaefer C, Berlin M, De Santis M, Vial T, Ieri A, Malm H, Eleftheriou G, MD, PhD, MSc, Bracha Stahl, MPharm, Philippe Rousso, MD, Ursula Winterfeld, Rothuizen LE, Buclin T, Pregnancy outcome following exposure to topical retinoids: A multicenter prospective study Journal of Clinical Pharmacology, 2012 52:1844-1851
Parr CL, Magnus MC, Karlstad Ø, Holvik K, Lund-Blix NA, Haugen M, Page CM, Nafstad P, Ueland PM, London SJ, Håberg SE, Nystad W. Vitamin A and D intake in pregnancy, infant supplementation, and asthma development: the Norwegian Mother and Child Cohort. American Journal of Clinical Nutrition 2018;107:789–798.
Piersma AH, Bode W, Verhoef A, Olling M. Teratogenicity of a Single Oral Dose of Retinyl Palmitate in the Rat, and the Role of Dietary Vitamin A Status
Pharmacology & Toxicology 1996 79(3): 131-135
Piersma AH, Hessel EV, Staal YC. Retinoic acid in developmental toxicology: Teratogen, morphogen and biomarker Reproductive Toxicology 2017 72: 53-61
Qi H, Ratnam M. Synergistic induction of folate receptor beta by all-trans retinoic acid and histone deacetylase inhibitors in acute myelogenous leukemia cells: mechanism and utility in enhancing selective growth inhibition by antifolates Cancer Research 2006 66(11):5875-5882 doi: 10.1158/0008-5472.CAN-05-4048
Penniston KL, Tanumihardjo SA The acute and chronic toxic effects of vitamin A. American Journal of Clinical Nutrition 2006;83:191–201.
Raghupathy R, Makhseed M, Azizieh F, Hassan N, Al-Azemi M, Al-Shamali E. Maternal Th1- and Th2-Type Reactivity to Placental Antigens in NormalHuman Pregnancy and Unexplained Recurrent Spontaneous Abortions Cellular Immunology1999 196:122–130.
Raghupathy R, Makhseed M, Azizieh F, Omu A, Gupta M, Farhat R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Human Reproduction 2000; 15:713–718.
Ritchie HE, Webster WS, Eckhoff C, Oakes DJ. Model predicting the teratogenic potential of retinyl palmitate, using a combined in vivo/in vitro approach. Teratology 1998 58: 113-123.
Robson J, Moss N, McGettigan P, Beardsley SJ, Lovegrove E, Dezateux C. First do no harm: valproate and medicines safety in pregnancy British Journal of General Practice 2020; 70 (699): 477-478.
Rohde CM, Manatt M, Clagett-Dame M, DeLuca H-F. Vitamin A antagonizes the action of vitamin D in rats. Journal of Nutrition 1999 129: 2246–2250,
Rosa FW: Teratogenicity of isotretinoin (Letter). Lancet 1983; 2:513
Rothman KJ, Moore LL,. Singer MR, Nguyen U-SDT, Mannino S, Milunsky A Teratogenicity of high vitamin a intake. New England Journal of Mediciine 1995; 333:1369-73.
Russell RM The vitamin A spectrum from deficiency to toxicity American Journal of Clinical Nutrition 2000;71:878–84.
Rutkowski M, and Grzegorczyk K adverse effects of antioxidative vitamins International Journal of Occupational Medicine and Environmental Health 2012;25(2):105 – 121 DOI 10.2478/S13382-012-0022-x
Salganik RI The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population Journal of the American College of Nutrition. 2001;20(5 Suppl):464S-472S.
SCF (Scientific Committee on Food), 2002. Opinion on the Tolerable Upper Intake Level of preformed vitamin A (retinol and retinyl esters). complet_chapitres.indd (europa.eu)
Schnorr CE, Da Silva Morrone M, Weber MH, Lorenzi R, Behr GA, Claudio J, Moreira F. The effects of vitamin A supplementation to rats during gestation and lactation upon redox parameters: Increased oxidative stress and redox modulation in mothers and their offspring. Food and Chemical Toxicology 2011; 49: 2645–2654
Söderlund MB, Fex GA, Nilsson-Ehle P. Concentrations of retinoids in early pregnancy and in newborns and their mothers. American Journal of Clinical Nutrition 2005;81:633– 636.
Shaw GM, Wasserman CR, Block G, Lammer EJ. High maternal vitamin A intake and risk of anomalies of structures with a cranial neural crest cell contribution. Lancet 1996 347:899–900.
