Second draft statement on the potential risks from ergot alkaloids in the maternal diet
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Introduction
1. The Scientific Advisory Committee on Nutrition (SACN) last considered maternal diet and nutrition in relation to offspring health, in its reports on ‘The influence of maternal, fetal and child nutrition on the development of chronic disease in later life’ (SACN, 2011) and on ‘Feeding in the first year of life’ (SACN, 2018). In the latter report, the impact of breastfeeding on maternal health was also considered. In 2019, SACN agreed to conduct a risk assessment on nutrition and maternal health focusing on maternal outcomes during pregnancy, childbirth and up to 24 months after delivery; this would include the effects of chemical contaminants and excess nutrients in the diet.
2. SACN agreed that, where appropriate, other expert Committees would be consulted and asked to complete relevant risk assessments e.g., in the area of food safety advice. This subject was initially discussed during the horizon scanning item at the January 2020 meeting with a scoping paper being presented to the Committee on the Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) in July 2020. This included background information on a provisional list of chemicals proposed by SACN. It was noted that the provisional list of chemicals was subject to change following discussion by COT who would be guiding the toxicological risk assessment process: candidate chemicals or chemical classes can be added or removed as the COT considered appropriate. The list was brought back to the COT with additional information in September 2020. Following a discussion at the COT meeting in September 2020, it was agreed that papers on a number of components should be prioritised. For this paper, the advice of the COT is sought on whether exposure to ergot alkaloids (EAs) would pose a risk to maternal health.
3. The Committee discussed the potential risk from EAs in the maternal diet (TOX/2022/36) at the COT meeting in July 2022. At this meeting, the Committee noted that only a single study on sirenomelia associated with EAs was included in the discussion paper. Members therefore asked for additional animal studies to be added, if available, to provide supporting evidence. Further information was also requested on the extend of gastrointestinal absorption and the prolactin effect. The requested information has been included in the respective sections of the first draft statement.
4. The first draft statement on ergot alkaloids can be found in Annex A.
Questions for the Committee
The Committee are asked to consider the following question:
a) Do the Committee have any comments on the structure or content of the draft Statement?
b) Do the Committee have any comments on the additional information presented in this draft statement?
c) Does the Committee wish to align with the JECFA ARfD of 0.4 μg/kg bw.
d) Does the Committee have any other comments?
Secretariat
February 2024
Annex A to TOX/2024/01
Introduction
1. The Scientific Advisory Committee on Nutrition (SACN) last considered maternal diet and nutrition in relation to offspring health, in its reports on ‘The influence of maternal, fetal and child nutrition on the development of chronic disease in later life’ (SACN, 2011) and on ‘Feeding in the first year of life’ (SACN, 2018). In the latter report, the impact of breastfeeding on maternal health was also considered. In 2019, SACN agreed to conduct a risk assessment on nutrition and maternal health focusing on maternal outcomes during pregnancy, childbirth and up to 24 months after delivery; this would include the effects of chemical contaminants and excess nutrients in the diet.
2. SACN agreed that, where appropriate, other expert Committees would be consulted and asked to complete relevant risk assessments e.g., in the area of food safety advice. This subject was initially discussed by the COT during the horizon scanning item at the January 2020 meeting with a scoping paper being presented to the Committee on the Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) in July 2020. This included background information on a provisional list of chemicals proposed by SACN. It was noted that the provisional list of chemicals was subject to change following discussion by COT who would be guiding the toxicological risk assessment process: candidate chemicals or chemical classes can be added or removed as the COT considered appropriate. The list was brought back to the COT with additional information in September 2020. Following a discussion at the COT meeting in September 2020, it was agreed that papers on a number of components should be prioritised. The following paper provides the advice of the COT on whether exposure to ergot alkaloids (EAs) would pose a risk to maternal health.
Background
3. Ergot alkaloids (EA) are secondary metabolites produced by the fungi families Clavicipitaceae and Trichocomaceae, with Claviceps purpurea being the most widespread EA producing species in Europe. Infection by these fungi can affect more than 400 plant species, including some economically important cereal grains such as rye, wheat, triticale, barley, millet and oats.
4. The physiological effects of EAs have been known for centuries, including their traditional use in obstetrics. Consumption of contaminated grains, flour or bread caused severe epidemics of a condition known as Erysipelas or St. Anthony’s fire. Since the first systematic investigations in the 1900s, many natural EAs and their synthetic analogues have been used as pharmaceutical agents to treat central nervous system diseases (Tasker and Wipf, 2021).
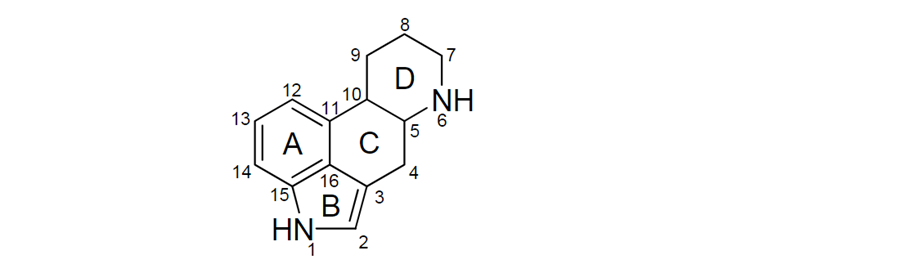
5. Most of the naturally occurring EAs contain a tetracyclic ergoline ring system (Figure 1) consisting of four fused rings with the N6 position carrying a methyl group, and a double bond at either C8,9 or at C9,10 (EFSA, 2012). There are 80 different naturally occurring EAs (Schiff, 2006). Based on their occurrence and the available toxicological data, the European Food Safety Authority (EFSA) considered six EAs in their risk assessment in 2005, namely ergotamine, ergocornine, α-ergocryptine, ergosine, ergocristine (peptide ergot alkaloids) and ergometrine (a lysergic acid amide). For Δ9,10-ergolenes the asymmetric centre at C8 (Figure 1) gives rise to two epimers, with a double bond at C9/10, β-Δ9,10-ergolenes (suffix -ine) and α-Δ9,10-isoergolenes (suffix -inine). While the -inine forms of EAs are considered biologically inactive, interconversion occurs regularly and hence EFSA included both forms of EAs (-ine and inine) in their assessment (EFSA, 2005, Tasker and Wipf, 2021).
Figure 1: Ergoline ring system including numbering and assignment of ring (EFSA, 2012).
6. Bromocriptine is a synthetic ergoline derivate and is used in the treatment of Parkinson’s disease and pituitary tumours (Herdman et al., 2001). Lysergic acid diethylamide (LSD) is a semi synthetic derivative of the EA-family, first introduced in the 1950s, the illegal drug has worldwide recreational (ab)use and is known to cause psychoactive effects.
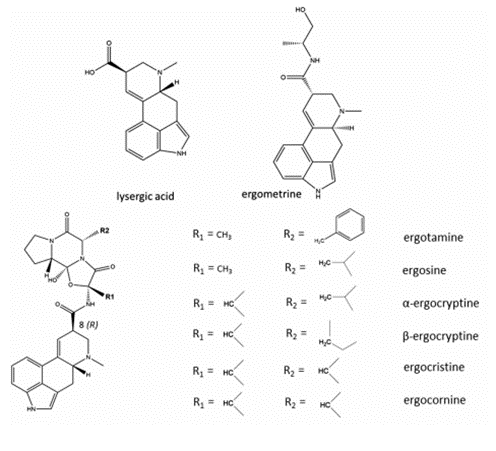
7. Ergometrine is a derivate of lysergic acid (LA), other EAs considered in this evaluation are peptide alkaloids with a cyclized tripeptide as a substituent at C8. The chemical structures of the most prevalent EAs are illustrated in Figure 2 (EFSA, 2012; JECFA,2023).
Figure 2: Chemical structures of different EAs (JECFA, 2023).
