Advancing in silico methods of assessing toxicological risk
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Introduction
1. The FSA and COT have been reviewing New Approach Methodologies (NAMs) to scope the best scientific methodologies available to be used in risk assessment of chemicals in foods and the environment, and to understand how these can be incorporated and accepted in a regulatory context.
2. In 2021, the FSA started funding a 4-year computational toxicology postdoctoral fellow Dr Arthur de Carvalho e Silva at the University of Birmingham and a three-year PhD Student Mr Alexander Kalian (London Interdisciplinary Doctoral Program-LIDo-TOX AI) at King’s College London.
3. The fellow and PhD student have been working alongside other government departments to understand how NAMs will improve indicative levels of safety in chemical risk assessment.
4. In addition, these new partnerships have helped with networking, research collaboration, training opportunities and furthering our knowledge in this area. The fellowship and studentship also compliment the work set out in the COT FSA UK NAMs Roadmap towards using new approach methodologies in chemical risk assessment.
5. The Fellow and the PhD student have prepared a yearly review as outlined below and will present their progress to date to the COT Members. An update was provided by Mr Kalian to Members at the October 2023 meeting.
Postdoctoral Fellow Update
Why and how are you associated with the FSA?
6. The FSA and COT have been reviewing new approach methodologies (NAMs) and developing a UK NAMs roadmap towards the integration and acceptance of NAMs for chemical risk assessment. One of the activities defined in this roadmap was to actively work on advancing in silico methods for assessing toxicological risk, specifically focused on food-related chemicals, but remaining open to work on other classes of chemicals relevant to the FSA’s risk assessments. In this context, I was recruited as a computational toxicology fellow and awarded a 4-year fellowship funded by the FSA, whilst supervised by a team of academic and applied NAM experts. The supervisory team is composed of Prof. Mark Viant and Prof. John Colbourne (University of Birmingham), Dr. George Loizou (HSE Science and Research Centre), and Dr. Olivia Osborne, Ms. Claire Potter, and Dr. David Gott (FSA).
Broad overview of the FSA fellowship and its aims
7. The programme of work of the fellowship consists of (i) scoping the FSA’s problem space in chemical risk assessment and mapping this to our computational NAMs solution space, thereby aiding the FSA to develop a strategy for the utilisation of NAMs (months 1-24); (ii) ensuring that the FSA is trained in the use of computational NAMs by delivering training courses, including an introduction to existing and emerging NAM technologies, and topics selected from the FSA’s NAM strategy (months 1-48); (iii) developing and evaluating confidence in a new hazard assessment workflow that integrates in vitro omics toxicity data, benchmark dose modelling and PBPK modelling to serve as the basis for quantitative risk assessment for human health, i.e. towards generating human health-based safety thresholds for the FSA and other regulators (months 1-36); and (iv) developing and delivering a second case study that fortifies the community-wide acceptance of 21st century methods in risk assessments, to accelerate the successful application of NAMs within the FSA (months 25-48).