Spiegler E, Kim Y-K, Wassef L, Shete V, Quadro L. Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues
Biochimica et Biophysica Acta. 2012; 1821(1): 88–98.
Stratford T, Logan C, Zile M, Maden M. Abnormal anteroposterior and dorsoventral patterning of the limb bud in the absence of retinoids. Mechanisms of Development 1999 81(1-2):115-25
Tamura K, Yokouchi Y, Kuroiwa A, Ide H. Retinoic acid changes the proximodistal developmental competence and affinity of distal cells in the developing chick limb bud. Developmental Biology 1997 188(2):224-234Tayyem RF, Mahmoud RI, Shareef MH, Marei LS. Nutrient intake patterns and breast cancer risk among Jordanian women: a case-control study. Epidemiology and Health Volume: 41,.Article ID: e2019010, 7 pages https://doi.org/10.4178/epih.e2019010
Van den Berg H, Hulshof KF, Deslypere JP. Evaluation of the effect of the use of vitamin supplements on vitamin A intake among (potentially) pregnant women in relation to the consumption of liver and liver products European Journal of Obstetrics, Gynecology and Reproductive Biology 1996 66(1):17-21.
Werler MW, Lammer EJ, Rosenberg L, Mitchell AA Maternal vitamin A supplementation in relation to selected birth defects Teratology 1990 Nov;42(5):497- 503 doi: 10.1002/tera.1420420506
Wiegand UW, Hartmann S, Hummler H. Safety of vitamin A: recent results. International Journal of Vitamin Nutrition Research 1998 68: 411-416.
Wilkinson RD. Safety of retinoic acid in acne therapy. Canadian Medical Association Journal 1975 113 606
Willhite CC, Hill RM, Irving DW, Isotretinoin-induced craniofacial malformations in humans and hamsters. J Craniofacial Genetics and Developmental Biology 1986;2(suppl): 193-209.
Willhite CC, Sharma RP, Allen PV, Berry DL. Percutaneous retinoid absorption and embryotoxicity. Journal of Investigational Dermatology. 1990; 95(5):523-9. doi: 10.1111/1523-1747.ep12504873.
Williams AL, Pace ND, DeSesso JM Teratogen update: Topical use and third-generation retinoids Birth Defects Research 2020 112(15):1105-1114.
WHO (2021) Vitamin A supplementation during pregnancy. 500 (who.int)
Yee MMF, Chin K-Y, Ima-Nirwana S, Wong SK. Vitamin A and Bone Health: A Review on Current Evidence. Molecules 2021, 26,1757.
Zachman RD, Grummer MA. The Interaction of Ethanol and Vitamin A as a Potential Mechanism for the Pathogenesis of Fetal Alcohol Syndrome. Alcoholism: Clinical and Experimental Research 1998; 22(2): 1544 – 1556
Zile MH Vitamin A and Embryonic Development: An Overview The Journal of Nutrition, 1998 128(2), 455S–458S.
https://doi.org/10.1093/jn/128.2.455S
Zomerdijk IM, Ruiter R, Houweling LMA, Herings RMC, Sturkenboom MCJM, S Straus SMJM, Stricker BH. Isotretinoin exposure during pregnancy: a population-based study in The Netherlands. BMJ Open 2014; 4:e005602. doi:10.1136/ bmjopen-2014-005602
Literature search
The following terms were input into PubMed and the relevant papers found as well as references therein were cited in this paper:
|
Vitamin A AND maternal health |
|
prerconception |
|
conception |
|
pregnancy |
|
postnatal |
|
fetus OR foetus |
|
teratogen* |
|
abortion |
|
absorption |
|
distribution |
|
metabolism |
|
excretion |
|
toxicity |
|
repro* |
|
interactions |
|
beta carotene |
|
preeclampsia |
|
cancer |
Appendix 1: Vitamin A content of foods, fortified food products and supplements
Table 1. Approximate Vitamin A concentrations in foods (FSA, 2021)
|
Food type |
Retinol Equivalent (µg /100 g) |
Type of Vitamin A |
|
Liver calf (fried in corn oil) |
25,217 |
Preformed retinol |
|
Liver, chicken, fried in corn oil |
10,500 |
Preformed retinol |
|
Giblets, turkey, boiled |
3,100 |
Preformed retinol |
|
Eel, yellow, raw |
1,200 |
Preformed retinol |
|
Ghee, butter |
1,233 |
Preformed retinol |
|
Fat spread, low fat (26 – 39 %), polyunsaturated |
962 |
Preformed retinol |
|
Carrots. raw |
1,961 |
Carotenoids |
|
Carrots, boiled |
1,850 |
Carotenoids |
|
Spinach, boiled |
1,101 |
Carotenoids |
|
Sweet potato, flesh only, boiled in unsalted water |
927 |
Carotenoids |
|
Curly Kale, raw |
525 |
Carotenoids |
|
Melon, Canteloupe-type, flesh only, weighed with skin |
194 |
Carotenoids |
|
Mangoes, ripe, flesh only, raw |
116 |
Carotenoids |
|