Toxicity
8. Due to their structural similarities to neurotransmitters, EAs are considered to be agonists or antagonists of noradrenaline, dopamine and serotonin neurotransmitters (Arroyo-Manzanares et al., 2017, Fitzgerald and Dinan, 2008). EAs have been reported to produce direct peripheral effects such as uterotonic action or vasoconstriction, indirect peripheral effects such as serotonin antagonism or adrenergic blockade, and central nervous system (CNS) effects such as induction of hypothermia and emesis (EFSA, 2012). EAs indirectly affect the CNS by modulating neurotransmitter function, either by mimicking them, blocking their receptors, or modifying their release or reuptake (Cassady et al., 1974).
Toxicokinetics
9. Data in animals and humans suggest that EAs are absorbed from the gastrointestinal (GI) tract and subjected to oxidative biotransformation, thought to involve P450 3A4 (CYP3A4) via hydroxylation in the liver. The intestinal absorption of hydrogenated ergot peptide alkaloids generally varies between 10 and 30 % (Eckert et al., 1978; Olver et al., 1980, Aellig et al., 1977; Little et al., 1982, Wyss et al., 1991). Although CYP3A4 metabolizes EAs via hydroxylation, EAs have also been shown to bind to it as an inhibitor substrate. Recent studies showed that many EAs are derivatives of LA and that P450 monooxygenase and its cluster clavine oxidase (CloA) plays a key role in their biosynthesis (Gerhards et al., 2014, Haarmann et al., 2006; Mulac and Humpf, 2011). Furthermore, an additional CYP is involved in the biosynthetic pathway of EAs in Claviceps purpurea (Haarmann et al., 2006).
10. No studies were available on the toxicokinetics of dietary EAs in humans. However, human data were available on ergotamine used as a pharmaceutical to treat Parkinson’s disease. Absorption of ergotamine from the GI tract was poor after oral/sublingual administration and bioavailability was further reduced by high pre-systemic hepatic metabolism. Ergotamine tartrate can also be given rectally to improve absorption, though bioavailability is still ≤ 5 %. Caffeine can be included in oral and rectal preparations to improve the absorption, however, the effectiveness of this is still unclear (Tfelt-Hansen et al., 2000; Silberstein and McCrory, 2003).
Acute Toxicity
11. In vivo studies identified that animal species differed in their sensitivity to EAs, with rabbits being the most sensitive species with LD50 values between 0.9 and 3.2 mg/kg body weight (bw) following intravenous injection. The LD50s were determined by a series of experiments on naturally occurring and (semi-) synthetic EAs by intravenous (i.v.), subcutaneous (s.c.) and oral exposures (in 2 % gelatine) in mouse, rat and rabbit (Griffith et al., 1978, abstract only cited by EFSA). All naturally occurring EAs demonstrated a low oral acute toxicity compared to i.v. administration, indicating low absorption and high pre-systemic metabolism via the oral route. Based on the LD50s (27.8 – 1200 mg/kg), EFSA concluded EAs to overall exhibit moderate oral acute toxicity (EFSA, 2012).
12. Repeat oral dose studies in rats demonstrated no significant differences in the toxicity of ergotamine, ergometrine and α-ergocryptine, with no-observed-adverse effect levels (NOAELs) ranging from 0.22 - 0.60 mg/kg bw per day (EFSA, 2012).
13. In humans, acute effects are directly related to receptor antagonism and include diarrhoea, and collapse.
14. Exposure to cereal grains contaminated with EAs can also lead to a condition called ergotism (Guggisberg, 2003). There are two main types of ergotism, gangrenous and convulsive. The gangrenous form is caused by the strong vasoconstrictive properties of some EAs, which result in restriction of blood flow to parts of the body (ischemia). In the convulsive form, tingling is followed by neurotoxic symptoms such as hallucinations, delirium, and epileptic-type seizures. It has been suggested that a deficiency in vitamin A together with high concentration of EAs could be a causative factor inducing convulsive ergotism. Additional symptoms of ergotism were lethargy or depression (Arroyo-Manzanares et al., 2017; EFSA, 2012).
15. Limited data were available for individual EAs and their toxic effects on humans. The available data mostly consists of receptor interaction analysis for single substances in dopamine over-expressing cells or tumour cells. Studies indicated that ergocryptine, ergocristine, and bromocryptine produced an elevation in baseline dopamine release of approximately 400 % with effective concentrations of approximately 30 µM (Larson et al.,1995; 1999). No toxic effects were observed in the studies.
16. Because EAs act on several neurotransmitter receptors, particularly adrenergic, dopaminergic and serotonergic receptors, EFSA considered neurotoxicity to be the main acute effect with symptoms such as restlessness, miosis or mydriasis (contraction and dilation of the pupils), muscular weakness, tremor and rigidity in mammals.
Chronic toxicity
Animal studies
17. A study by Valente et al. (2020) showed EAs to be structurally similar to biogenic amines, such as 5-hydroxytryptamine (serotonin), which allowed EAs to interact with serotonin receptors and act as agonists or antagonists when consumed by bovine species. In contrast, a study by Kalkman et al. (1982) showed vasoconstriction based on adrenergic-like receptors in rats injected intravenously with ergometrine.
18. A study by Korn et al. (2014) reported spontaneous alopecia, erosions, crusts and necrosis, specifically of the tail area exclusively in young rabbits aged 113 ± 20 days (14 out of 103 rabbits) in rabbits from a colony, fed with hay and a commercial pelleted feed. The results of the study indicated that EAs may have been the cause of the tail necrosis with immunoassays on blood samples showing mean and maximum EAs concentration of 410 µg/kg and 1,700 µg/kg, respectively. In addition, EAs were detected in the faeces of the affected rabbits at levels up to 200 μg/kg. The mean and maximum dietary intakes of total EAs were 17 and 71 μg/kg bw, respectively. Other toxins, such as fusarium toxin, were also detected in the feed, but at levels which, according to the authors, did not explain the observed effects.
19. Repeated dosing with various EAs, resulted in ischaemia, particularly in the extremities (e.g., tails) of rats, decreased body weight gain and changes in the levels of some hormones. Tail gangrene was observed in rats 5 - 7 days after a single i.p. exposure to 25 mg/kg bw ergotoxine (a mixture including ergocornine, α- and β-ergocryptine, and ergocristine) (Griffith et al., 1978, abstract only cited by EFSA). The NOAELs were 0.22 - 0.60 mg/kg bw per day. No major quantitative difference in the toxicity of ergotamine, ergometrine and α-ergocryptine was observed (EFSA, 2012).
Studies in humans
20. No data were available on the chronic toxicity of EAs from dietary exposure in humans. However, limited information was available from the use of ergot containing medications. Case studies of long-term use of EA medication for migraine headaches reported severe lower extremity claudication (pain in the limbs) due to chronic arterial insufficiency (Garcia et al., 2000; Bogun et al., 2011; Fröhlich et al., 2010; Silberstein and McCrory, 2003). In all instances treatment was discontinued and patients were also asked to discontinue the use of caffeine and cigarettes. Anti-platelet therapy was used to successfully reverse the symptoms.
21. To minimize toxicity and avoid adverse effects such as nausea, vomiting, weakness, muscle pains, paraesthesiae and coldness of the extremities from acute migraine treatment with ergotamine, the dosage was limited to no more than 10 mg per week (Orton and Richardson, 1982; Perrin et al., 1985).
22. Bromocriptine is a synthetic compound with an affinity to dopamine receptors due to its structural similarities to a variety of EAs. It is therefore used as a treatment for Parkinson’s disease and type II diabetes. However, several case reports involving a total of 790 patients, showed adverse side effects in 37 % of patients with severe Parkinson’s disease at doses up to 100 mg/day (Lieberman at al., 1985; Bernard et al., 2015).
Genotoxicity and Carcinogenicity
23. No genotoxicity or mutagenic effects were demonstrated for ergotamine tartrate (Et) in the Salmonella typhimurium (St) assay (10-10,000 µg/plate for 48 hours) and mouse lymphoma TK+/- assay (7.7-108 µg/mL for 4h) (Seifried et al., 2006). An in vitro study by Roberts and Rand (1977) indicated that ergotamine induced chromosomal abnormalities in human lymphocytes and leukocytes. In a study by Dighe and Vaidya (1988) ergotamine, ergonovine and methylergonovine effectively induced sister chromatid exchange (SCE) frequencies in vitro in cultured Chinese hamster ovary (CHO) cells, while ergocristine and α-ergocryptine showed a weak and no effect, respectively.