Progress with the first case study
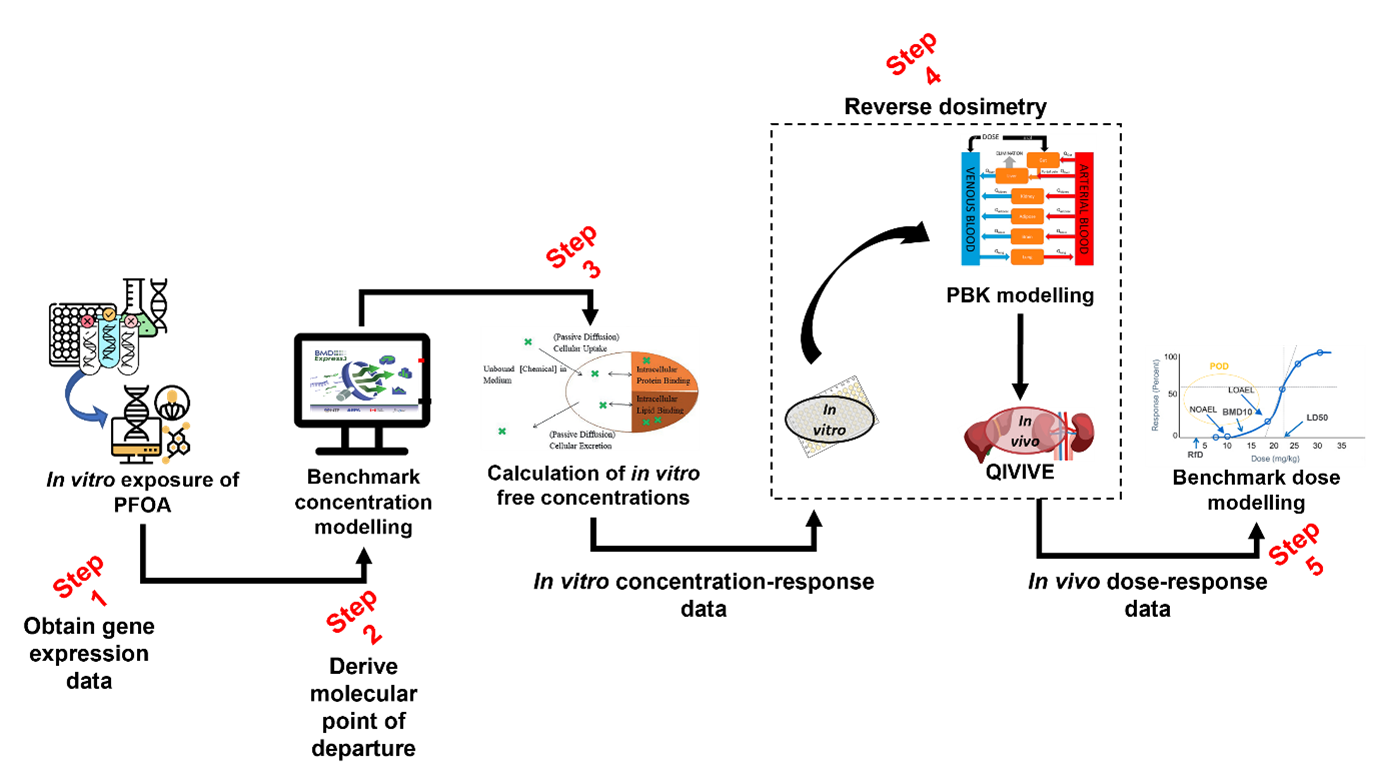
8. The workflow implemented and tested is described in Figure 1. Through its five steps, it seeks to utilise multiple NAM approaches, ultimately to generate a human-health based point of departure for risk assessment. The NAMs being employed include:
● NAMs in relation to the type of testing platform - in vitro hepatic microtissues;
● NAMs in relation to the type of data/read-outs - transcriptomics data, providing an untargeted measurement of extensive gene expression;
● NAMs in relation to data analysis - PBPK modelling.
9. The systematic integration of various data generation and modelling approaches has required a broad range of expertise, with steps 1-2 predominantly guided by the University of Birmingham team and steps 3-5 by the HSE Science & Research Centre.
10. To date, two case studies have been conducted. The first case study focused on the plasticiser di-2-ethylhexyl terephthalate (DEHTP). The main objective was to derive a health-based guidance value mainly focusing on steps 3-5 of the workflow (Figure 1). Concentration-response data obtained from ToxCast, via the Chemicals Dashboard (US EPA), was used. The second case study had as the chemical of choice, a perfluorinated substance, perfluorooctanoic acid (PFOA). The main objective was to integrate our in-silico workflow with transcriptomics data to derive a health-based guidance value for PFOA that could be compared with that previously published by the European Food Safety Authority (EFSA). Transcriptomics data published by Health Canada (Rowen-Carrol et al., 2021) was used as a data source from in vitro exposures of human liver microtissues to PFOA.
Figure 1. In silico workflow to generate a human health-based point of departure for risk assessment.
Progress with papers and conferences
11. The DEHTP and PFOA case studies are both intended for publication. It will report the utility of the workflow and the integration of the calculation of in vitro free concentrations as an important step towards deriving a health-based guidance value. The case studies are being written and being revised by the co-authors. Our recent work on PFOA has been presented on several occasions. To list a few, PARC Science Day (poster presentation), NURA Dynamic Discussions (oral presentation, online), HSE’s workshop (oral presentation, online), EFSA’s workshop (oral presentation, online), EUROTOX 2023 (poster presentation), ASPIS Open Symposium 2023 (poster presentation). PFOA was submitted as a nomination to the Lush Prize under the Young Researcher category and has been one of the five projects awarded in 2022. The third case study is now under consideration by the supervisory team, which is keen to work with tropane alkaloids as this class of substances is of high interest to the FSA.
Secretariat
January 2024
References
Rowan-Carroll A, Reardon A, Leingartner K, Gagné R, Williams A, Meier MJ, et al. Toxicological Sciences. 2021;181(2):199-214.