Apricots, dried |
105 |
Carotenoids |
|
Peaches, raw, flesh and skin |
19 |
Carotenoids |
Consumption and exposure assessments for vitamin A in various food sources
1. The following tables (Tables 2 to 13a) details consumption of selected foods containing vitamin A and indicate estimated exposure to vitamin A. The exposure estimates are derived from individual consumption of these foods and take into account various forms of the foods as well as recipes. For example, liver from different animal sources contain varying amounts of vitamin A (Table 1). As such, exposure estimates take account of only some of the concentrations shown in Table 1. All variations of foods available within the NDNS database were used to obtain the consumption and exposure estimates.
Table 2. Chronic exposure of Vitamin A (retinol equivalents) in women from food sources only (Bates et al., 2014; 2016; 2018)**.
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Age group |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
16 – 49 yrs |
760 |
2,600 |
11 |
39 |
|
19 – 64 yrs |
830 |
2,800 |
12 |
43 |
*Rounded to 2 significant figures
**Based on total population
Liver
Table 3. Chronic consumption of all types of liver (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; 2018)^.
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
25 |
22 |
38 |
0.33 |
0.56 |
*Rounded to 2 significant figures
**Consumption or exposure estimates made with a small number of consumers may not be accurate. The number of consumers is less than 60, this should be treated with caution and may not be representative for a large number of consumers.
^Based on food consumers of all types of liver
Table 3a. Chronic exposure of Vitamin A from all types of liver (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; 2018)^.
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
25 |
3,500 |
7,500 |
50 |
97 |
*Rounded to 2 significant figures
**Consumption or exposure estimates made with a small number of consumers may not be accurate. The number of consumers is less than 60, this should be treated with caution and may not be representative for a large number of consumers.
^Based on food consumers on all types of liver
Butter
Table 4. Chronic consumption of butter (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; 2018).
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
1,474 |
5.9 |
25 |
0.09 |
0.37 |
*Rounded to 2 significant figures
Table 4a. Chronic exposure of Vitamin A from butter (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; 2018).
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
1,474 |
40 |
230 |
0.60 |
3.5 |
*Rounded to 2 significant figures
Table 4b. Chronic consumption of ghee (butter and vegetable oil-based) (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; and Roberts et al., 2018).
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers |
Mean |
97.5th Percentile |
Mean |
97.5th Percentile |
|
123 |
3.0 |
12 |
0.043 |
0.18 |
*Rounded to 2 significant figures
Table 4c. Chronic exposure of Vitamin A from ghee (butter and vegetable oil-based)(with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; and Roberts et al., 2018).
|
|
(µg/person/day)*
|
(µg/person/day)*
|
(µg/kg bw/day)*
|
(µg/kg bw/day)*
|
|
Consumers |
Mean |
97.5th Percentile |
Mean |
97.5th Percentile |
|
123 |
9.6 |
120 |
0.14 |
1.8 |
*Rounded to 2 significant figures
Table 4d. Chronic consumption of ghee (butter-based) (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; and Roberts et al., 2018).
|
|
(g/person/day)*
|
(g/person/day)*
|
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers |
Mean |
97.5th Percentile |
Mean |
97.5th Percentile |
|
107 |
3.3 |
13 |
0.047 |
0.2 |
*Rounded to 2 significant figures
Table 4e. Chronic exposure of Vitamin A from ghee (butter-based) (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; and Roberts et al., 2018).
|
|
(µg/person/day)*
|
(µg/person/day)*
|
(µg/kg bw/day)*
|
(µg/kg bw/day)*
|
|
Consumers |
Mean |
97.5th Percentile |
Mean |
97.5th Percentile |
|
107 |
11 |
130 |
0.16 |
1.9 |
*Rounded to 2 significant figures
Milk
Table 5. Chronic consumption of cow’s milk (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; 2018).