24. However, due to limited and contradictory data on the genotoxic and mutagenic effects of EAs, EFSA (2012) considered the available genotoxicity studies to be insufficient, except for ergotamine, concluding that the available data on ergotamine did not indicate bacterial or mammalian cell mutation.
25. Due to its inhabitation of prolactin secretion, bromocriptine has been shown to cause an increase in uterine tumours in rats with a lifetime of relative hyperprolactinemia. However, as the mechanism proposed is the reduced luteotrophic effect of prolactin in the rat, and due to the distinctly different mechanisms involved in female reproductive hormonal regulation between humans and rat, these rat tumours are generally considered to have no significance to humans (Harleman et al., 2012)
26. EAs are not considered to be carcinogenic and have not been assessed by the International Agency for Research on Cancer (IARC). However, they have been suggested to be possible cytostatic agents (De Ruyck et al., 2015). Experiments in rodents showed that ergotamine, ergocryptine and ergocornine were able to suppress the growth of pituitary tumours in vivo (MacLeod and Lehmeyer, 1973)
27. More recently a range of mRNA microarray studies investigated the cytotoxic activity of EAs on a range of human cancer cell types and reported strong inhibitory effects for 1-propylagroclavine and dihydroergocristine against genes associated with the progression of leukaemia. The cytotoxic effect pathway is not yet fully understood, but preliminary results suggested that EAs have the potential to be used as possible treatment of otherwise drug-resistant and refractory tumours via the inhibition of prolactin release from the anterior pituitary gland (Cassady et al., 1974; Mrusek et at., 2015).
Reproductive and Developmental toxicity
Pregnancy in animals
28. Several studies reported adverse effects of EAs on the reproductive process in rodents, including prevention of pregnancy predominantly due to interference with implantation, and embryotoxicity (Page et al., 2019; Klotz et al., 2019). Meanwhile studies with stallions showed exposure to EAs could impair or alter normal sexual maturation, which could lead to fertility issues when these colts become sexually mature. (Fayrer-Hosken et al., 2013)
29. The adverse effects on the reproductive process in rodents include prevention of pregnancy by interfering with implantation, embryotoxicity, and inhibition of lactation have generally been observed at higher doses than the lowest-observed-adverse-effect levels (LOAELs) in repeat dose studies for reproductive toxicity (EFSA, 2012). Studies in livestock also reported reduced reproductive performance, particularly in female cattle, after EAs exposure. Regional vasoconstriction and corresponding decreased blood flow to reproductive tissues was observed, along with a decreased dry matter intake, and/or increased body temperature, leading the authors to conclude that the effect of EAs was both direct and indirect (Poole and Poole, 2019).
30. Limited information was available on the effects of EAs exposure during pregnancy, in particular the effects on the vascular system supporting the growing fetus. A study by Duckett et al. (2014) examined fetal growth in sheep following maternal exposure to EAs at a concentration of 0.8 µg of ergovaline/g diet during gestation, this is the equivalent to 0.011μg/kg bw per day based on a 70 kg pregnant ewe. The results demonstrated that exposure to EAs during mid and/or late gestation in ewes reduced fetal growth. A more recent study in ewes indicated that maternal blood supply to the placenta appeared to be shielded from adverse effects of EAs, but umbilical vasculature was not, which could adversely influence normal fetal growth (Klotz et al., 2019). In utero exposure to EAs in pregnant ewes, especially during phase two of gestation was seen to alter fetal growth, muscle fibre formation, and miRNA expression (Greene et al., 2019). Ergovaline was shown to be a potent vasoconstrictor in the bovine umbilical and uterine arteries and reduces blood flow to developing placental tissues and fetuses (Klotz et al., 2015). Placental weight reduction was highly correlated with fetal birthweight and high exposure to EAs in ruminants can result in additional adverse effects such as hyperexcitability, hypermetria, and tremors (Klotz et al., 2015, Britt et al., 2019).
Pregnancy in humans
31. Data from trials on the use of EAs (ergometrine and methylergometrine) as uterotonic medication suggested that EAs may decrease mean blood loss from both mother and child by at least 500 mL and increase maternal haemoglobin levels in the blood. However, the results also suggested the treatment increased the incidence of adverse effects such as increased blood pressure and pain after birth (Liabsuetrakul et al., 2018).
32. One epidemiological study linked the use of purified ergotamine to congenital abnormalities during pregnancy in humans. Ergotamine was used to treat acute migraine and a mean daily dose of 1.5 mg ergotamine during the 2nd month of pregnancy led to a higher risk for neural-tube defects, spina bifida, posterior cleft palate, congenital cataract and clubfoot (Czeizel, 1989). Two further case studies reported an association between the use of ergotamine during early pregnancy and the development of Möbius sequence in children (Smets et al., 2004; Graf and Shepard, 1997). Möbius sequence is a rare congenital disorder defined by the paralysis of the 6th and 7th cranial nerves in combination with various odontological, craniofacial, ophthalmological and orthopaedic conditions (Kjeldgaard Pedersen et al., 2017). Vascular disruption has been suggested as one possible explanation for the pathogenesis of Möbius sequence. Ergotamine has also been reported to cause vasospasm and a prolonged and marked increase in uterine tone (Smets et al., 2004; Graf and Shepard, 1997).
33. A single case study suggested that exposure to methylergonovine maleate (EA derivate) at a critical stage of organogenesis was a possible cause in the development of sirenomelia. Sirenomelia is a rare and deadly condition characterized by fusion of the lower limbs, lower spinal column defects, severe malformations of the urogenital and lower GI tract, and an aberrant abdominal umbilical artery (Cozzolino et al., 2016)
Lactation in animals
34. Intraperitoneal injection of 1 mg ergocornine methane sulfonate in lactating rats temporarily inhibited milk production, the effect being prevented by treatment with prolactin (Zeilmakla and Carlsen, 1962). The potency of EAs to inhibit prolactin secretion in rats decreased in the following order: ergocornine > ergocryptine > dihydro-ergocryptine > dihydro-ergocornine > ergotamine > ergometrine (Griffith et al., 1978, abstract only). Shaar and Clemens (1972) suggested that EAs directly affect the pituitary therefore preventing prolactin secretion, resulting in partial inhibition of lactation. Decreases in prolactin levels were observed in rats of both sexes with increasing amount of α-ergocryptine in the diet. In males, thyroxine (T4) was also significantly decreased and accompanied by a decrease in free thyroxine (FT4) and thyroid-stimulating hormone (TSH). The authors suggested a decrease in T4 may reduce basal metabolic rate (Janssen et al., 2000).
Lactation in humans
35. In a randomised clinical trial to evaluate the effects of EAs on milk secretion postpartum, 30 women received an injection of 0.2 mg methylergobasine immediately after delivery followed by 3 tablets of 1 mg of ergotamine tartrate given daily (orally) for 6 days post-partum. Results showed that the treatment had no significant effect on either the weight of the infant or the quantity of milk consumed (Jolivet et al., 1978; abstract only). A study by Arroyo-Manzanares et al (2017) addressed the similarities of the actions of EAs to those of monoamine neurotransmitters and provided evidence that EAs have the ability to act on the secretion of adrenocorticotropic hormone (ACTH), prolactin (PRL), luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Prolactin has an important biological function in lactation, reproduction, immune responses, and metabolism and inhibition of milk production and the inhibition of prolactin production has been seen in humans, laboratory animals, and livestock animals (Arroyo-Manzanares et al., 2017; Prendiville et al., 2000).