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
1,814 |
150 |
460 |
2.2 |
7.1 |
*Rounded to 2 significant figures
Table 5a. Chronic exposure of Vitamin A from cow’s milk (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; 2018).
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
1,814 |
36 |
120 |
0.54 |
1.8 |
*Rounded to 2 significant figures
Egg yolk
Table 6. Chronic consumption of egg yolk (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; 2018)**.
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
903 |
8.5 |
25 |
0.15 |
0.38 |
*Rounded to 2 significant figures
**Assumption – average egg contains 29 % yolk.
Table 6a. Chronic exposure of Vitamin A from egg yolk (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; 2018)**.
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
903 |
12 |
34 |
0.17 |
0.52 |
*Rounded to 2 significant figures
**Assumption – average egg contains 29 % yolk.
Carrots
Table 7. Chronic consumption of carrots (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; 2018).
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
1,327 |
21 |
74 |
0.31 |
1.1 |
*Rounded to 2 significant figures
Table 7a. Chronic exposure of Vitamin A from carrots (with recipes) in women aged 16 - 49 (Bates et al., 2014; 2016; 2018)*.
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
1,327 |
330 |
1,300 |
4.9 |
20 |
*Rounded to 2 significant figures
Peppers
Table 8. Chronic consumption of peppers (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
1,049 |
14 |
60 |
0.21 |
0.91 |
*Rounded to 2 significant figures
Table 8a. Chronic exposure of Vitamin A from peppers (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
1,049 |
12 |
51 |
0.18 |
0.76 |
*Rounded to 2 significant figures
Spinach
Table 9. Chronic consumption of spinach (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
222 |
19 |
61 |
0.24 |
0.97 |
*Rounded to 2 significant figures
Table 9a. Chronic exposure of Vitamin A from spinach (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
222 |
103 |
517 |
1.6 |
8.4 |
*Rounded to 2 significant figures
Cantaloupe melon
Table 12. Chronic consumption of Cantaloupe melon (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
42 |
46 |
131 |
0.78 |
2.5 |
*Rounded to 2 significant figures
**Consumption or exposure estimates made with a small number of consumers may not be accurate. The number of consumers is less than 60, this should be treated with caution and may not be representative for a large number of consumers
Table 12a. Chronic exposure of Vitamin A from Cantaloupe melon (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
42 |
135 |
384 |
2.3 |
7.5 |
*Rounded to 2 significant figures
**Consumption or exposure estimates made with a small number of consumers may not be accurate. The number of consumers is less than 60, this should be treated with caution and may not be representative for a large number of consumers
Mango
Table 13. Chronic consumption of mango (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
235 |
18 |
105 |
0.26 |
13 |
*Rounded to 2 significant figures
Table 13a. Chronic exposure of Vitamin A from mango (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
234 |
15 |
94 |
0.22 |
1.3 |
*Rounded to 2 significant figures
Apricot
Table 14. Chronic consumption of apricot (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
88 |
5.7 |
27 |
0.084 |
0.40 |
*Rounded to 2 significant figures
Table 14a. Chronic exposure of Vitamin A from Apricot (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
88 |
3.8 |
20 |
0.057 |
0.30 |
*Rounded to 2 significant figures
Peach
Table 15. Chronic consumption of peaches (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(g/person/day)* |
(g/person/day)* |
(g/kg bw/day)* |
(g/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
77 |
24 |
110 |
0.34 |
1.3 |
*Rounded to 2 significant figures
Table 15a. Chronic exposure of Vitamin A from peaches (with recipes) (Bates et al., 2014; 2016; 2018).
|
|
(µg/person/day)* |
(µg/person/day)* |
(µg/kg bw/day)* |
(µg/kg bw/day)* |
|
Consumers** |
Mean |
97.5th percentile |
Mean |
97.5th percentile |
|
77 |
4.6 |
21 |
0.67 |
0.28 |
*Rounded to 2 significant figures
Fortified foods
2. Foods are sometimes fortified with vitamin A such as butter and other fat spreads, milk and nutritional powders and cereal products. Some foods such as spreads and sports drinks are fortified with beta carotenes which are used for colouration of the product.