Health-based guidance values
36. EFSA (2012) considered the vasoconstrictive effect as the critical effect for EAs and derived a BMDL10 of 0.33 mg/kg bw per day, based on the finding of tail muscular atrophy in rats fed for 13 weeks with ergotamine. EFSA applied an overall uncertainty factor (UF) of 300, the default UF of 100 for intra- and interspecies differences and an additional UF of 3 to account for deficiencies in the database, to derive an acute reference dose (ARfD) of 1 μg/kg bw (rounded to one significant figure). In line with EFSA’s recommendations, an additional UF of 2 was applied to the derivation of the tolerable daily intake (TDI) for the extrapolation from a sub-chronic to a chronic study. Therefore, an overall UF of 600 was applied to the same BMDL10 of 0.33 mg/kg bw per day to establish a TDI of 0.6 μg/kg bw per day. EFSA concluded that the available data were not sufficient to determine the relative potencies of individual EAs, but the limited data available for some EAs showed no apparent differences in potencies.
37. In 2021, the Joint FAO/WHO Expert Committee on Food Additives (JEFCA), identified ergotamine maleate as the cause of uterine contractions in humans during late pregnancy and postpartum and decided to investigate the role of EAs in the diet (JEFCA, 2021). As ergometrine has the highest uterotonic effect and potency for uterine contractions JECFA established an ARfD based on the lowest oral therapeutic dose of 0.2 mg ergometrine maleate (equivalent to 2.5 μg/kg bw, expressed as ergometrine). An UF of 2 was applied for extrapolating a pharmacological lowest observed effect level (LOEL) to a no-observed effect level (NOEL) and an UF of 3.16 to account for possible interindividual toxicodynamic differences. Applying a composite UF of 6.3 an ARfD of 0.4 μg ergometrine/kg bw was established. JECFA also considered two 4-week studies on ergotamine tartrate and α-ergocryptine in rats and derived a reference point (BMDL10) of 1.3 mg/kg bw, based on muscular degeneration in the tail. However, JECFA considered the human pharmacological effect level of 2.5 μg/kg bw and resulting NOEL to provide a much more sensitive reference point than a downstream toxic effect in animals. A TDI of 1 μg/kg bw per day was initially established by selecting the lowest BMDL10 value of 0.6 mg/kg bw per day. However, JECFA concluded that a TDI should not be higher than the ARfD and hence decided to establish a group TDI for the sum of total EAs in the diet at the same value as the group ARfD of 0.4 μg/kg bw per day.
Sources of EAs exposure
38. The European Union (EU) established maximum levels (ML) of ergot sclerotia and EAs, effective as of January 2022. For milled products derived from barley, wheat, spelt or oats with an ash content of less than 900 mg/100 g, a limit of 100 µg EAs/kg applies, which will be further reduced to 50 µg/kg in July 2024. For the same types of grain products with a higher ash content or sold directly to the end consumer, the maximum level of EAs was set at 150 µg/kg. The maximum level of EAs in wheat gluten is 400 µg/kg. As an open pollination species, rye is generally more susceptible to infestation, which is reflected in a higher maximum level. Milled rye products are subject to an EAs limit of 500 µg/kg, which will be further reduced to 250 µg/kg in July 2024. A maximum level of 20 µg/kg for EAs in grain-based food for infants and toddlers has also been introduced. The levels brought in by the EU for EAs as well as any subsequent changes to these limits do not apply in Great Britian (GB), however they do apply in Northern Ireland (NI).
39. EFSAs estimated chronic dietary exposure in the adult population between 0.007 and 0.08 μg/kg bw per day for average consumers and 0.014 and 0.19 μg/kg bw per day for high consumers. The acute dietary exposure in the adult population ranged between 0.02 and 0.23 μg/kg bw per day for average consumers, and between 0.06 and 0.73 μg/kg bw per day for high consumers. The highest exposure (chronic and acute) was in countries with relatively high consumption of rye bread and rolls. Assessment of the dietary exposure to EAs in specific groups of the population indicated no significant differences between vegetarians and the general population. However, a slightly higher dietary exposure to EAs was noted in consumers of unprocessed grains compared to the general population (EFSA, 2012).
40. The German Federal Institute for Risk Assessment (BfR) based their risk assessment on the consumption of rye flour contaminated with ergotamine and ergometrine. The BfR estimated that on average, ergotamine accounted for a maximum of 46 % of the total alkaloid content. The consumption of 250 g of the most contaminated rye flour would result in an intake of 834 µg ergotamine per day per person. The consumption of highly contaminated rye flour therefore exceeds the maximum therapeutic daily dose tolerated for a month-long therapy of 670 µg ergotamine tartrate per day (BfR, 2004).
41. Caraballo et al. (2019) reported concentrations of up to 47 µg/kg in grains and grain-based composites. In cereal and flour, particularly rye, EAs concentrations of over 7 mg/kg have been reported (Krska and Crews, 2008). Arroyo-Manzanares (2017) carried out an extensive survey on European products and tested 1,065 samples of cereals and cereal products (rye, wheat, and multigrain-based food that contain rye and wheat) intended for human consumption. In total, 59 % of samples tested positive for EAs, with EAs present in 84 % of rye, 67 % of wheat and 48 % of multigrain-based food. The levels ranged from 1 to 12,340 μg/kg. Storm et al. (2011) detected EAs in Danish rye flour samples with an average and maximum concentration of 46 μg/kg and 234 μg/kg, respectively. Crews et al. (2007) detected EAs in 25 of 28 samples, including all 11 types of rye crispbreads with concentration up to 340 μg/kg, while Müller et al. (2009) found EAs in 92 % of the tested rye products with a maximum content of 740 μg/kg. Reinhold et al. (2011) tested 500 food samples from Germany, approximately 50 % were positive for EAs with a highest concentration of 1,063 μg/kg. A more recent survey by Bryła et al. (2015) detected EAs in 83 % of the tested rye grain, 94 % of rye flour, and 100 % of rye bran and flake samples. Ergocryptine, ergocristine, and ergotamine were the most common EAs detected in the majority of surveys and foods sampled. A study by Dusemund et al. (2006) concluded that ergometrine contributed 5 % of the total alkaloid content and that consumption of 250 g of the most contaminated rye flour would result in an intake of 91 µg ergometrine per day per person. This would be below the lowest therapeutic dose equivalent to 400 µg egometrine hydrogen maleate per day.
Exposure Assessment
42. Estimated exposures to EAs were derived using data from the 2014 Total Diet Study-Mycotoxin analysis and consumption data from the National Diet and Nutrition Survey (NDNS) for all groups and subpopulation groups.
43. The total diet study (TDS) data (Stratton et al., 2017) was based on 28 food groups which were further divided to produce 138 food categories. Total EAs and the epimers were determined by LC/MS/MS (Carbonell-Rozas et al., 2021) of which ergocristine, ergotamine, ergocornine, ergosine, ergocryptine, ergometrine, ergocristinine, ergotaminine, ergocorninine, ergosinine, ergocryptinine and ergometrinine were the most frequent forms detected. More data on each specific subset are available in the TDS study report (Diet Study (TDS) – Mycotoxin Analysis Report, 2017).
44. The results of Ergot alkaloids analysis indicated that in some food groups EA was found below the limit of quantification (LOQ), therefore the results of the exposure assessment have been provided as lower bound (LB) and upper bound (UB).
45. The food groups contributing most to EAs exposure were a) wholemeal and granary bread, b) white sliced bread and c) other bread. Mean and 97.5th percentile estimated exposure to EAs from the individual food groups for women of child-bearing age (16- 49 years) can be found in Table 1 (acute) and Table 2 (chronic).
Table 1: Acute exposure to ergot alkaloids in women of childbearing age; food groups not containing EAs have been removed.
|
Food groups |
Exposure (ng/kg bw) LB – UB, Mean |
Exposure (ng/kg bw) LB – UB, P97.5 |
|
White sliced bread |
9.2-9.3 |
41.0 |
|
White unsliced bread |
6.0-6.1 |
34.0-35.0 |
|
Brown bread |
2.3 |
33.0 |
|
Wholemeal and granary bread |
24.0 |
91.0 |
|
Other bread |
15.0 |
68.0 |
|
Misc cereals FLOUR |
1.5-1.7 |
12.0-13.0 |
|
Misc cereals Buns cakes and pastries |
1.9-2.7 |
9.4-13.0 |
|
Misc cereals Savoury biscuits |
0.9 |
7.6-7.7 |
|
Misc cereals Sweet biscuits |
1.5-1.6 |
8.4-9.0 |
|
Misc cereals Chocolate biscuits |
0.6 |
5.4-5.8 |
|
Misc cereals Breakfast cereals |
3.0 |
18.3-18.4 |
|
Misc cereals RICE |
0-6.2 |
0-25.0 |
|
Misc cereals Other cereal products |
1.0-1.7 |
8.8-15.0 |
|
Misc cereals PASTA |
2.2-6.5 |
8.9-27.0 |
|
Misc cereals Pizza |
7.2-7.5 |
54.0-56.0 |
|
Total (sum of all values) |
52.0-57.0 |
120.0-130.0 |
LB= lower bound; UB= upper bound.