Table 16. Estimated exposure from fortified food products containing Vitamin A (Tesco, Sainsbury’s, Asda, Boots, Holland & Barret, Morrisons, M&S 2021)
|
Food product |
Vitamin A concentration |
Vitamin A concentration (µg per serving) |
Exposure^
|
|
Butters and Spreads |
µg per 100 g |
µg per 10 g serving |
µg/kg bw/day* |
|
Flora Original Spread 500 g
|
814 |
81.4 |
1.2 |
|
Flora Buttery Spread 500g |
233 |
23.3 |
0.33 |
|
Flora Light Spread 500 g |
839 |
83.9 |
1.2 |
|
Flora ProActiv Buttery Taste Spread 500 g |
120 |
12 |
0.17 |
|
Bertolli Original Spread 500 g |
800 |
80 |
1.1 |
|
Bertolli With Butter 400 g |
800 |
80 |
1.1 |
|
Benecol Buttery Spread 500 g |
900 |
90 |
1.3 |
|
Pure Vegan Dairy Free Olive Spread 500 g |
800 |
80 |
1.1 |
|
Pure Vegan Dairy Free Sunflower Spread 500 g |
800 |
80 |
1.1 |
|
Nutritional Drink powders |
µg per 100 g |
µg per serving |
|
|
Complan Nutritional Drink Strawberry 4 X 55 g |
551 |
303 per 55 g |
4.3 |
|
Complan Nutritional Drink Drink Chocolate 4 X 55 g |
522 |
287 per 55 g |
4.1 |
|
Complan Nutritional Drink Banana 4 X 55 g |
550 |
303 per 55 g |
4.3 |
|
Complan Nutritional Drink Original 425 g |
547 |
301 per 55 g |
4.3 |
|
Slimfast Vitality Meal Replacement Shake Chocolate Intensity 400 g |
81.9 (as prepared) |
243 (as prepared) per 40 g |
3.5 |
|
USN Diet Fuel Ultralean Strawberry Flavoured Meal Replacement Shake |
474 |
256 per 54 g |
3.6 |
|
Nutritional Drinks |
µg per 100 ml |
µg per serving |
|
|
Tropicana+ Vitamin Victory Juice 750 ml |
208 |
312 per150 ml |
4.4 |
|
Benefit Drinks Cleanse Prune Juice |
320 |
800 per 250 ml |
11 |
|
Oshee Vitamin Cocktail 250 ml |
160 |
400 per 250 ml |
5.7 |
|
Slim-Fast Milkshake Strawberry 6 x 325 ml |
73.8 |
240 per 325 ml |
3.4 |
|
Dr Witt Multivitamin Drink 1 Litre |
216 |
432 per 200 ml |
6.1 |
|
Nutrient Powder (foods) |
µg per 100g |
µg per serving |
|
|
Funktional Foods Spirulina Powder 100 g |
3,685 |
369 per 10 g |
5.2 |
|
Funktional Foods Wheatgrass Powder 100 g |
1,289 |
258 per 10 g |
3.7 |
|
Dried Milk |
µg per 100 ml as prepared |
µg per serving as prepared (200 ml) |
|
|
Sainsbury's Skimmed Milk Powder 300 g |
66.7 |
133 |
1.9 |
|
Tesco Instant Dried Skimmed Milk 340 g |
71 |
142 |
2.0 |
|
Marvel Dried Milk Powder 278 g |
66 |
132 |
1.9 |
|
Cereal bars |
µg per 100 g
|
µg per serving |
|
|
Oshee Vitamin Muesli Bar Hazlenut & Raisin 40 g |
300 |
120 per 40 g |
1.7 |
|
Oshee Vitamin Muesli Bar Plum & Cranberry 40 g |
300 |
120 per 40 g |
1.7 |
|
Slimfast Meal Replacement Very Berry Bar 4 x 60 g |
400 |
240 per 60 g |
3.4 |
|
Other products |
µg per 100 g
|
µg per serving |
|
|
Blockhead Sugar Free Vitamin D, C, B & A Gum |
|
800 per 2 pieces |
11 |
|
Tetley Super Fruit Multi Vitamins Berry 20 Tea Bags 40 g |
30 |
30 per 100 ml |
0.43 |
|
Potters Malt Extract with Cod Liver Oil Butterscotch 650 g |
1,720 |
172 per 10 g |
2.4 |
|
Boots Malt Extract + Cod Liver Oil – 650 g |
1,400 |
140 per 10 g |
2.0 |
^Exposure is calculated from the recommended serving size and the average body weight of women aged 16- 49 years (70.3kg)
*Rounded to 2 significant figures
Supplements
Table 17. List of a sample of supplements containing vitamin A (Sources: Lloyds Pharmacy, Boots Pharmacy and Superdrug)