Where rounding produced the same value for the upper and lower bound, single figures have been used within the table.
Table 2: Chronic exposure to ergot alkaloids in women of childbearing age; food groups not containing EAs have been removed.
|
Food groups |
Exposure (ng/kg bw) LB – UB, Mean |
Exposure (ng/ng bw) LB – UB, P97 .5th percentile |
|
White sliced bread |
4.0-4.1 |
21.4-21.5 |
|
White unsliced bread |
2.1 |
12.8-12.9 |
|
Brown bread |
0.8 |
10.0 |
|
Wholemeal and granary bread |
0.011 |
52.0 |
|
Other bread |
0.0055 |
29.0 |
|
Misc cereals FLOUR |
0.6 |
4.7-5.2 |
|
Misc cereals Buns cakes and pastries |
0.7-0.9 |
3.8-5.2 |
|
Misc cereals Savoury biscuits |
0.3 |
3.1 |
|
Misc cereals Sweet biscuits |
0.6 |
3.6-3.9 |
|
Misc cereals Chocolate biscuits |
0.2 |
1.8-1.9 |
|
Misc cereals Breakfast cereals |
1.9 |
8.9-9.0 |
|
Misc cereals RICE |
0-2.4 |
0-12 |
|
Misc cereals Other cereal products |
0.3-0.5 |
2.8-4.8 |
|
Misc cereals PASTA |
0.7-2.1 |
3.5-10.0 |
|
Misc cereals Pizza |
1.9-2.0 |
15.0 |
|
Total (sum of all values) |
31.0-35.0 |
72.0-80.0 |
LB= lower bound; UB= upper bound
Where rounding produced the same value for the upper and lower bound, single figures have been used within the table.
Exposures in subpopulation groups
Vegans and Vegetarians
46. The numbers of vegans (n=10) and vegetarians (n=112) among the total number of consumers (n= 2556) were relatively small.
47. For vegans (n=10) the LB (lower bound) and UB (upper bound) mean and 97.5th percentile acute exposures were 64.0 – 70.0 ng/kg bw and 127.0 – 130.0 ng/kg bw, respectively. For vegetarians (n=112) the LB and UB mean and 97.5th percentile exposures were 61.0 – 67.0 ng/kg bw and 135.0 – 140.0 ng/kg bw, respectively.
48. For vegans (n=10) the LB and UB mean and 97.5th percentile chronic exposures were 44.0– 49.0 ng/kg bw and 84.0 – 87.0 ng/kg bw, respectively. For vegetarians (n=112) the LB and UB mean and 97.5th percentile exposures were 38.0 – 43.0 ng/kg bw and 78.0 – 92.0 ng/kg bw, respectively.
Ethnicity
49. The number of Asian or Asian British women of childbearing age (n=135) and Black or Black British women of childbearing age (n=82) were relatively small compared to White women of childbearing age (n=2234).
50. The LB and UB mean and 97.5th percentile acute exposures were 57.8-68.0 ng/kg bw and 110 – 130.0 ng/kg bw for Asian women, respectively. The LB and UB mean and 97.5th percentile acute exposures were 47.0-55.0 ng/kg bw and 100 – 110.0 ng/kg bw for Black women, respectively. For White women the LB and UB mean and 97.5th percentile exposures were 52.0-56.0 ng/kg bw and 120.0 – 130.0 ng/kg bw, respectively.
51. The LB and UB mean and 97.5th percentile chronic exposures were 34.0-46.0 ng/kg bw and 71.0-97.0 ng/kg bw for Asian women, respectively. The LB and UB mean and 97.5th percentile acute exposures were 27.0-33.0 ng/kg bw and 73.0-85.0 ng/kg bw for Black women, respectively. For White women the LB and UB mean and 97.5th percentile exposures were 30.0-34.0 ng/kg bw and 73.0-79.0 ng/kg bw, respectively.
Risk Characterisation
52. The available data suggest that EAs produce direct peripheral effects (uterotonic action or vasoconstriction), indirect peripheral effects (serotonin antagonism or adrenergic blockade), and central nervous system effects (induction of hypothermia and emesis or control of the secretion of pituitary hormones). Due to their structural similarities to neurotransmitters EAs act as agonists or antagonists to noradrenaline, dopamine and serotonin neurotransmitters.
53. Limited data were available for individual EAs and their toxic effects on humans, available data was mostly consistent of receptor interaction analysis for single substances in dopamine over-expressing cells or tumour cells.
54. EAs are not considered carcinogenic and have not been assessed by the International Agency for Research on Cancer (IARC), and due to limited and contradictory data on the genotoxic and mutagenic effects of EAs, EFSA (2012) considered the available genotoxicity studies to be insufficient. However available data on ergotamine did not indicate bacterial or mammalian cell mutation.
55. Exposure to EAs has been associate with pregnancy hindrance by interfering with egg implantation and embryotoxicity in rodents, negative effects on maternal blood supply to the placenta in ewes and possibly sirenomelia associated with in utero exposure in humans. EAs can negatively affect lactation due to their hormone mimicking activity, in particular LH/FSH balance and prolactin (Della-Giustina eta al., 2005).
56. EFSA (2012) established an ARfD of 1 μg/kg bw and a TDI of 0.6 μg/kg bw per day for EAs. JEFCA establish a group TDI for the sum of total EAs in the diet at the same value as the group ARfD of 0.4 μg/kg bw.
57. Using TDS mycotoxin data, mean and 97.5th percentile total acute estimated exposures were 52 to 57 ng/kg bw and 120 to 130 ng/kg bw respectively. Mean and 97.5th percentile total chronic estimated exposures were 31 to 35 ng/kg bw and 72 to 80 ng/kg bw respectively. All estimated exposures are below the respective ARfD and TDI by EFSA and are therefore not of toxicological concern. The estimated exposures are also below the HBGVs established by JECFA.
58. The exposures from food are below any therapeutic doses that have shown adverse effects.
59. The food groups contributing most to the overall exposures were wholemeal and granary bread, white sliced bread and other bread. However, it should be noted that the dietary exposure estimates are based on a limited number of food groups and that data from ready-to-eat foods analysis are scarce. A contribution to overall EAs exposure from other foods can therefore not be excluded.
60. The total exposure was produced using a sum of all categories and therefore makes the assumptions all food groups are consumed which may not be realistic. Furthermore, this means that the 97.5th percentile produces a relatively conservative estimate, especially for chronic exposure as this would make the assumptions that there is a high consumption of all foods groups listed, although it should be noted this approach is beneficial for the Margin of Safety (MoS).
61. The current assessment was based on consumption data from the NDNS for women of maternal/childbearing age and therefore may not be representative of maternal diet. The relatively small data set and limited number of EAs evaluated further add a level of uncertainty to the results.
Conclusions
62. Applying occurrence data from the 2011 TDS for EAs and consumption data from the NDNS for woman of childbearing age, all estimated mean and 97.5th percentile exposures are below the respective ARfD of 1 µg/kg bw and TDI of 0.6 μg/kg bw and are therefore not of toxicological concern. These exposures are also below any therapeutic dose reported to have adverse effects of natural or synthetic EAs.
63. However, it should be noted that the assessment was based on a relatively small sample size (food groups, EAs tested). In addition, the consumption data may not be representative of the maternal diet as the data were for women of childbearing age. The 97.5th percentile provides a conservative estimate, assuming high consumption across all food groups and therefore ensures the margin of safety is sufficient.
COT statement
??/24
February 2024
Abbreviations
|
ACTH |
Adrenocorticotropic hormone |
|
ARfD |
Acute reference dose |
|
BfR |
Bundesinstitut fuer Risikobewertung/German Federal Institute for Risk Assessment |
|
BMDL |
Benchmark Dose Lower Confidence Limit |
|
CNS |
Central nervous system |
|
CONTAM |
Panel on Contaminants in the Food Chain |
|
COT |
Committee on the Toxicity of Chemicals in Food, Consumer Products and the Environment |
|
DM |
Dry matter |
|
DNA |
Deoxyribonucleic acid |
|
EAs |
Ergot alkaloids |
|
EC |
European Commission |
|
EFSA |
European Food Safety Authority |
|
EU |
European Union |
|
FAO |
Food and Agriculture Organization of the United Nations |
|
FSH |
Follicle-stimulating hormone |
|
GI tract |
Gastrointestinal tract |
|
HBGV |
Health based guidance value |
|
IARC |
International Agency for Research on Cancer |
|
i.v. |
Intravenous |
|
JECFA |
Joint FAO/WHO Expert Committee on Food Additives |
|
LA |
Lysergic acid |
|
LB |
Lower bound |
|
LC |
Liquid chromatography |
|
LH |
Luteinizing hormone |
|
LOAEL |
Lowest observed adverse effect level |
|
MOS |
Margin of Safety |
|
Misc |
Miscellaneous |
|
MS |
Mass spectrometry |
|
NDNS |
National Diet & Nutrition Survey |
|
ng/g |
nanograms per gram |
|
NOAEL |
No observed adverse effect level |
|
PRL |
Prolactin |
|
SACN |
Scientific Advisory Committee on Nutrition |
|
sc |
Subcutaneous |
|
SCE |
Sister chromatid exchange |
|
TDI |
Tolerable daily intake |
|
TDS |
Total Diet Study |
|
UB |
Upper bound |
|
UF |
Uncertainty factor |
|
WHO |
World Health Organisation |
|
μg |
μg = microgram |
|
μg/g |
microgram per gram |
|
μg/kg |
microgram per kilogram |
|
μg/L |
microgram per litre |
References
Aellig WH, Nüesch E (1977). Comparative pharmacokinetic investigations with tritium-labeled ergot alkaloids after oral and intravenous administration man. Int J Clin Pharmacol Biopharm. 15(3):106-12. PMID: 403149. Comparative pharmacokinetic investigations with tritium-labeled ergot alkaloids after oral and intravenous administration man - PubMed (nih.gov).
Arroyo-Manzanares N., Gámiz-Gracia L., García-Campaña A.M., Di Mavungu J.D, De Saeger S. (2017). Ergot Alkaloids: Chemistry, Biosynthesis, Bioactivity, and Methods of Analysis. Fungal Metabolites 887-929. DOI: 10.1007/978-3-319-25001-4_1
Bernard N, Jantzem H, Becker M, Pecriaux C, Bénard-Laribière A, Montastruc JL, Descotes J, Vial T (2015). Severe adverse effects of bromocriptine in lactation inhibition: a pharmacovigilance survey. Royal College of Obstetricians and Gynaecologists, 122(9): 1244-51. DOI https://doi.org/10.1111/1471-0528.13352
Bogun N, Mathies R, Baesecke J (2011). Angiospastic occlusion of the superficial femoral artery by chronic ergotamine intake. Deutsche Medizinische Wochenschrift, 136(1-2): 23-6. DOI: https://doi.org/10.1055/s-0030-1269435
Bryła M, Szymczyk K, Jędrzejczak R, Roszko M (2015). Application of liquid chromatography/ ion trap mass spectrometry technique to determine ergot alkaloids in grain products. Food Technol Biotechnol 53:18–28. DOI https://doi.org/10.17113/ftb.53.01.15.3770
Britt JL, Greene MA, Bridges Jr WC, Klotz JL, Aiken GE, Andrae JG, Pratt SL, Long NM, Schrick FN, Strickland JR, Wilbanks SA, Miller Jr MF, Koch BM, Duckett SK (2019). Ergot alkaloid exposure during gestation alters. I. Maternal characteristics and placental development of pregnant ewes. Journal of Animal Science, 97(4):1874-90. DOI: https://doi.org/10.1093%2Fjas%2Fskz068
Caraballo D, Toloso J, Ferrer E, Berrada H (2019). Dietary exposure assessment to mycotoxins through total diet studies. A review. Food and Chemical Toxicology, 128: 8-20. DOI: https://doi.org/10.1016/j.fct.2019.03.033
Carlsen RA, Zeilmaker GH, Shelesnyak MC. (1961). Termination of early (pre-nidation) pregnancy in the mouse by single injection of ergocornine methanesulfonate. J Reprod Fertil. 2:369–73. DOI: https://doi.org/10.1530/jrf.0.0020369
Cassady JM, Li GS, Spitzner EB, Floss H, Clemens JA (1974). Ergot alkaloids. Ergolines and related compounds as inhibitors of prolactin release. J Med Chem. (3):300-7. DOI: https://doi.org/10.1021/jm00249a009
Chestnut D.H. Chronic Pain during and after Pregnancy (2020). Chestnut's Obstetric Anesthesia. DOI Book.
Cozzolino M, Riviello C, Fichtel G, Di Tommaso M (2016). Brief Report Exposure to Methylergonovine Maleate as a Cause of Sirenomelia. Birth Defects Research Part A: Clinical and Molecular Teratology, 106(7): 643-7. DOI: https://doi.org/10.1002/bdra.23503
COT (2017). Review of potential risks from mycotoxins in the diet of infants aged 0 to 12 months and children aged 1 to 5 years. Available at: tox2017-30_0.pdf (food.gov.uk).
Crews C, Anderson WAC, Rees G, Krska R (2009). Ergot alkaloids in some rye-based UK cereal products. Food Addit Contam Part B Surveill 2:79–85. DOI: https://doi.org/10.1080/02652030903042509
Czeizel A (1989). Teratogenicity of ergotamine J Med Genet 26(1):69-70. DOI:
http://dx.doi.org/10.1136/jmg.26.1.69-a
Della-Giustina K and Chow G (2003). Medications in pregnancy and lactation. Emergency Medicine Clinics of North America, 21(3): 585-613. DOI:
https://doi.org/10.1016/s0733-8627(03)00037-3
De Ruyck K, De Boevre M, Huybrechts I, De Saeger S (2015). Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutation Research/Reviews in Mutation Research, 766: 32-41. DOI: https://doi.org/10.1016/j.mrrev.2015.07.003
Duckett SK, Andrae JG, Prat SL (2014). Exposure to Ergot alkaloids during gestation reduces fetal growth in sheep. Frontiers in Chemistry, 21: 2:68. DOI: https://doi.org/10.3389/fchem.2014.00068
Dighe, R., Vaidya, V.G., (1988). Induction of sister chromatid exchanges by ergot compounds in Chinese hamster ovary cells in vitro. Teratog. Carcinog. Mutagen. 8, 169–174. DOI: https://doi.org/10.1002/tcm.1770080306
Duesterhoeft S.M., Ernst L.M., Siebert J.R., Kapur RK (2007). Five cases of caudal regression with an aberrant abdominal umbilical artery: Further support for a caudal regression-sirenomelia spectrum. Am J Med Genet A. 15;143A(24):3175-84. DOI: 10.1002/ajmg.a.32028
Dusemund B, Altmann H-J and Lampen A, (2006). II. Toxikologische Bewertung Mutterkornalkaloidkontaminierter Roggenmehle. Journal of Consumer Protection and Food Safety, 1, 150-152. „ „Mutterkornalkaloide in Lebensmitteln“ | Journal of Consumer Protection and Food Safety (springer.com)
Eckert, H., Kiechel, J.R., Rosenthaler, J., Schmidt, R., Schreier, E. (1978). Biopharmaceutical Aspects. In: Berde, B., Schild, H.O. (eds) Ergot Alkaloids and Related Compounds. Handbook of Experimental Pharmacology / Handbuch der experimentellen Pharmakologie, vol 49. Springer, Berlin, Heidelberg. DOI: 10.1007/978-3-642-66775-6_11
EFSA (2012). Scientific Opinion on Ergot alkaloids in food and feed. EFSA Journal, 10(7): 2798. Available at: Ergot alkaloids in food and feed | EFSA (europa.eu)
Commission Regulation (EU) 2021/1399 of 24 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of ergot sclerotia and ergot alkaloids in certain foodstuffs (Text with EEA relevance). Available at: Regulation - 2021/1399 - EN - EUR-Lex (europa.eu).