|
Supplement |
Maternal supplement?^ |
Vitamin A form |
Recommended intake (mg/day) |
Daily exposure (mg/kg bw)* |
|
Vitabiotics pregnacare tablets range |
Yes |
Beta carotene |
2,000 |
0.028 |
|
Vitabiotics Pregnacare Liquid |
Yes |
Beta carotene |
1,000 |
0.014 |
|
Vitabiotics pregnacare breastfeeding range |
Yes |
Beta carotene |
2,000 |
0.028 |
|
Seven Seas all stages during pregnancy |
Yes |
Beta carotene |
1,000 |
0.014 |
|
Seven Seas pregnancy follow on |
Yes |
Beta carotene |
1,000 |
0.014 |
|
Proceive Advanced Fertility Supplement Max Women |
Yes |
Beta carotene |
7,000 |
0.10 |
|
Seven Seas Adult Complete Multivitamins 28 |
No |
Vitamin A Acetate |
800 |
0.011 |
|
Healthspan women's multivitamin super fruit 30 gummies |
No |
Vitamin A Palmitate |
800 |
0.011 |
|
Pink simply radiant multivitamin for her gummies 60 gummies |
No |
Vitamin A |
750 |
0.011 |
|
Superdrug Multivitamin With Iron |
No |
Vitamin A Acetate |
800 |
0.011 |
|
Bassets Adult Multivitamin Pastilles |
No |
Vitamin A |
800 |
0.011 |
|
Vitabiotics wellwoman original 30 capsules |
No |
Beta carotene |
2,000 |
0.028 |
|
Boots multivitamins |
No |
Vitamin A Acetate |
800 |
0.011 |
|
Supplement |
Maternal supplement?^ |
Vitamin A form |
Recommended intake (µg/day) |
Daily exposure (mg/kg bw)* |
|
Centrum Advance multivitamins |
No |
Vitamin A (RE) (25% as beta-carotene) |
800 |
0.011 |
|
Centrum Fruity Chewable |
No |
Vitamin A (RE) |
660 |
0.009 |
|
Centrum MultiGummies |
No |
Vitamin A (RE) |
660 |
0.009 |
|
Centrum Women |
No |
Vitamin A (RE) |
667 |
0.009 |
|
SimplySupplements Cod Liver Oil 1,000 mg |
No |
Vitamin A |
300 - 900 |
0.004-0.013 |
|
Holland & Barrett Cod Liver Oil Pure Liquid 500 ml |
No |
Vitamin A (RE) |
691 |
0.0098 |
|
Seven Seas Cod Liver Oil One-A-Day Omega-3 Fish Oil & Vitamin D 120 Capsules |
No |
Vitamin A (RE) |
750 |
0.011 |
|
Solgar Super Cod Liver Oil Complex - 60 Tablets |
No |
Retinyl palmitate |
906 |
0.014 |
*Exposure is calculated from the daily recommended intake and the average body weight of women aged 16 - 49 years (70.3kg)
^Indicates whether the supplement is marketed specifically to pregnant or breastfeeding women
References
Bates, B.; Lennox, A.; Prentice, A.; Bates, C.; Page, P.; Nicholson, S.; Swan, G. (2014) National Diet and Nutrition Survey Results from Years 1, 2, 3 and 4 (combined) of the Rolling Programme (2008/2009 – 2011/2012) Available at: Main heading (publishing.service.gov.uk)
Bates, B.; Cox, L.; Nicholson, S.; Page, P.; Prentice, A.; Steer, T.; Swan, G. (2016) National Diet and Nutrition Survey Results from Years 5 and 6 (combined) of the Rolling Programme (2012/2013 – 2013/2014) Available at: Main heading (publishing.service.gov.uk)
Food Standards Agency (2021). McCance and Widdowson’s The Composition of Foods Integrated Dataset 2021. McCance_Widdowsons_Composition_of_Foods_Integrated_Dataset_2021..xlsx (live.com)
Roberts, C.; Steer, T.; Maplethorpe, N.; Cox, L.; Meadows, S.; Page, P.; Nicholson, S.; Swan, G. (2018) National Diet and Nutrition Survey Results from Years 7 and 8 (combined) of the Rolling Programme (2014/2015 – 2015/2016) Available at: National Diet and Nutrition Survey (publishing.service.gov.uk)