Fitzgerald P and Dinan TG (2008). Prolactin and dopamine: what is the connection? A review article. Journal of Psychopharmacology, 22(2 Suppl), 12-9. DOI: https://doi.org/10.1177/0269216307087148
Fayrer-Hosken R.A., Hill N.S., Heusner G.L., Traylor-Wiggins W., Turner K (2013). The effects of ergot alkaloids on the breeding stallion reproductive system. Equine Vet J Suppl 12 (45):44-7. DOI: https://doi.org/10.1111/evj.12164
Fröhlich G, Kaplan V, Amann-Vesti B (2010). Holy fire in an HIV-positive man: a case of 21st century ergotism. Canadian Medical Association Journal, 182 (4): 378-80. DOI: https://doi.org/10.1503%2Fcmaj.091362
FSA (2014). Monitoring the Presence of Ergot Alkaloids in Cereals and a Study of a Possible Relationship between Occurrence of Sclerotia Content and Levels of Ergot Alkaloids. Available at: Monitoring the presence of ergot alkaloids in cereals | Food Standards Agency.
Garcia GD, Goff Jr JM, Hadro NC, I-Donnell SD, Greatorex PS (2000). Chronic ergot toxicity: A rare cause of lower extremity ischemia. Journal of Vascular Surgery, 31(6): 1245-7. DOI: https://doi.org/10.1067/mva.2000.105668
Gerhards N, Neubauer L, Tudzynski P, Li SM (2014). Biosynthetic Pathways of Ergot Alkaloids. Toxins, 6(12):3281-95. DOI: https://doi.org/10.3390%2Ftoxins6123281
Greene MA, Britt JL, Powell RP, Feltus FA, Bridges WC, Bruce T, Klotz JL, Miller MF, Duckett SK (2019). Ergot alkaloid exposure during gestation alters: 3. Fetal growth, muscle fiber development, and miRNA transcriptome. Journal of Animal Science, 97(7):3153-68. DOI: https://doi.org/10.1093/jas/skz153
Graf WD and Shepard TH (1997). Uterine contraction in the development of Möbius syndrome. Journal of Child Neurology,12(3): 225-7. DOI: https://doi.org/10.1177/088307389701200315
Griffith, R.W., Grauwiler, J., Hodel, Ch., Leist, K.H., Matter, B., 1978. In: Berde, B., Schild, H.O. (Eds.), Toxicologic Considerations. Ergot Alkaloids and Related Compounds. Springer Verlag, Berlin, Heidelberg, New York, pp. 805–851, abstract only. DOI: https://rd.springer.com/book/10.1007/978-3-642-66775-6
Guggisberg H (1954). Changes in the therapeutic use of ergot. Ther Umsch. 11(4):77-9. DOI n/a.
Haarmann T, Ortel I, Tudzynski P, Keller U (2006). Identification of the cytochrome P450 monooxygenase that bridges the clavine and ergoline alkaloid pathways. ChemBioChem – Chemistry Europe. 7(4):645-52. DOI: https://doi.org/10.1002/cbic.200500487
Hartleman J, Hargreaves A, Andersson H, Kirk S (2012). A Review of the Incidence and Coincidence of Uterine and Mammary Tumors in Wistar and Sprague-Dawley Rats Based on the RITA Database and the Role of Prolactin. Toxicologic Pathology. 40(6):926-930. DOI: https://doi.org/10.1177/0192623312444621
Janssen GB, Beems RB, Elvers LH, Speijers GJ (2000b). Subacute toxicity of α-ergocryptine in Sprague-Dawley rats. 2: metabolic and hormonal changes. Food Chem Toxicol. 38:689–95. DOI: https://doi.org/10.1016/S0278-6915(00)00055-7
JECFA (2021). Safety evaluation of certain food additives and contaminants. Summary report of the ninety-first meeting. Available at: Evaluation of certain food additives and contaminants: ninety-first report of the Joint FAO/WHO Expert Committee on Food Additives.
JECFA (2023). Safety evaluation of certain contaminants in food: prepared by the ninety-first meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available at: Safety evaluation of certain contaminants in food: prepared by the ninety-first meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA)
Jolivet A., Robyn C., Huraux-Rendu C., Gautray J.P. (1978). Effect of ergot alkaloid derivatives on milk secretion in the immediate postpartum period. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 7(1):129-134. DOI clinical trial.
Kalkman, H.O.; Van Gelderen, E.M.; Timmermans, P.B.; Van Zwieten, P.A. (1982) Involvement of α1- and α2-adrenoceptors in the vasoconstriction caused by ergometrine. Eur. J. Pharmacol. 78, 107–111. DOI: https://doi.org/10.1016/0014-2999(82)90377-6
Klotz J. (2015).Activities and Effects of Ergot Alkaloids on Livestock Physiology and Production. Toxins (Basel) 7(8), 2801-2821. DOI: https://doi.org/10.3390/toxins7082801
Klotz JL, Britt JL, Miller MF, Snider MA, Aiken GE, Long NM, Pratt SL, Andrae GJ, Duckett SK (2019). Ergot alkaloid exposure during gestation alters: II. Uterine and umbilical artery vasoactivity. Journal of Animal Science, 97(4): 1891-1902. DOI: https://doi.org/10.1093/jas/skz069
Korn AK, Gross M, Usleber E Thom N, Köhler K, Erhardt G (2014). Dietary ergot alkaloids as a possible cause of tail necrosis in rabbits. Mycotoxin Residues, 30(4):241–50. DOI: https://doi.org/10.1007%2Fs12550-014-0208-0
Krska R and Crews C (2008). Significance, chemistry and determination of ergot alkaloids: a review. Food Additives and Contaminants: Part A, chemistry, analysis, control, exposure and risk assessment, 25(6):722-31. DOI: https://doi.org/10.1080/02652030701765756
Larson, B.T., Harmon, D.L., Piper, E.L., Griffis, L.M., Bush, L.P. (1999). Alkaloid binding and activation of D2 dopamine receptors in cell culture. J. Anim. Sci. 77, 942–947. DOI: https://doi.org/10.2527/1999.774942x
Larson, B.T., Samford, M.D., Camden, J.M., Piper, E.L., Kerley, M.S., Paterson, J.A., Turner, J.T., (1995). Ergovaline binding and activation of D2 dopamine receptors in GH4ZR7 cells. J. Anim. Sci. 73, 1396–1400. DOI: https://doi.org/10.2527/1995.7351396x
Liabsuetrakul T., Choobun T., Peeyananjarassri K., Monir Islam Q. (2018). Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database Syst Rev 7;6(6):CD005456. DOI: https://doi.org/10.1002/14651858.CD005456.pub3
Lieberman AN and Goldstein M (1985). Bromocriptine in Parkinson disease. Pharmacological Review. 37(2) 217-27. Bromocriptine in Parkinson disease - PubMed (nih.gov) (No DOI available).
MacLeod RM and Lehmeyer J (1973). Suppression of Pituitary Tumor Growth and Function by Ergot Alkaloids. Cancer Research 33, 849-855, DOI not available.
Mrusek M et al (2015). Identification of cellular and molecular factors determining the response of cancer cells to six ergot alkaloids. Invest New Drugs. 33(1):32-44. DOI: https://doi.org/10.1007/s10637-014-0168-4
Mulac D and Humpf HU (2011). Cytotoxicity and accumulation of ergot alkaloids in human primary cells. Toxicology, 282(3):112–21. DOI: https://doi.org/10.1016/j.tox.2011.01.019
Müller C, Kemmlein S, Klaffke H, Krauthause W, Preiss-Weigert A, Wittkowski R (2009). A basic tool for risk assessment: a new method for the analysis of ergot alkaloids in rye and selected rye products. Mol Nutr Food Res 53:500–507. DOI: https://doi.org/10.1002/mnfr.200800091
Kjeldgaard Pedersen L, Rikke Damkjær Maimburg R, Hertz JM, Gjørup H, Klit Pedersen T, Møller-Madsen B, Rosendahl Østergaard J (2017). Moebius sequence – a multidisciplinary clinical approach. Orphanet Journal of Rare Diseases, 12(1):4. DOI: https://doi.org/10.1186/s13023-016-0559-z
Little PJ, Jennings GL, Skews H, Bobik A (1982). Bioavailability of dihydroergotamine in man. Br J Clin Pharmacol, 13(6):785-90. DOI: 10.1111/j.1365-2125.1982.tb01866.x.
Olver IN, Jennings GL, Bobik A, Esler M (1980). Low bioavailability as a cause of apparent failure of dihydroergotamine in orthostatic hypotension. British Medical Journal. 281(6235):275-276. DOI: 10.1136/bmj.281.6235.275-a.
Orton DA and Richardson RJ (1982). Ergotamine absorption and toxicity. Postgraduate Medical Journal, 58(675): 6- 11. DOI: https://doi.org/10.1136%2Fpgmj.58.675.6
Page R, Lester T, Rorie R, Rosenkrans Jr C (2019). Ergot Alkaloid Effects on Bovine Sperm Motility In Vitro. Advance in Reproductive Science, 7(1): 7-15. DOI: https://doi.org/10.4236/arsci.2019.71002
Poole R and Poole DH (2019). Impact of Ergot Alkaloids on Female Reproduction in Domestic Livestock Species. Toxins, 11(6): 364. DOI: https://doi.org/10.3390/toxins11060364
Prendiville WJ, Elbourne D, McDonals S (2000). Active versus expectant management in the third stage of labor. Cochrane Database Systematic Reviews, 3. DOI: https://doi.org/10.1002/14651858.cd000007
Perrin VL (1985). Clinical pharmacokinetics of ergotamine in migraine and cluster headache. Clinical Pharmacokinetics 10(4): 334–52. DOI:https://doi.org/10.2165/00003088-198510040-00004
Reinhold L, Reinhardt K (2011). Mycotoxins in foods in Lower Saxony (Germany): results of official control analyses performed in 2009. Mycotoxin Res 27:137–143. DOI: https://link.springer.com/article/10.1007/s12550-011-0086-7
Roberts G and Rand MJ (1977). Chromosomal damage induced by some ergot derivatives in vitro. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 48 (2):205-214. DOI: https://doi.org/10.1016/0027-5107(77)90162-2
Carbonell-Rozas L, Gamiz-Gracia L, Lara FJ, Garcia-Campana AM (2021). Determination of the main ergot alkaloids and their epimers in oat-based functional foods by ultra-high performance liquid chromatography tandem mass spectrometry. Molecules, 26(12): 3717. DOI: https://doi.org/10.3390/molecules26123717
Seifried, H.E., Seifried, R.M., Clarke, J.J., Junghans, T.B., San, R.H.C. (2006). A compilation of two decades of mutagenicity test results with the Ames Salmonella typhimurium and L5178Y mouse lymphoma cell mutation assays. Chem. Res. Toxicol. 19, 627–644. DOI: https://doi.org/10.1021/tx0503552
Shaar CJ, Clemens JA (1972). Inhibition of lactation and prolactin secretion in rats by ergot alkaloid. Endocrinology. 90:285–88 [abstract only]. DOI: https://doi.org/10.1210/endo-90-1-285
Silberstein SD and McCrory DC (2003). Ergotamine and dihydroergotamine: history, pharmacology, and efficacy. Headache, 43(2): 144-66. DOI: https://doi.org/10.1046/j.1526-4610.2003.03034.x
Smets K, Zecic A, Willems J (2004). Ergotamine as a possible cause of Möbius sequence: additional clinical observation. Journal of Child Neurology, 19(5): 398. DOI: https://doi.org/10.1177/088307380401900518
Storm ID, Have Rasmussen P, Strobel BW, Hansen HCB (2008). Ergot alkaloids in rye flour determined by solid-phase cation-exchange and high-pressure liquid chromatography with fluorescence detection. Food Addit Contam 25:338–346. DOI: https://doi.org/10.1080/02652030701551792
Stratton J, Anderson S, Leon I, Hepworth P, Chapman S, Christy J, Jardine S, Philips D, Setter R, Clough J, MacDonals S (2017). Diet Study (TDS) – Mycotoxin Analysis. Final Report, FS102081. Fera Science Ltd, York (UK). Available at : Diet Study (TDS) – Mycotoxin Analysis: Final report (food.gov.uk)
Tasker NR and Wipf P (2021). Biosynthesis, total synthesis, and biological profiles of Ergot alkaloids. Alkaloids Chemical Biology, 85:1-112. DOI: https://doi.org/10.1016/bs.alkal.2020.08.001
Tfelt-Hansen, P., Saxena, P.R., Dahlof, C., Pascual, J., Lainez, M., Henry, P.,Diener, H.,Schoenen, J., Ferrari, M.D., Goadsby, P.J., (2000). Ergotamine in the acute treatment of migraine: a review and European consensus. Brain 123 (Pt 1), 9–18. DOI: 10.1093/brain/123.1.9
Valente Eel, Klotz JL, Ahn G, McLeod KR, Herzing HM, King M, Harmon DL (2020) Ergot alkaloids reduce circulating serotonin in the bovine. Journal of Animal Science, 98(12). DOI: http://dx.doi.org/10.1093/jas/skaa362
Wyss, P.A., Rosenthaler, J., Nüesch, E., Aellig, W.H (1991). Pharmacokinetic investigation of oral and IV dihydroergotamine in healthy subjects. Eur J Clin Pharmacol 41, 597–602. DOI: https://doi.org/10.1007/BF00314992
Appendix A
Literature Search Terms for Ergot Alkaloids (January 2022 - June 2022)
acute toxicity
chronic toxicity
reproductive toxicity
biomarkers (exposure/ toxicity)
maternal health
preconception
conception
pregnancy
post-natal
lactation
fetus/ foetus/ fetal /foetal
placenta
pre-term
preeclampsia
cancer/ carcinogen(icity)
teratogen(icity)
absorption
distribution
metabolism
Appendix B
Food groups analysed in the TDS for EAs
Table 5: List of all food groups analysed for ergot alkaloids (EAs).
|
Food groups and Category |
Occurrence data total EAs (µg/kg) |
|
Bread |
Bread |
|
White sliced bread |
14.08 |
|
White unsliced bread |
11.88 |
|
Brown bread |
27.29 |
|
Wholemeal and granary bread |
33.69 |
|
Other bread |
23.29 |
|
Miscellaneous cereals |
Miscellaneous cereals |
|
Flour |
19.46 |
|
Buns cakes and pastries |
8.25 |
|
Savoury biscuits |
2.23 |
|
Sweet biscuits |
9.34 |
|
Chocolate biscuits |
4.90 |
|
Breakfast cereals |
3.07 |
|
Rice |
7.08 |
|
Other cereal products |
0.00 |
|
Pasta |
0.64 |
|
Pizza |
0.00 |
|
Group sample |
6.94 |
|
Non-alcoholic beverages |
Non-alcoholic beverages |
|
Branded food drinks |
4.30 |
|
(With bottles water) |
(With bottles water) |
|
Alternatives to milk |
0.00 |
|
Alcoholic drinks |
Alcoholic drinks |
|
Beer |
0.00 |
|
Alcoholic drinks |
Alcoholic drinks |
|
Cider |
0.00 |
|
Snacks |
Snacks |
|
Other snacks (not potato based) |
3.65 |
|
Sandwiches |
Sandwiches |
|
Sandwiches |
13.46 |
|
Sandwiches |
Sandwiches |
|
Group sample |
n/a |